The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/HO-1/HMGB1 Axis
Abstract
1. Introduction
2. Materials and Methods
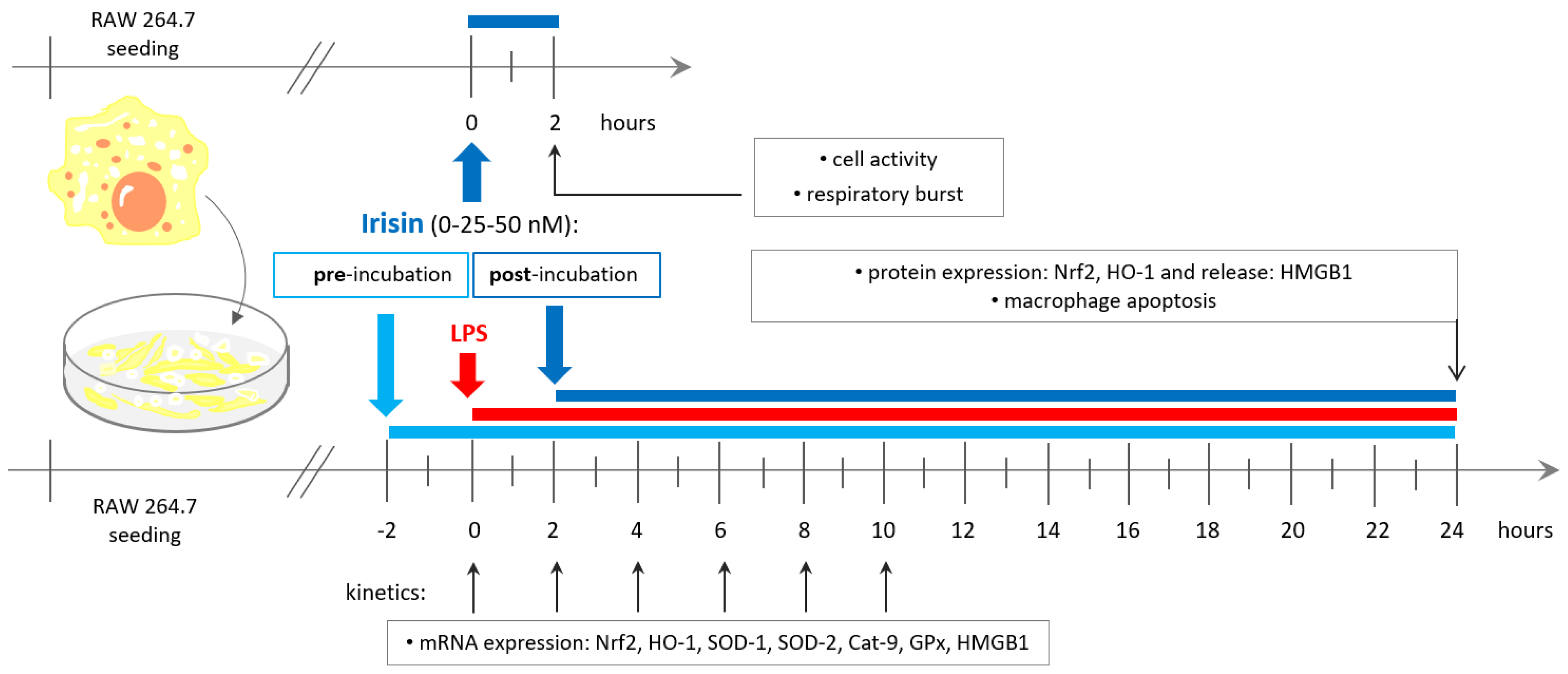
2.1. Cell Culture and Experimental Design
2.2. Cell Activity and Respiratory Burst Generation
2.3. Apoptosis Assay
2.4. Quantitative Real-Time PCR (qPCR)
2.5. Western Blot Analysis of Nrf2 and HO-1 Protein Expression
2.6. Examination of HMGB1 Release
2.7. Statistical Analysis
3. Results
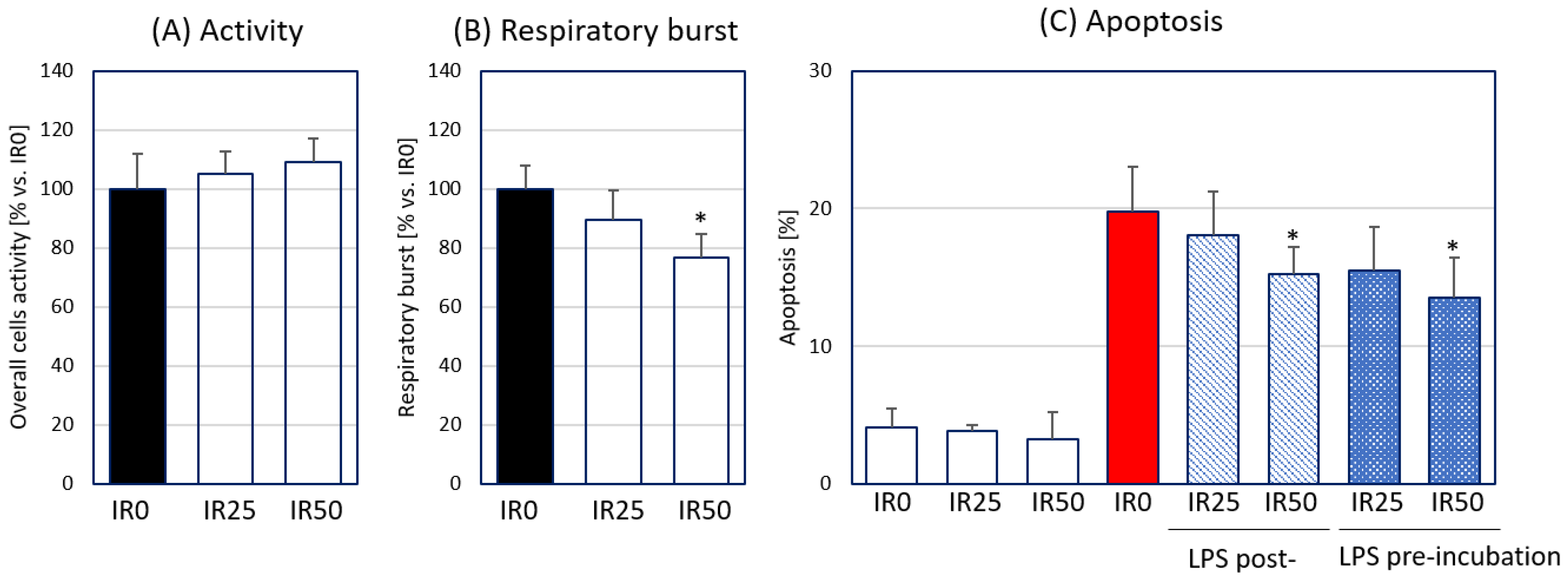
3.1. Irisin Attenuates Respiratory Burst Generated by Macrophages and Their Apoptosis
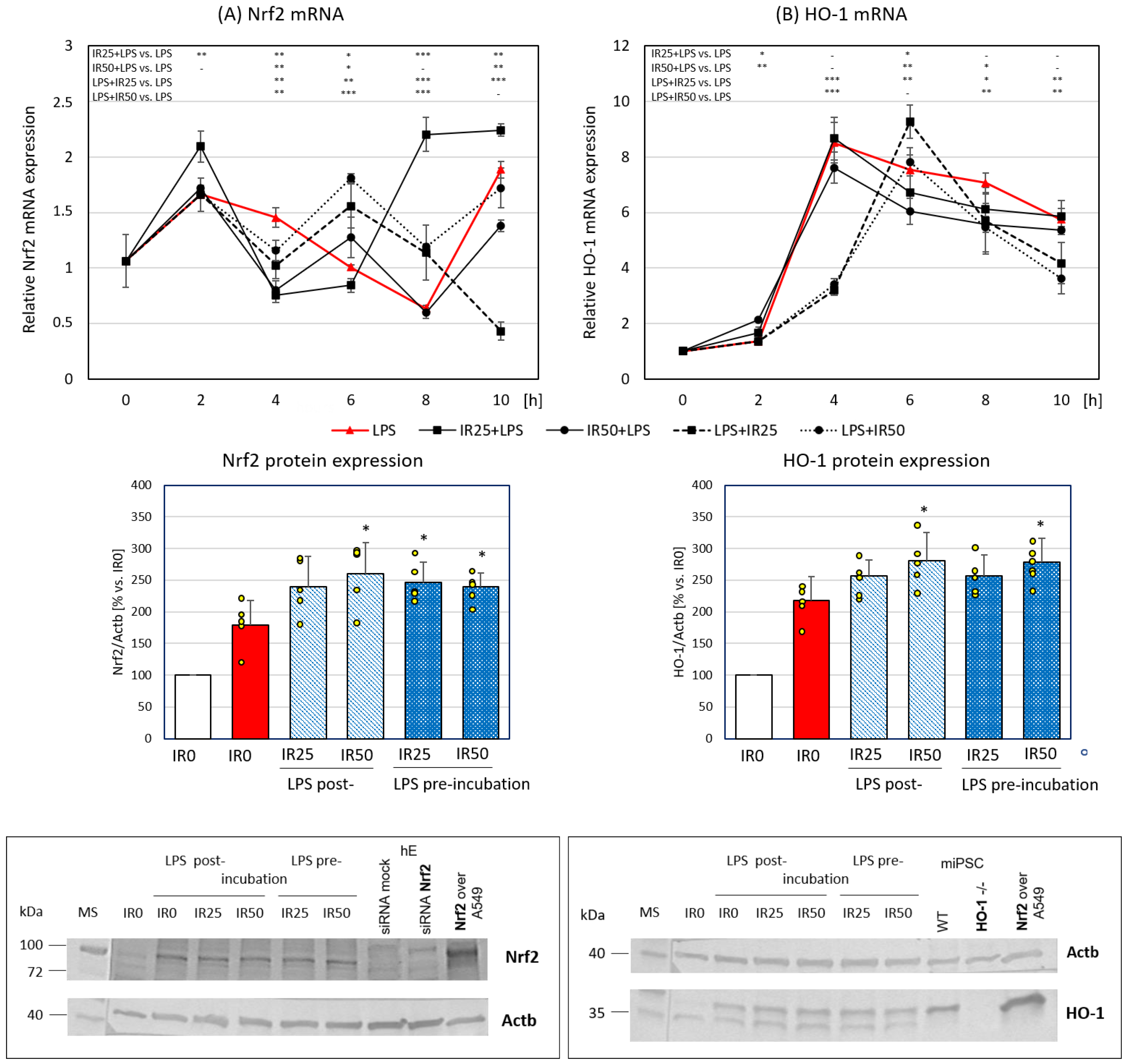
3.2. Irisin Effects on Nrf2 and HO-1 Gene and Protein Expression
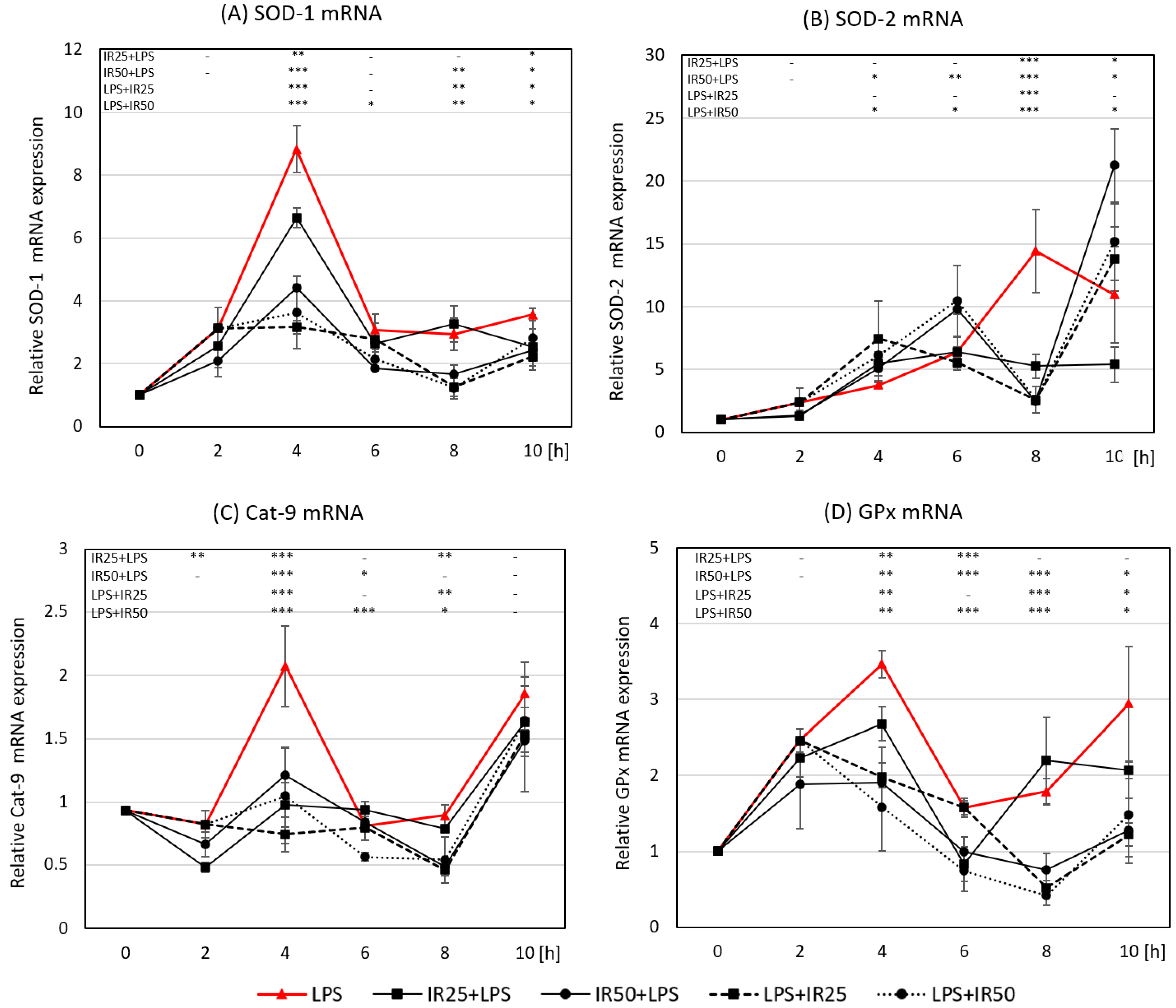
3.3. Irisin Effects on the Expression of Crucial Antioxidant Enzymes SOD-1, SOD-2, GPx, and Cat-9
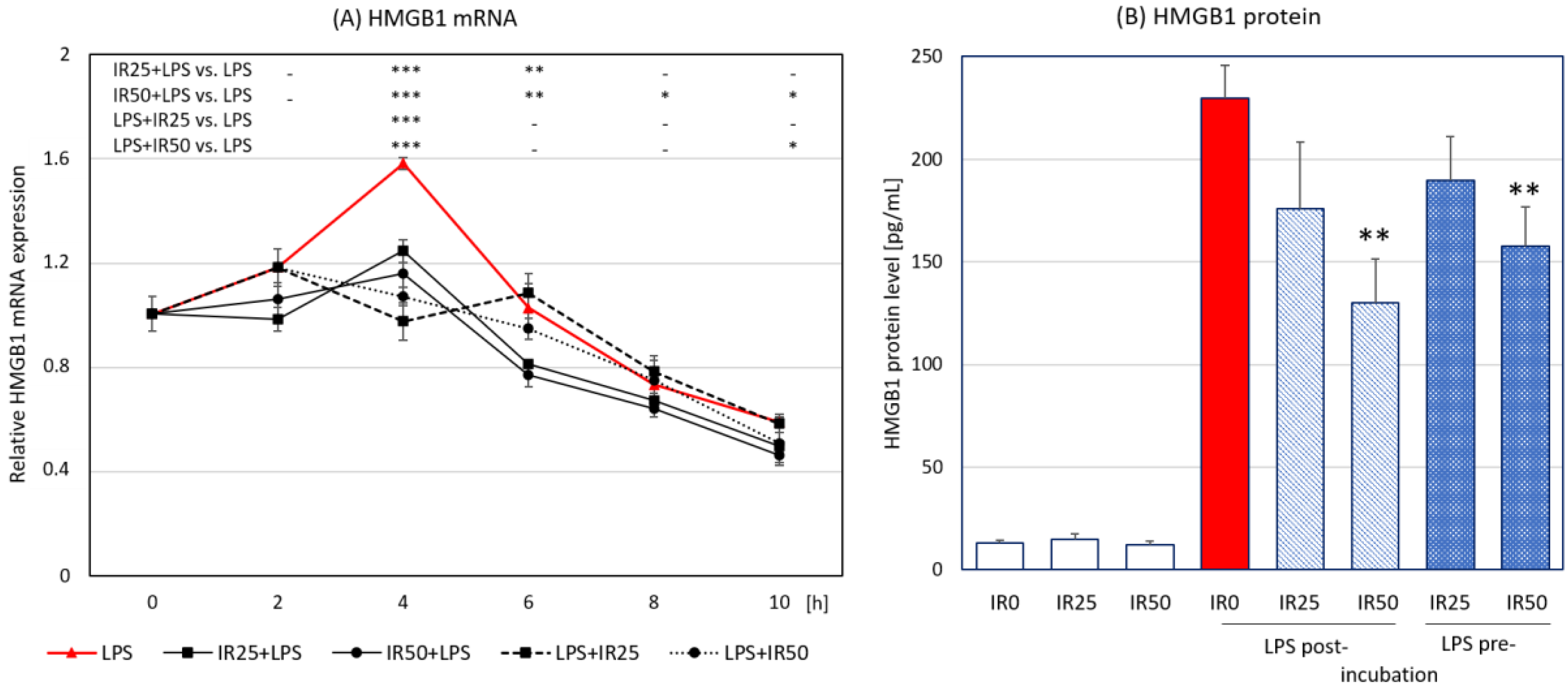
3.4. Irisin Effects on HMGB1 Expression and Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Virág, L.; Jaén, R.I.; Regdon, Z.; Boscá, L.; Prieto, P. Self-defense of macrophages against oxidative injury: Fighting for their own survival. Redox Biol. 2019, 26, 101261. [Google Scholar] [CrossRef]
- Hawkes, W.C.; Alkan, Z. Regulation of Redox Signaling by Selenoproteins. Biol. Trace Element Res. 2010, 134, 235–251. [Google Scholar] [CrossRef]
- Sander, L.E.; Garaude, J. The mitochondrial respiratory chain: A metabolic rheostat of innate immune cell-mediated antibacterial responses. Mitochondrion 2018, 41, 28–36. [Google Scholar] [CrossRef]
- Marikovsky, M.; Ziv, V.; Nevo, N.; Harris-Cerruti, C.; Mahler, O. Cu/Zn Superoxide Dismutase Plays Important Role in Immune Response. J. Immunol. 2003, 170, 2993–3001. [Google Scholar] [CrossRef]
- Paiva, C.N.; Bozza, M.T. Are Reactive Oxygen Species Always Detrimental to Pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef]
- Bozinovski, S.; Seow, H.J.; Crack, P.J.; Anderson, G.P.; Vlahos, R. Glutathione Peroxidase-1 Primes Pro-Inflammatory Cytokine Production after LPS Challenge In Vivo. PLoS ONE 2012, 7, e33172. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Castaneda, O.A.; Lee, S.-C.; Ho, C.-T.; Huang, T.-C. Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds. J. Food Drug Anal. 2017, 25, 111–118. [Google Scholar] [CrossRef]
- Moussa, Z.; Judeh, Z.M.; Ahmed, S.A. Nonenzymatic Exogenous and Endogenous Antioxidants. In Free Radical Medicine and Biology; Das, K., Das, S., Biradar, M.S., Bobbarala, V., Tata, S.S., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I. Irisin acts as a regulator of macrophages host defense. Life Sci. 2017, 176, 21–25. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Kozlowska, K.; Pochec, E.; Bilski, J.; Brzozowski, T. Myokine irisin-induced protection against oxidative stress in vitro. Involvement of heme oxygenase-1 and antioxidazing enzymes superoxide dismutase-2 and glutathione peroxidase. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2018, 69, 117–125. [Google Scholar] [CrossRef]
- Korta, P.; Pocheć, E.; Mazur-Bialy, A.I. Irisin as a Multifunctional Protein: Implications for Health and Certain Diseases. Medicina 2019, 55, 485. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Pocheć, E.; Zarawski, M. Anti-Inflammatory Properties of Irisin, Mediator of Physical Activity, Are Connected with TLR4/MyD88 Signaling Pathway Activation. Int. J. Mol. Sci. 2017, 18, 701. [Google Scholar] [CrossRef]
- Mazur-Bialy, A.I.; Bilski, J.; Pochec, E.; Brzozowski, T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2017, 68, 243–251. [Google Scholar]
- Mazur-Bialy, A.I.; Mazur-Bialy, A.I. Superiority of the Non-Glycosylated Form Over the Glycosylated Form of Irisin in the Attenuation of Adipocytic Meta-Inflammation: A Potential Factor in the Fight Against Insulin Resistance. Biomolecules 2019, 9, 394. [Google Scholar] [CrossRef]
- Vijayan, V.; Wagener, F.A.D.T.G.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Alexander, C.; Rietschel, E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione peroxidase family—An evolutionary overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef]
- El Gazzar, M. HMGB1 modulates inflammatory responses in LPS-activated macrophages. Inflamm. Res. 2007, 56, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, R.; Peng, Z.; Hu, B.; Rao, X.; Li, J. HMGB1 participates in LPS-induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF-κB signaling pathways. Int. J. Mol. Med. 2019, 45, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Tsoyi, K.; Lee, T.Y.; Lee, Y.S.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Chang, K.C. Heme-Oxygenase-1 Induction and Carbon Monoxide-Releasing Molecule Inhibit Lipopolysaccharide (LPS)-Induced High-Mobility Group Box 1 Release in Vitro and Improve Survival of Mice in LPS- and Cecal Ligation and Puncture-Induced Sepsis Model in Vivo. Mol. Pharmacol. 2009, 76, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nat. Cell Biol. 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, Y.M.; Park, S.W.; Kim, H.J.; Lee, J.H.; Lee, D.-U.; Chang, K.C. Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice. Food Chem. Toxicol. 2013, 55, 386–395. [Google Scholar] [CrossRef]
- Tang, D.; Shi, Y.; Kang, R.; Li, T.; Xiao, W.; Wang, H.; Xiao, X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leukoc. Biol. 2007, 81, 741–747. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Jiang, H. Nrf-2–HO-1–HMGB1 axis: An important therapeutic approach for protection against myocardial ischemia and reperfusion injury. Int. J. Cardiol. 2014, 172, 223–224. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Xie, J.; Xu, W.; Jiang, H. Beta-1-Adrenergic Receptors Mediate Nrf2-HO-1-HMGB1 Axis Regulation to Attenuate Hypoxia/Reoxygenation-Induced Cardiomyocytes Injury in Vitro. Cell. Physiol. Biochem. 2015, 35, 767–777. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, C.; Wang, G.; Xu, R.; Zhu, Y. Higenamine regulates Nrf2-HO-1-Hmgb1 axis and attenuates intestinal ischemia–reperfusion injury in mice. Inflamm. Res. 2015, 64, 395–403. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.; Bian, Y.; Li, Y.; Liu, L.; Zhang, H.; Xie, K.; Wang, G.; Yu, Y. Hydrogen Gas Protects Against Intestinal Injury in Wild Type But Not NRF2 Knockout Mice With Severe Sepsis by Regulating HO-1 and HMGB1 Release. Shock 2017, 48, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Faridvand, Y.; Nozari, S.; Vahedian, V.; Safaei, N.; Pezeshkian, M.; Haddadi, P.; Mamipour, M.; Rezaie-Nezhad, A.; Jodati, A.R.; Nouri, M. Nrf2 activation and down-regulation of HMGB1 and MyD88 expression by amnion membrane extracts in response to the hypoxia-induced injury in cardiac H9c2 cells. Biomed. Pharmacother. 2019, 109, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Pérez, J.; Martínez-Rosas, M.; Conde-Castañón, C.A.; Toscano-Garibay, J.D.; Ruiz-Pérez, N.J.; Flores-Chávez, P.L.; Mera Jiménez, E.M.; Flores-Estrada, J. Epigallocatechin 3-Gallate Has a Neuroprotective Effect in Retinas of Rabbits with Ischemia/Reperfusion through the Activation of Nrf2/HO-1. Int. J. Mol. Sci. 2020, 21, 3716. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Fernandez, P.; Guillen, M.I. Anti-Inflammatory Actions of the Heme Oxygenase-1 Pathway. Curr. Pharm. Des. 2003, 9, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zhang, J.; Ren, Y.; Du, Z.; Li, Q.; Wang, Y.; Wei, S.; Yang, L.; Zhang, J.; Liu, C.; et al. Irisin alleviates liver ischemia-reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019, 20, 296–306. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, J.; Wang, M.; Bi, J.; Wang, T.; Qiu, M.; Lv, Y.; Wu, Z.; Wu, R. Identification of irisin as a therapeutic agent that inhibits oxidative stress and fibrosis in a murine model of chronic pancreatitis. Biomed. Pharmacother. 2020, 126, 110101. [Google Scholar] [CrossRef]
- Lu, J.; Da Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef]
- Eyu, Y.; Etang, D.; Kang, R. Oxidative stress-mediated HMGB1 biology. Front. Physiol. 2015, 6, 93. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Tracey, K.J. Extracellular role of HMGB1 in inflammation and sepsis. J. Intern. Med. 2004, 255, 320–331. [Google Scholar] [CrossRef]
- Huang, W.; Tang, Y.; Li, L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 2010, 51, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhang, Z.; Zhang, P.; Zheng, C.; Zhou, W.; Cui, W.; Xu, L.; Gao, J. Downregulation of HMGB1 is required for the protective role of Nrf2 in EMT-mediated PF. J. Cell. Physiol. 2018, 234, 8862–8872. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur-Bialy, A.I.; Pocheć, E. The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/HO-1/HMGB1 Axis. Antioxidants 2021, 10, 88. https://doi.org/10.3390/antiox10010088
Mazur-Bialy AI, Pocheć E. The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/HO-1/HMGB1 Axis. Antioxidants. 2021; 10(1):88. https://doi.org/10.3390/antiox10010088
Chicago/Turabian StyleMazur-Bialy, Agnieszka Irena, and Ewa Pocheć. 2021. "The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/HO-1/HMGB1 Axis" Antioxidants 10, no. 1: 88. https://doi.org/10.3390/antiox10010088
APA StyleMazur-Bialy, A. I., & Pocheć, E. (2021). The Time-Course of Antioxidant Irisin Activity: Role of the Nrf2/HO-1/HMGB1 Axis. Antioxidants, 10(1), 88. https://doi.org/10.3390/antiox10010088




