Rapid Liquid Chromatography—Tandem Mass Spectrometry Analysis of Two Urinary Oxidative Stress Biomarkers: 8-oxodG and 8-isoprostane
Abstract
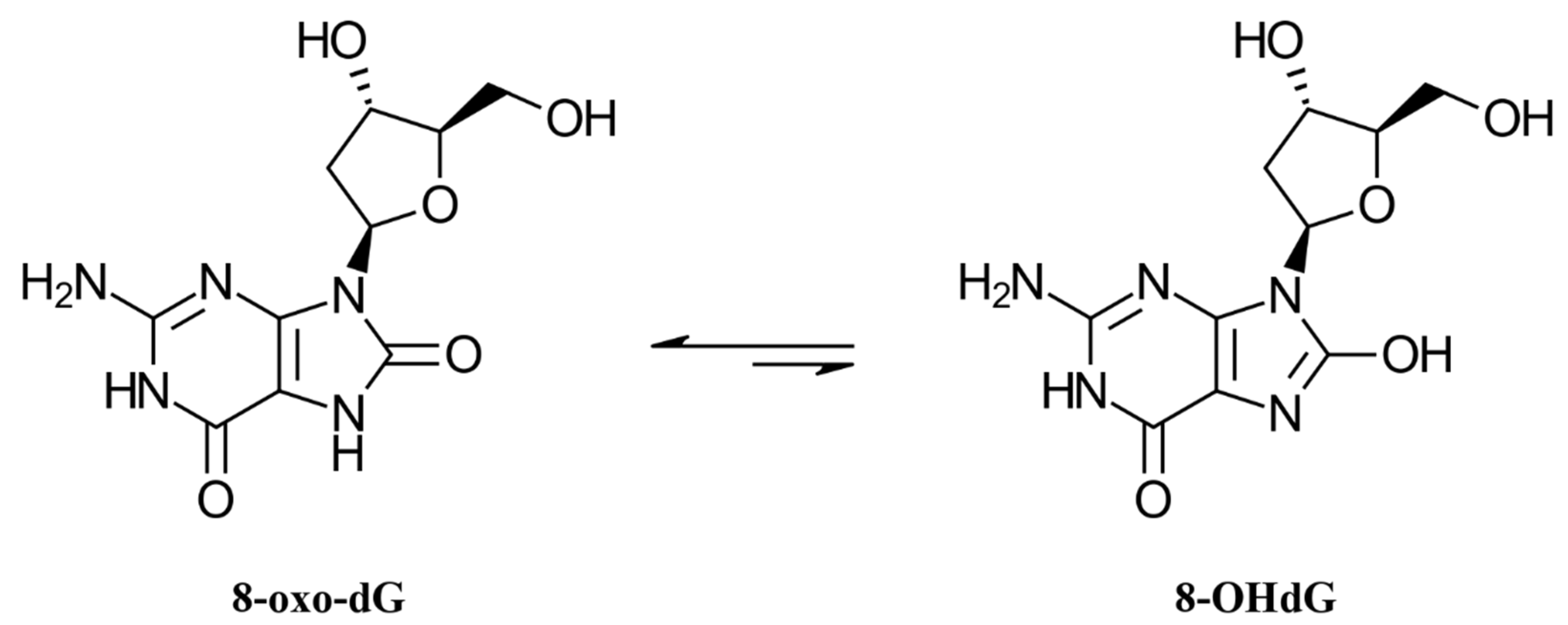
1. Introduction
2. Materials and Methods
2.1. Standards, Chemicals, and Material
2.2. Urine Samples for Method Validation
2.3. Calibration Curve and Quality Controls (QC)
2.4. Solid-Phase Extraction (SPE)
2.5. LC-MS/MS Analysis
2.6. Application to Urine Samples Obtained from Healthy Participants
2.7. Participant Description
2.8. Other Bioanalytical Methods
2.9. Data Presentation and Statistical Analysis
3. Results
3.1. LC-MS/MS Analysis
3.2. Sensitivity, Linearity, Accuracy, and Precision
3.3. Extraction Recovery and Matrix Effects
3.4. Stability
3.5. Oxidative Stress Biomarkers’ Concentrations in Healthy Participants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharifi-Rad, M.; Kumar, N.V.A.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Fokou, P.V.T.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, F.U.; Chhipa, A.S.; Sagar, N.; Pathak, C. Oxidative Stress and Inflammation Can Fuel Cancer. In Role of Oxidative Stress in Pathophysiology of Diseases; Maurya, P.K., Dua, K., Eds.; Springer: Singapore, 2020; pp. 229–258. ISBN 9789811515682. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Herbert, K.E.; Lunec, J. Urinary 8-oxo-2′-deoxyguanosine—Source, significance and supplements. Free Radic. Res. 2000, 32, 381–397. [Google Scholar] [CrossRef]
- Cho, B.P.; Kadlubar, F.F.; Culp, S.J.; Evans, F.E. Nitrogen-15 nuclear magnetic resonance studies on the tautomerism of 8-hydroxy-2’-deoxyguanosine, 8-hydroxyguanosine, and other C8-substituted guanine nucleosides. Chem. Res. Toxicol. 1990, 3, 445–452. [Google Scholar] [CrossRef]
- Cooke, M.S.; Loft, S.; Olinski, R.; Evans, M.D.; Bialkowski, K.; Wagner, J.R.; Dedon, P.C.; Møller, P.; Greenberg, M.M.; Cadet, J. Recommendations for Standardized Description of and Nomenclature Concerning Oxidatively Damaged Nucleobases in DNA. Chem. Res. Toxicol. 2010, 23, 705–707. [Google Scholar] [CrossRef][Green Version]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef]
- Loft, S.; Olsen, A.; Møller, P.; Poulsen, H.E.; Tjønneland, A. Association between 8-oxo-7,8-dihydro-2′-deoxyguanosine Excretion and Risk of Postmenopausal Breast Cancer: Nested Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1289–1296. [Google Scholar] [CrossRef]
- Loft, S.; Svoboda, P.; Kawai, K.; Kasai, H.; Sørensen, M.; Tjønneland, A.; Vogel, U.; Møller, P.; Overvad, K.; Raaschou-Nielsen, O. Association between 8-oxo-7,8-dihydroguanine excretion and risk of lung cancer in a prospective study. Free Radic. Biol. Med. 2012, 52, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Musiek, E.S.; Morrow, J.D. F2-Isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers 2005, 10, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Dai, Q.; Roberts, L.J. The isoprostanes—25 years later. Biochim. Biophys. Acta 2015, 1851, 433–445. [Google Scholar] [CrossRef]
- Kadiiska, M.B.; Gladen, B.C.; Baird, D.D.; Germolec, D.; Graham, L.B.; Parker, C.E.; Nyska, A.; Wachsman, J.T.; Ames, B.N.; Basu, S.; et al. Biomarkers of Oxidative Stress Study II: Are oxidation products of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic. Biol. Med. 2005, 38, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. F2-Isoprostanes in Human Health and Diseases: From Molecular Mechanisms to Clinical Implications. Antioxid. Redox Signal. 2008, 10, 1405–1434. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.S.; Roberts, L.J. F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic. Biol. Med. 2011, 50, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, H.E.; Nadal, L.L.; Broedbaek, K.; Nielsen, P.E.; Weimann, A. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 801–808. [Google Scholar] [CrossRef]
- Korkmaz, K.S.; Butuner, B.D.; Roggenbuck, D. Detection of 8-OHdG as a diagnostic biomarker. J. Lab. Precis. Med. 2018, 3, 95. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Klawitter, J.; Haschke, M.; Shokati, T.; Klawitter, J.; Christians, U. Quantification of 15-F2t -isoprostane in human plasma and urine: Results from enzyme-linked immunoassay and liquid chromatography/tandem mass spectrometry cannot be compared. Rapid Commun. Mass Spectrom. 2011, 25, 463–468. [Google Scholar] [CrossRef]
- Wu, C.; Chen, S.-T.; Peng, K.-H.; Cheng, T.-J.; Wu, K.-Y. Concurrent quantification of multiple biomarkers indicative of oxidative stress status using liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2016, 512, 26–35. [Google Scholar] [CrossRef]
- Zhao, G.; Fu, Y.; Yu, J.; Wang, S.; Duan, K.; Xie, F.; Liu, H. A Simple Method for the Determination of 8-Oxoguanosine, 8-Oxo-2′-Deoxyguanosine and 8-Iso-Prostaglandin F2α in Human Urine by UHPLC–MS/MS. Chromatography 2017, 80, 401–408. [Google Scholar] [CrossRef]
- Saito, A.; Hamano, M.; Kataoka, H. Simultaneous analysis of multiple urinary biomarkers for the evaluation of oxidative stress by automated online in-tube solid-phase microextraction coupled with negative/positive ion-switching mode liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2018, 41, 2743–2749. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.P.; Kannan, K. Simultaneous Analysis of Seven Biomarkers of Oxidative Damage to Lipids, Proteins, and DNA in Urine. Environ. Sci. Technol. 2018, 52, 6647–6655. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, S.; Wang, P.G. Matrix effects and application of matrix effect factor. Bioanalysis 2017, 9, 1839–1844. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- Marchi, I.; Viette, V.; Badoud, F.; Fathi, M.; Saugy, M.; Rudaz, S.; Veuthey, J.-L. Characterization and classification of matrix effects in biological samples analyses. J. Chromatogr. A 2010, 1217, 4071–4078. [Google Scholar] [CrossRef]
- Prasain, J.K.; Arabshahi, A.; Taub, P.R.; Sweeney, S.; Moore, R.; Sharer, J.D.; Barnes, S. Simultaneous quantification of F2-isoprostanes and prostaglandins in human urine by liquid chromatography tandem-mass spectrometry. J. Chromatogr. B 2013, 913–914, 161–168. [Google Scholar] [CrossRef]
- Barregard, L.; Møller, P.; Henriksen, T.; Mistry, V.; Koppen, G.; Rossner, P.; Sram, R.J.; Weimann, A.; Poulsen, H.E.; Nataf, R.; et al. Human and Methodological Sources of Variability in the Measurement of Urinary 8-Oxo-7,8-dihydro-2′-deoxyguanosine. Antioxid. Redox Signal. 2013, 18, 2377–2391. [Google Scholar] [CrossRef]
- Zanolin, M.E.; Girardi, P.; Degan, P.; Rava, M.; Olivieri, M.; Di Gennaro, G.; Nicolis, M.; De Marco, R. Measurement of a Urinary Marker (8-hydroxydeoxy-Guanosine, 8-OHdG) of DNA Oxidative Stress in Epidemiological Surveys: A Pilot Study. Int. J. Biol. Markers 2015, 30, 341–345. [Google Scholar] [CrossRef]
- Sakano, N.; Wang, D.-H.; Takahashi, N.; Wang, B.; Sauriasari, R.; Kanbara, S.; Sato, Y.; Takigawa, T.; Takaki, J.; Ogino, K. Oxidative Stress Biomarkers and Lifestyles in Japanese Healthy People. J. Clin. Biochem. Nutr. 2009, 44, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. Fatty acid oxidation and isoprostanes: Oxidative strain and oxidative stress. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, M. Ueber Den Niederschlag, Welchen Pikrinsäure Im Normalen Harn Erzeugt, Und Über Eine Neue Reaction Des Kreatinins. Z. Physiol. Chem. 1886, 10, 391–400. [Google Scholar]
- Schick, S.F.; Blount, B.C.; Jacob, P.; Saliba, N.A.; Bernert, J.T.; El Hellani, A.; Jatlow, P.; Pappas, R.S.; Wang, L.; Foulds, J.; et al. Biomarkers of exposure to new and emerging tobacco delivery products. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L425–L452. [Google Scholar] [CrossRef]
- Graille, M.; Wild, P.; Sauvain, J.-J.; Hemmendinger, M.; Canu, I.G.; Hopf, N.B. Urinary 8-OHdG as a Biomarker for Oxidative Stress: A Systematic Literature Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3743. [Google Scholar] [CrossRef]
- Graille, M.; Wild, P.; Sauvain, J.-J.; Hemmendinger, M.; Canu, I.G.; Hopf, N.B. Urinary 8-isoprostane as a biomarker for oxidative stress. A systematic review and meta-analysis. Toxicol. Lett. 2020, 328, 19–27. [Google Scholar] [CrossRef]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef]
- Cho, B.P. Structure of oxidatively damaged nucleic acid adducts: PH dependence of the13C NMR spectra of 8-oxoguanosine and 8-oxoadenosine. Magn. Reson. Chem. 1993, 31, 1048–1053. [Google Scholar] [CrossRef]
- Jang, Y.H.; Goddard, W.A.; Noyes, K.T.; Sowers, L.C.; Hwang, S.; Chung, D.S. First Principles Calculations of the Tautomers and pKaValues of 8-Oxoguanine: Implications for Mutagenicity and Repair. Chem. Res. Toxicol. 2002, 15, 1023–1035. [Google Scholar] [CrossRef]
- Janicka, M.; Kot-Wasik, Á.; Kot, J.; Namieśnik, J. Isoprostanes-Biomarkers of Lipid Peroxidation: Their Utility in Evaluating Oxidative Stress and Analysis. Int. J. Mol. Sci. 2010, 11, 4631–4659. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Il’Yasova, D.; Scarbrough, P.; Spasojevic, I. Urinary biomarkers of oxidative status. Clin. Chim. Acta 2012, 413, 1446–1453. [Google Scholar] [CrossRef]
- Helmersson, J.; Vessby, B.; Larsson, A.; Basu, S. Association of Type 2 Diabetes with Cyclooxygenase-Mediated Inflammation and Oxidative Stress in an Elderly Population. Circulation 2004, 109, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Tatsch, E.; De Carvalho, J.A.M.; Hausen, B.S.; Bollick, Y.S.; Torbitz, V.D.; Duarte, T.; Scolari, R.; Duarte, M.M.F.; Londero, S.W.K.; Vaucher, R.A.; et al. Oxidative DNA damage is associated with inflammatory response, insulin resistance and microvascular complications in type 2 diabetes. Mutat. Res. Mol. Mech. Mutagen. 2015, 782, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Altemose, B.; Robson, M.G.; Kipen, H.M.; Strickland, P.O.; Meng, Q.; Gong, J.; Huang, W.; Wang, G.; Rich, D.Q.; Zhu, T.; et al. Association of air pollution sources and aldehydes with biomarkers of blood coagulation, pulmonary inflammation, and systemic oxidative stress. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Squillacioti, G.; Bellisario, V.; Grosso, A.; Ghelli, F.; Piccioni, P.; Grignani, E.; Corsico, A.; Bono, R. Formaldehyde, Oxidative Stress, and FeNO in Traffic Police Officers Working in Two Cities of Northern Italy. Int. J. Environ. Res. Public Health 2020, 17, 1655. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Díaz-Castro, J.; Kajarabille, N.; García, C.; Guisado, I.M.; De Teresa, C.; Guisado, R. Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 2011, 51, 373–380. [Google Scholar] [CrossRef]
- Mrakic-Sposta, S.; Gussoni, M.; Moretti, S.; Pratali, L.; Giardini, G.; Tacchini, P.; Dellanoce, C.; Tonacci, A.; Mastorci, F.; Borghini, A.; et al. Effects of Mountain Ultra-Marathon Running on ROS Production and Oxidative Damage by Micro-Invasive Analytic Techniques. PLoS ONE 2015, 10, e0141780. [Google Scholar] [CrossRef]
- Larsen, E.L.; Poulsen, H.E.; Michaelsen, C.; Kjær, L.K.; Lyngbæk, M.; Andersen, E.S.; Petersen-Bønding, C.; Lemoine, C.; Gillum, M.; Jørgensen, N.R.; et al. Differential time responses in inflammatory and oxidative stress markers after a marathon: An observational study. J. Sports Sci. 2020, 38, 2080–2091. [Google Scholar] [CrossRef]
- Helmersson, J.; Ärnlöv, J.; Larsson, A.; Basu, S. Low dietary intake of β-carotene, α-tocopherol and ascorbic acid is associated with increased inflammatory and oxidative stress status in a Swedish cohort. Br. J. Nutr. 2008, 101, 1775–1782. [Google Scholar] [CrossRef]
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.-P.; Sinaiko, A.R. Fruit and Vegetable Consumption and Its Relation to Markers of Inflammation and Oxidative Stress in Adolescents. J. Am. Diet. Assoc. 2009, 109, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cai, H.; Xiang, Y.-B.; Cai, Q.; Yang, G.; Liu, D.; Sanchez, S.; Zheng, W.; Milne, G.L.; Shu, X.-O. Intra-Person Variation of Urinary Biomarkers of Oxidative Stress and Inflammation. Cancer Epidemiol. Biomark. Prev. 2010, 19, 947–952. [Google Scholar] [CrossRef] [PubMed]



| Wu et al. (2016) [22] | Zhao et al. (2017) [23] | Saito et al. (2018) [24] | Martinez and Kannan (2018) [25] | Our Study | |
|---|---|---|---|---|---|
| Parameters | |||||
| LOD | |||||
| 8-oxodG | 20 pg/mL | 170 pg/mL | 12.6 pg/mL | 30 pg/mL | 10 pg/mL |
| 8-isoprostane | 8 pg/mL | 40 pg/mL | 3.4 pg/mL | 10 pg/mL | 20 pg/mL |
| LOQ | |||||
| 8-oxodG | 50 pg/mL | 570 pg/mL | 20 pg/mL | 100 pg/mL | 30 pg/mL |
| 8-isoprostane | 30 pg/mL | 130 pg/mL | 29 pg/mL | 20 pg/mL | 50 pg/mL |
| Linearity | |||||
| 8-oxodG | R2 > 0.998 | R2 > 0.999 | R2 > 0.999 | R2 > 0.999 | R2 > 0.999 |
| 8-isoprostane | R2 > 0.998 | R2 > 0.999 | R2 > 0.999 | R2 > 0.999 | R2 > 0.999 |
| Intra-/inter-day accuracy | |||||
| 8-oxodG 8-isoprostane | 98.8–102.2%/ | n.a./ | 91.1–97%/ | 92–101%/ | 92–114%/ |
| 98.5–101.6% | n.a. | n.a. | n.a. | 92–103% | |
| 98.5–101.7%/ | n.a./ | 95.7–100%/ | 93–103%/ | 97–114%/ | |
| 99–102.1% | n.a. | n.a. | n.a. | 97–114% | |
| Intra-/inter-day precision | |||||
| 8-oxodG | <8.1%/<8.5% | <1.9%/<3.9% | <5%/<6.1% | <9%/n.a. | <5.7%/<10% |
| 8-isoprostane | <4.6%/<5.1% | <2.3%/<5.3% | <2.1%/<4.5% | <9%/n.a. | <7.0%/<8.1% |
| SPE recovery | |||||
| 8-oxodG | 90.1–90.7% | 90.1–100% | n.a. | n.a. | 97% |
| 8-isoprostane | 94.3–95% | 89.2–108% | n.a. | n.a. | 91% |
| Matrix effects1 | |||||
| 8-oxodG | 89.2% | n.a. | n.a. | n.a. | 20% |
| 8-isoprostane | 96.6% | n.a. | n.a. | n.a. | 70% |
| Characteristic | Non-Smokers (n = 56) |
|---|---|
| Age (years) | 43.5 (35.5–54.25) * |
| Sex | |
| Men | 30 (54) ** |
| Women | 26 (46) |
| BMI (kg/m2) | 26 (23–28) |
| ≤25 | 22 (39) |
| >25 | 34 (61) |
| TNE (nmol/mg creatinine) | 0.01 (0.00–0.02) |
| Urine | Compound | Concentration 1 | Intra-Day Accuracy 2 and Precision 3 | Inter-Day Accuracy and Precision | ||
|---|---|---|---|---|---|---|
| “Light urine” | 8-oxodG | 0.5 | 94 | 0.9 | 94 | 2.5 |
| 1 | 92 | 1.9 | 92 | 2.7 | ||
| 10 | 99 | 0.5 | 99 | 2.4 | ||
| 8-isoprostane | 0.1 | 97 | 5.9 | 97 | 8.1 | |
| 0.2 | 99 | 4.4 | 99 | 3.4 | ||
| 0.5 | 100 | 3.3 | 100 | 2.7 | ||
| “Dark urine” | 8-oxodG | 0.5 | 113 | 5.4 | 103 | 10 |
| 1 | 107 | 5.7 | 100 | 8.4 | ||
| 10 | 98 | 0.8 | 99 | 4.4 | ||
| 8-isoprostane | 0.1 | 114 | 7.0 | 114 | 2.1 | |
| 0.2 | 100 | 5.5 | 100 | 4.9 | ||
| 0.5 | 102 | 2.0 | 102 | 2.5 | ||
| 8-oxodG | 8-isoprostane | ||||
|---|---|---|---|---|---|
| All Participants | BMI | All Participants | BMI | ||
| 4.04 * (3.42–5.37) | BMI ≤ 25 (n = 22) | 4.28 (3.62–6.11) | 0.20 (0.14–0.28) | BMI ≤ 25 (n = 22) | 0.19 (0.14–0.30) |
| BMI > 25 (n = 34) | 3.96 (2.81–4.97) | BMI > 25 (n = 34) | 0.21 (0.14–0.27) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambiagio, N.; Sauvain, J.-J.; Berthet, A.; Auer, R.; Schoeni, A.; Hopf, N.B. Rapid Liquid Chromatography—Tandem Mass Spectrometry Analysis of Two Urinary Oxidative Stress Biomarkers: 8-oxodG and 8-isoprostane. Antioxidants 2021, 10, 38. https://doi.org/10.3390/antiox10010038
Sambiagio N, Sauvain J-J, Berthet A, Auer R, Schoeni A, Hopf NB. Rapid Liquid Chromatography—Tandem Mass Spectrometry Analysis of Two Urinary Oxidative Stress Biomarkers: 8-oxodG and 8-isoprostane. Antioxidants. 2021; 10(1):38. https://doi.org/10.3390/antiox10010038
Chicago/Turabian StyleSambiagio, Nicolas, Jean-Jacques Sauvain, Aurélie Berthet, Reto Auer, Anna Schoeni, and Nancy B. Hopf. 2021. "Rapid Liquid Chromatography—Tandem Mass Spectrometry Analysis of Two Urinary Oxidative Stress Biomarkers: 8-oxodG and 8-isoprostane" Antioxidants 10, no. 1: 38. https://doi.org/10.3390/antiox10010038
APA StyleSambiagio, N., Sauvain, J.-J., Berthet, A., Auer, R., Schoeni, A., & Hopf, N. B. (2021). Rapid Liquid Chromatography—Tandem Mass Spectrometry Analysis of Two Urinary Oxidative Stress Biomarkers: 8-oxodG and 8-isoprostane. Antioxidants, 10(1), 38. https://doi.org/10.3390/antiox10010038





