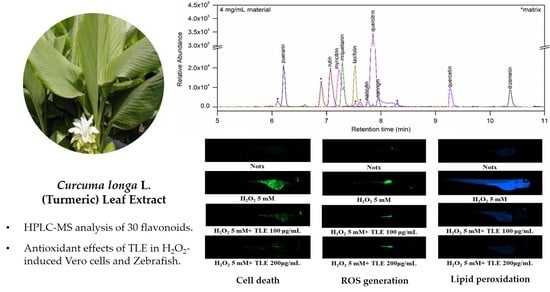
Antioxidant Effects of Turmeric Leaf Extract against Hydrogen Peroxide-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Turmeric Leaf Extraction
2.3. High-Performance Liquid Chromatography Mass Spectrometry (HPLC-MS)
2.4. Cell Culture
2.5. Assessment of Cell Viability
2.6. Intracellular ROS Generation in Vero Cells
2.7. Cell Cycle Analysis in Vero Cells
2.8. Nuclear Staining with Hoechst 33342 and PI
2.9. Origin and Maintenance of Zebrafish
2.10. Waterborne Exposure of Embryo with or without TLE and H2O2 in Zebrafish
2.11. Estimation of Oxidative Stress-Induced Intracellular ROS and Lipid Peroxidation as well as Cell Death in Zebrafish Embryos and Image Analysis
2.12. Statistical Analysis
3. Results
3.1. Flavonoids in TLE
3.2. Effect of TLE on Cell Viability
3.3. Effect of TLE on Intracellular ROS Generation in Vero Cells
3.4. Effect of TLE on the Sub-G1 Phase Cell Population in Vero Cells
3.5. Nuclear Staining with Hoechst 33342 and PI
3.6. Inhibitory Effects of TLE on H2O2-Induced Cell Death, ROS Production, and Lipid Peroxidation in Zebrafish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, K.; Bhori, M.; Kasu, Y.A.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.-L.; Wong, R.S.-M.; Lam, K.-H.; Hung, L.-K.; Wong, M.M.; Yung, L.-H.; Ho, Y.-W.; Wong, W.-Y.; Hau, D.K.-P.; Gambari, R.; et al. The role of reactive oxygen species in the biological activity of antimicrobial agents: An updated mini review. Chem. Biol. Interact. 2020, 320, 109023. [Google Scholar] [CrossRef] [PubMed]
- Amra, B. Antioxidant Enzymes and their Role in Preventing Cell Damage. ASNH 2020, 4. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M. The role of oxidative stress in carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 239–267. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Zhang, Q.; Yang, F.; Yin, Z.; Wang, L.; Li, Q. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways. Vet. Microbiol. 2019, 232. [Google Scholar] [CrossRef]
- Molavi, B.; Mehta, J.L. Oxidative stress in cardiovascular disease molecular basis of its deleterious effects, its detection, and therapeutic considerations. Curr. Opin. Cardiol. 2004, 19, 488–493. [Google Scholar] [CrossRef]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10, 18–25. [Google Scholar] [CrossRef]
- Rosca, M.G.; Mustata, T.G.; Kinter, M.T.; Ozdemir, A.M.; Kern, T.S.; Szweda, L.I.; Brownlee, M.; Monnier, V.M.; Weiss, M.F. Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am. J. Physiol. Ren. Physiol. 2005, 289, 420–430. [Google Scholar] [CrossRef]
- Filomeni, G.; de Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Verma, R.V.; Kumari, P.; Maurya, R.K.; Kumar, V.; Verma, R.B.; Singh, R.K. Medicinal properties of turmeric (Curcuma longa L.): A review. Int. J. Chem. Stud. 2018, 6, 1354–1357. [Google Scholar] [CrossRef]
- Uchio, R.; Higashi, Y.; Kohama, Y.; Kawasaki, K.; Hirao, T.; Muroyama, K.; Murosaki, S. A hot water extract of turmeric (Curcuma longa) suppresses acute ethanol-induced liver injury in mice by inhibiting hepatic oxidative stress and inflammatory cytokine production. J. Nutr. Sci. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Bomdyal, R.S.; Shah, U.M.; Doshi, S.Y.; Shah, A.V.; Khirade, P.S. Antibacterial activity of curcumin (turmeric) against periopathogens—An in vitro evaluation. J. Adv. Clin. Res. Insights 2017, 4, 175–180. [Google Scholar] [CrossRef]
- Na, L.-X.; Li, Y.; Pan, H.-Z.; Zhou, X.-L.; Sun, D.-J.; Meng, M.; Li, X.-X.; Sun, C.-H. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: A double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Rubya, A.J.; Kuttan, G.; Babub, K.D.; Rajasekharanb, K.N.; Kutta, R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef]
- Liu, Y.; Nair, M.G. Curcuma longa and Curcuma mangga leaves exhibit functional food property. Food Chem. 2012, 135, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.Y.; Lee, H.Y. Enhancement of Antioxidant Activities of Curcuma longa Leaves by Ultra High Pressure Extraction. Korean J. Med. Crop Sci. 2014, 22, 121–126. [Google Scholar] [CrossRef][Green Version]
- Braga, M.C.; Vieira, E.C.S.; de Oliveira, T.F. Curcuma longa L. leaves: Characterization (bioactive and antinutritional compounds) for use in human food in Brazil. Food Chem. 2018, 265, 308–315. [Google Scholar] [CrossRef]
- Yan, S.W.; Asmah, R. Comparison of total phenolic contents and antioxidant activities of turmeric leaf, pandan leaf and torch ginger flower. Int. Food Res. J. 2010, 17, 417–423. [Google Scholar]
- Wang, L.; Jayawardena, T.U.; Yang, H.W.; Lee, H.G.; Kang, M.C.; Sanjeewa, K.K.A.; Oh, J.Y.; Jeon, Y.J. Isolation, Characterization, and Antioxidant Activity Evaluation of a Fucoidan from an Enzymatic Digest of the Edible Seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, S.M.; Woo, H.S.; Park, J.Y.; Ko, B.S.; Heo, J.D.; Ryu, Y.B.; Lee, W.S. Chemical constituents and anti-inflammatory activity of the aerial parts of Curcuma longa. J. Funct. Foods 2016, 26, 485–493. [Google Scholar] [CrossRef]
- Ahn, D.R.; Lee, E.B.; Kim, B.J.; Lee, S.Y.; Lee, T.G.; Ahn, M.S.; Lim, H.W.; Cha, D.S.; Jeon, H.; Kim, D.K. Antioxidant and lifespan extending property of quercetin-3-O-dirhamnoside from Curcuma longa L. in Caenorhabditis elegans. J. Appl. Biol. Chem. 2014, 57, 709–714. [Google Scholar] [CrossRef]
- Kim, S.R.; Ko, S.C.; Kim, Y.S.; Ha, S.K.; Park, H.Y.; Park, Y.K.; Lee, S.H. Determination of Curcuma longa L. (Turmeric) Leaf Extraction Conditions Using Response Surface Methodology to Optimize Extraction Yield and Antioxidant Content. J. Food Qual. 2019, 2019. [Google Scholar] [CrossRef]
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000, 80, 561–566. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.K.; Choi, J.W.; Jang, H.; Seol, J.W. Anti-inflammatory effects of natural flavonoid diosmetin in IL-4 and LPS-induced macrophage activation and atopic dermatitis model. Int. Immunopharmacol. 2020, 89. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, H.; Liu, B.; Zhang, Q.; Li, M.; Zhu, R. Diosmetin inhibits cell proliferation and induces apoptosis by regulating autophagy via the mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cells. Oncol. Lett. 2016, 12, 4385–4392. [Google Scholar] [CrossRef]
- Yang, Y.; Gong, X.B.; Huang, L.G.; Wang, Z.W.; Wan, R.Z.; Zhang, P.; Zhang, Q.Y.; Chen, Z.; Zhang, B.S. Diosmetin exerts anti-oxidative, anti-inflammatory and antiapoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget 2017, 8, 30723–30733. [Google Scholar] [CrossRef]
- Yang, K.; Li, W.F.; Yu, J.F.; Yi, C.; Huang, W.F. Diosmetin protects against ischemia/reperfusion-induced acute kidney injury in mice. J. Surg. Res. 2017, 214, 69–78. [Google Scholar] [CrossRef]
- Caglayan, C.; Kandemir, F.M.; Yildirim, S.; Kucukler, S.; Eser, G. Rutin protects mercuric chloride-induced nephrotoxicity via targeting of aquaporin 1 level, oxidative stress, apoptosis and inflammation in rats. J. Trace Elem. Med. Biol. 2019, 54, 69–78. [Google Scholar] [CrossRef]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Qiu, T.A.; Miranda, J.G.; Hudson-Smith, N.V.; We, J.; Krause, M.O.P.; Fortner, J.D.; Haynes, C.L. Research highlights: Unveiling the mechanisms underlying nanoparticle-induced ROS generation and oxidative stress. Environ. Sci. Nano 2016, 3, 940–945. [Google Scholar] [CrossRef]
- Upadhyay, S.; Vaish, S.; Dhiman, M. Hydrogen peroxide-induced oxidative stress and its impact on innate immune responses in lung carcinoma A549 cells. Mol. Cell. Biochem. 2019, 450, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Heo, S.J.; Yang, H.W.; Ko, E.K.; Jung, M.S.; Cha, S.H.; Ahn, G.N.; Jeon, Y.J.; Kim, K.N. Protective Effect of 3-Bromo-4,5-Dihydroxybenzaldehyde from Polysiphonia morrowii Harvey against Hydrogen Peroxide-Induced Oxidative Stress In Vitro and In Vivo. J. Microbiol. Biotechnol. 2019, 29, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Kasinathan, A.; Ganesan, R.; Balasubramanian, A.; Bhaskaran, J.; Suresh, S.; Srinivasan, R.; Aravind, K.B.; Sivalingam, N. Curcumin induces apoptosis and cell cycle arrest via the activation of reactive oxygen species–independent mitochondrial apoptotic pathway in Smad4 and p53 mutated colon adenocarcinoma HT29 cells. Nutr. Res. 2018, 51, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, W.W.; Yang, H.W.; Ryu, B.M.; Cui, W.R.; Lee, S.C.; Lee, T.G.; Jeon, Y.J. Protective Effect of Water Extract of Citrus Pomace against AAPH-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish. Prev. Nutr. Food Sci. 2018, 23, 301–308. [Google Scholar] [CrossRef]
- Adam, W.C.; Peter, A.M.; James, R.S. The Role of Oxidative Stress in the Metabolic Syndrome. Rev. Cardiovasc. Med. 2011, 12, 21–29. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.S.; Jeon, Y.J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef]
- Jang, J.S.; Piao, S.; Cha, Y.N.; Kim, C.K. Taurine Chloramine Activates Nrf2, Increases HO-1 Expression and Protects Cells from Death Caused by Hydrogen Peroxide. J. Clin. Biochem. Nutr. 2009, 45, 37–43. [Google Scholar] [CrossRef]
- Belloc, F.; Dumain, P.; Boisseau, R.M.; Jalloustre, C.; Reiffers, J.; Bernard, P.; Lacombe, F. A flow cytometric method using Hoechst 33342 and propidium iodide for simultaneous cell cycle analysis and apoptosis determination in unfixed cells. Cytometry 1994, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Trang, D.T.; Hoang, T.K.V.; Nguyen, T.T.M.; Cuong, P.V.; Dang, N.H.; Dang, H.D.; Quang, T.N.; Dat, N.T. Essential Oils of Lemongrass (Cymbopogon citratus Stapf) Induces Apoptosis and Cell Cycle Arrest in A549 Lung Cancer Cells. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, I.H.; Campos, V.N.S.; Vale, A.A.M.; Maciel-Silva, V.L.; Leite, C.M.; Lopes, A.J.O.; Mourão, P.S.; Lima, F.-D.C.A.; Batista, A.A.; de Azevedo dos Santos, A.P.; et al. Ruthenium (II) complexes with N, O-chelating proline and threonine ligands cause selective cytotoxicity by the induction of genomic instability, cell cycle arrest and apoptosis in breast and prostate tumor cells. Toxicol. In Vitro 2020, 62, 1046–1079. [Google Scholar] [CrossRef]
- Wang, K.S.; Chan, C.K.; Hidayat, A.F.A.; Wong, A.H.; Kadir, H.A. Clinacanthus nutans induced reactive oxygen species-dependent apoptosis and autophagy in HCT116 human colorectal cancer cells. Pharmacogn. Mag. 2019, 15, 87–97. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Le, H.D.; Kim, T.N.T.; Nguyen, T.M.; Cornet, V.; Lambert, J.; Kestemont, P. Anti-Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants 2020, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.P.; Mansano, A.S.; Venturini, F.P.; Santos, F.; Zucolotto, V. Antioxidant metabolism of zebrafish after sub-lethal exposure to graphene oxide and recovery. Fish Physiol. Biochem. 2019, 45, 1289–1297. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Kim, H.S.; Lee, W.W.; Cui, W.; Lee, H.G.; Kim, Y.T.; Ko, J.Y.; Jeon, Y.J. Protective effect of polysaccharides from Celluclast-assisted extract of Hizikia fusiforme against hydrogen peroxide-induced oxidative stress in vitro in Vero cells and in vivo in zebrafish. Int. J. Biol. Macromol. 2018, 112, 483–489. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, M.; Kang, M.-C.; Lee, H.H.L.; Cho, C.H.; Choi, I.; Park, Y.; Lee, S.-H. Antioxidant Effects of Turmeric Leaf Extract against Hydrogen Peroxide-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish. Antioxidants 2021, 10, 112. https://doi.org/10.3390/antiox10010112
Kim S, Kim M, Kang M-C, Lee HHL, Cho CH, Choi I, Park Y, Lee S-H. Antioxidant Effects of Turmeric Leaf Extract against Hydrogen Peroxide-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish. Antioxidants. 2021; 10(1):112. https://doi.org/10.3390/antiox10010112
Chicago/Turabian StyleKim, Sera, Mingyeong Kim, Min-Cheol Kang, Hyun Hee L. Lee, Chi Heung Cho, Inwook Choi, Yongkon Park, and Sang-Hoon Lee. 2021. "Antioxidant Effects of Turmeric Leaf Extract against Hydrogen Peroxide-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish" Antioxidants 10, no. 1: 112. https://doi.org/10.3390/antiox10010112
APA StyleKim, S., Kim, M., Kang, M.-C., Lee, H. H. L., Cho, C. H., Choi, I., Park, Y., & Lee, S.-H. (2021). Antioxidant Effects of Turmeric Leaf Extract against Hydrogen Peroxide-Induced Oxidative Stress In Vitro in Vero Cells and In Vivo in Zebrafish. Antioxidants, 10(1), 112. https://doi.org/10.3390/antiox10010112