On Variation in Mindfulness Training: A Multimodal Study of Brief Open Monitoring Meditation on Error Monitoring
Abstract
:1. Introduction
2. Method
2.1. Participants
2.2. Procedural Overview
2.3. Audio Induction
2.4. Flanker Task
2.5. Trait Mindfulness
2.6. Manipulation Check
2.7. Psychophysiological Recording and Data Reduction
2.8. Data Analyses Overview
3. Results
3.1. Baseline Mindfulness and Manipulation Check
3.2. Behavioral Data
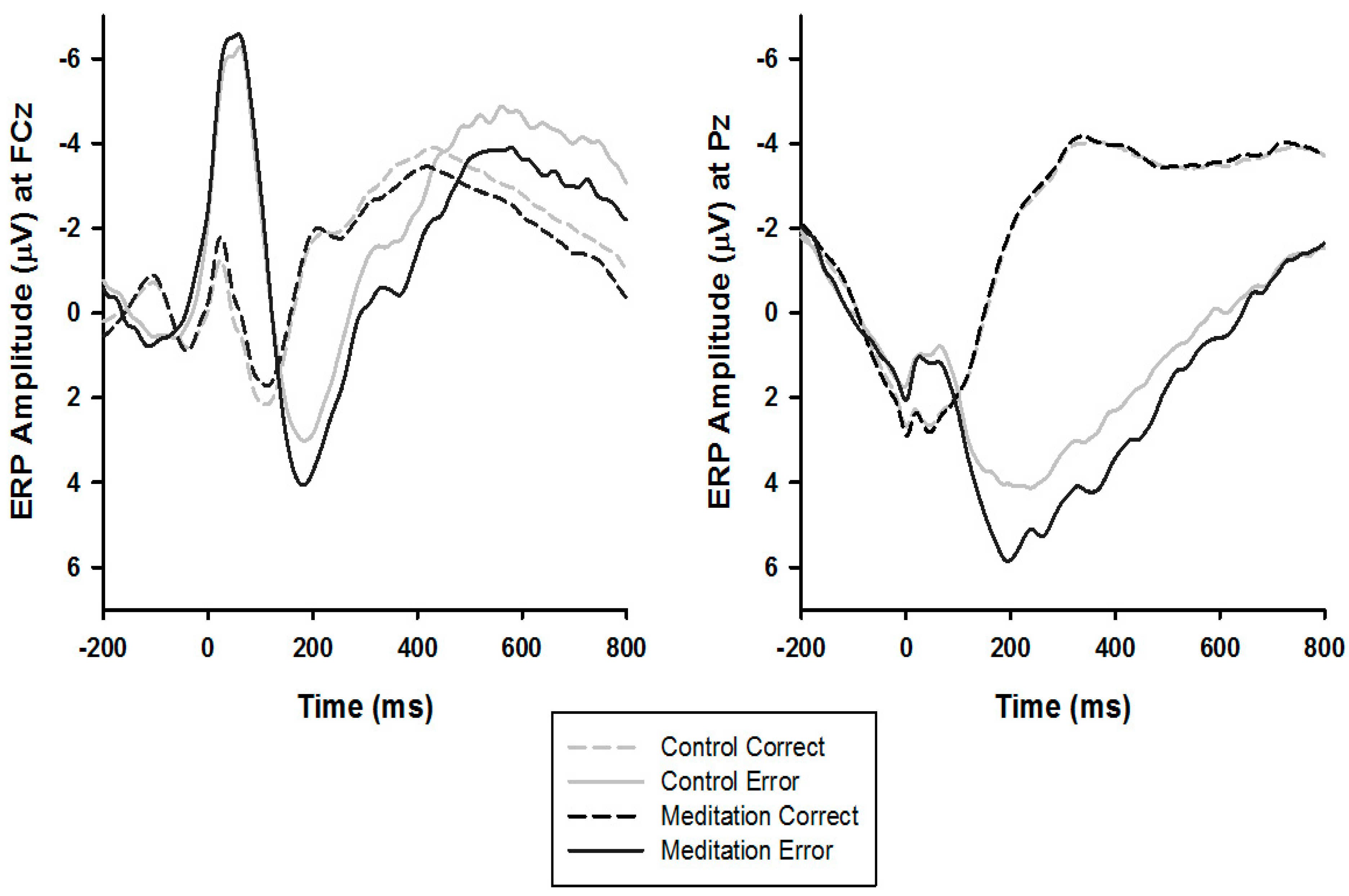
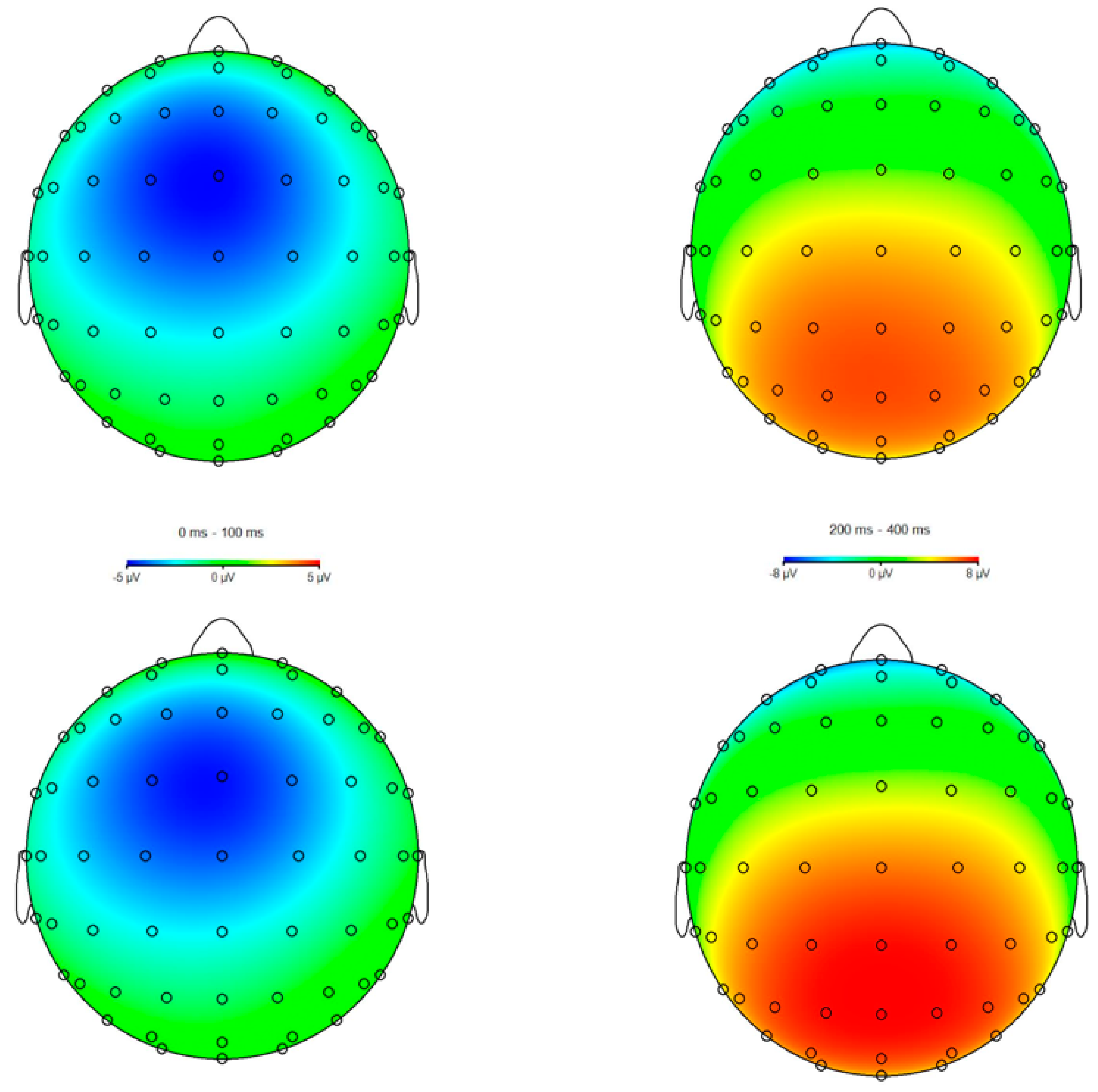
3.3. ERPs
3.4. Relationships between ERPs, Behavioral Performance, and Trait Mindfulness
4. Discussion
4.1. ERN Modulation
4.2. Pe Modulation
4.3. Trait Mindfulness and the ERN/Pe
4.4. Methodological Implications, Limitations, and Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bishop, S.J.; Duncan, J.; Lawrence, A.D. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J. Neurosci. 2004, 24, 10364–10368. [Google Scholar] [CrossRef]
- Kabat-Zinn, J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life; Hyperion: New York, NY, USA, 1994. [Google Scholar]
- Boyce, B. The Mindfulness Revolution: Leading Psychologists, Scientists, Artists, and Meditation Teachers on the Power of Mindfulness in Daily Life; Shambhala Publications: Boston, MA, USA, 2011; ISBN 978-1-59030-889-9. [Google Scholar]
- Ryan, T. A Mindful Nation; Hay House, Inc.: Carlsbad, CA, USA, 2012; ISBN 978-1-4019-3931-1. [Google Scholar]
- Joiner, T. Mindlessness: The Corruption of Mindfulness in a Culture of Narcissism; Oxford University Press: Oxford, UK, 2017; ISBN 978-0-19-020064-0. [Google Scholar]
- Purser, R.E.; Forbes, D.; Burke, A. (Eds.) Handbook of Mindfulness: Culture, Context, and Social Engagement; Mindfulness in Behavioral Health; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-44017-0. [Google Scholar]
- Van Dam, N.T.; van Vugt, M.K.; Vago, D.R.; Schmalzl, L.; Saron, C.D.; Olendzki, A.; Meissner, T.; Lazar, S.W.; Kerr, C.E.; Gorchov, J.; et al. Mind the Hype: A Critical Evaluation and Prescriptive Agenda for Research on Mindfulness and Meditation. Perspect. Psychol. Sci. 2018, 13, 36–61. [Google Scholar] [CrossRef]
- Farias, M.; Wikholm, C. Has the science of mindfulness lost its mind? BJPsych Bull. 2016, 40, 329–332. [Google Scholar] [CrossRef]
- Davidson, R.J.; Kaszniak, A.W. Conceptual and Methodological Issues in Research on Mindfulness and Meditation. Am. Psychol. 2015, 70, 581–592. [Google Scholar] [CrossRef]
- Gehring, W.J.; Goss, B.; Coles, M.G.H.; Meyer, D.E.; Donchin, E. A Neural System for Error Detection and Compensation. Psychol. Sci. 1993, 4, 385–390. [Google Scholar] [CrossRef]
- Taylor, S.F.; Stern, E.R.; Gehring, W.J. Neural systems for error monitoring: Recent findings and theoretical perspectives. Neuroscientist 2007, 13, 160–172. [Google Scholar] [CrossRef]
- Ullsperger, M.; Danielmeier, C.; Jocham, G. Neurophysiology of performance monitoring and adaptive behavior. Physiol. Rev. 2014, 94, 35–79. [Google Scholar] [CrossRef]
- Van Veen, V.; Carter, C.S. Conflict and Cognitive Control in the Brain. Curr. Dir. Psychol. Sci. 2006, 15, 237–240. [Google Scholar] [CrossRef]
- Yeung, N.; Botvinick, M.M.; Cohen, J.D. The Neural Basis of Error Detection: Conflict Monitoring and the Error-Related Negativity. Psychol. Rev. 2004, 111, 931–959. [Google Scholar] [CrossRef]
- Hasenkamp, W.; Barsalou, L.W. Effects of meditation experience on functional connectivity of distributed brain networks. Front. Hum. Neurosci. 2012, 6, 38. [Google Scholar] [CrossRef]
- Hasenkamp, W.; Wilson-Mendenhall, C.D.; Duncan, E.; Barsalou, L.W. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. NeuroImage 2012, 59, 750–760. [Google Scholar] [CrossRef]
- Langer, E.J. Mindfulness; Da Capo Press: Boston, MA, USA, 2014. [Google Scholar]
- Rabbitt, P.M. Errors and error correction in choice-response tasks. J. Exp. Psychol. 1966, 71, 264–272. [Google Scholar] [CrossRef]
- Ullsperger, M. Neural Bases of Performance Monitoring. In The Wiley Handbook of Cognitive Control; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 292–313. ISBN 978-1-118-92049-7. [Google Scholar]
- Falkenstein, M.; Hohnsbein, J.; Hoormann, J.; Blanke, L. Effects of crossmodal divided attention on late ERP components: I. Simple and choice reaction tasks. Electroencephalogr. Clin. Neurophysiol. 1991, 78, 438–446. [Google Scholar] [CrossRef]
- Overbeek, T.J.M.; Nieuwenhuis, S.; Ridderinkhof, K.R. Dissociable Components of Error Processing: On the Functional Significance of the Pe Vis-à-vis the ERN/Ne. J. Psychophysiol. 2005, 19, 319–329. [Google Scholar] [CrossRef]
- Gehring, W.; Liu, Y.; Orr, J.; Carp, J. The error-related negativity (ERN/Ne). In The Oxford Handbook of Event-Related Potential Components; Kappenman, E.S., Luck, J.S., Eds.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Yeung, N.; Cohen, J.D. The Impact of Cognitive Deficits on Conflict Monitoring: Predictable Dissociations Between the Error-Related Negativity and N2. Psychol. Sci. 2006, 17, 164–171. [Google Scholar] [CrossRef]
- Holroyd, C.B.; Coles, M.G.H. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 2002, 109, 679–709. [Google Scholar] [CrossRef]
- Inzlicht, M.; Bartholow, B.D.; Hirsh, J.B. Emotional foundations of cognitive control. Trends Cogn. Sci. 2015, 19, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, A.; Riesel, A.; Hajcak, G. Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motiv. Emot. 2012, 36, 84–100. [Google Scholar] [CrossRef]
- Shackman, A.J.; Salomons, T.V.; Slagter, H.A.; Fox, A.S.; Winter, J.J.; Davidson, R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011, 12, 154–167. [Google Scholar] [CrossRef]
- Weinberg, A.; Kotov, R.; Proudfit, G.H. Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. J. Abnorm. Psychol. 2015, 124, 172–185. [Google Scholar] [CrossRef]
- Hajcak, G.; McDonald, N.; Simons, R.F. To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology 2003, 40, 895–903. [Google Scholar] [CrossRef]
- Hajcak, G.; Foti, D. Errors Are Aversive: Defensive Motivation and the Error-Related Negativity. Psychol. Sci. 2008, 19, 103–108. [Google Scholar] [CrossRef]
- Herrmann, M.J.; Römmler, J.; Ehlis, A.-C.; Heidrich, A.; Fallgatter, A.J. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe). Brain Res. Cogn. Brain Res. 2004, 20, 294–299. [Google Scholar] [CrossRef]
- O’Connell, R.G.; Dockree, P.M.; Bellgrove, M.A.; Kelly, S.P.; Hester, R.; Garavan, H.; Robertson, I.H.; Foxe, J.J. The role of cingulate cortex in the detection of errors with and without awareness: A high-density electrical mapping study. Eur. J. Neurosci. 2007, 25, 2571–2579. [Google Scholar] [CrossRef]
- Ullsperger, M.; Harsay, H.A.; Wessel, J.R.; Ridderinkhof, K.R. Conscious perception of errors and its relation to the anterior insula. Brain Struct. Funct. 2010, 214, 629–643. [Google Scholar] [CrossRef] [Green Version]
- Murphy, P.R.; Robertson, I.H.; Allen, D.; Hester, R.; O’Connell, R.G. An electrophysiological signal that precisely tracks the emergence of error awareness. Front. Hum. Neurosci. 2012, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Nieuwenhuis, S.; Ridderinkhof, K.R.; Blom, J.; Band, G.P.; Kok, A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology 2001, 38, 752–760. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. (Regul. Ed.) 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Falkenstein, M.; Hoormann, J.; Christ, S.; Hohnsbein, J. ERP components on reaction errors and their functional significance: A tutorial. Biol. Psychol. 2000, 51, 87–107. [Google Scholar] [CrossRef]
- Di Gregorio, F.; Maier, M.E.; Steinhauser, M. Errors can elicit an error positivity in the absence of an error negativity: Evidence for independent systems of human error monitoring. Neuroimage 2018, 172, 427–436. [Google Scholar] [CrossRef]
- Steinhauser, M.; Yeung, N. Decision Processes in Human Performance Monitoring. J. Neurosci. 2010, 30, 15643–15653. [Google Scholar] [CrossRef] [Green Version]
- Compton, R.J.; Arnstein, D.; Freedman, G.; Dainer-Best, J.; Liss, A.; Robinson, M.D. Neural and behavioral measures of error-related cognitive control predict daily coping with stress. Emotion 2011, 11, 379–390. [Google Scholar] [CrossRef]
- Compton, R.J.; Robinson, M.D.; Ode, S.; Quandt, L.C.; Fineman, S.L.; Carp, J. Error-monitoring ability predicts daily stress regulation. Psychol. Sci. 2008, 19, 702–708. [Google Scholar] [CrossRef]
- Hall, J.R.; Bernat, E.M.; Patrick, C.J. Externalizing Psychopathology and the Error-Related Negativity. Psychol. Sci. 2007, 18, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Ruchsow, M.; Spitzer, M.; Grön, G.; Grothe, J.; Kiefer, M. Error processing and impulsiveness in normals: Evidence from event-related potentials. Cogn. Brain Res. 2005, 24, 317–325. [Google Scholar] [CrossRef]
- Shiels, K.; Hawk, L.W., Jr. Self-regulation in ADHD: The role of error processing. Clin. Psychol. Rev. 2010, 30, 951–961. [Google Scholar] [CrossRef] [Green Version]
- Hirsh, J.B.; Inzlicht, M. Error-related negativity predicts academic performance. Psychophysiology 2010, 47, 192–196. [Google Scholar] [CrossRef]
- Kim, M.H.; Grammer, J.K.; Marulis, L.M.; Carrasco, M.; Morrison, F.J.; Gehring, W.J. Early math and reading achievement are associated with the error positivity. Dev. Cogn. Neurosci. 2016, 22, 18–26. [Google Scholar] [CrossRef]
- Moser, J.S.; Moran, T.P.; Schroder, H.S.; Donnellan, M.B.; Yeung, N. On the relationship between anxiety and error monitoring: A meta-analysis and conceptual framework. Front. Hum. Neurosci. 2013, 7, 466. [Google Scholar] [CrossRef]
- Teper, R.; Inzlicht, M. Meditation, mindfulness and executive control: the importance of emotional acceptance and brain-based performance monitoring. Soc. Cogn. Affect. Neurosci. 2013, 8, 85–92. [Google Scholar] [CrossRef]
- Andreu, C.I.; Moënne-Loccoz, C.; López, V.; Slagter, H.A.; Franken, I.H.A.; Cosmelli, D. Behavioral and electrophysiological evidence of enhanced performance monitoring in meditators. Mindfulness 2017, 8, 1603–1614. [Google Scholar] [CrossRef]
- Bailey, N.W.; Raj, K.; Freedman, G.; Fitzgibbon, B.M.; Rogasch, N.C.; Van Dam, N.T.; Fitzgerald, P.B. Mindfulness meditators do not show differences in electrophysiological measures of error processing. Mindfulness 2019, 10, 1360–1380. [Google Scholar] [CrossRef]
- Larson, M.J.; Steffen, P.R.; Primosch, M. The impact of a brief mindfulness meditation intervention on cognitive control and error-related performance monitoring. Front. Hum. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Schoenberg, P.L.A.; Hepark, S.; Kan, C.C.; Barendregt, H.P.; Buitelaar, J.K.; Speckens, A.E.M. Effects of mindfulness-based cognitive therapy on neurophysiological correlates of performance monitoring in adult attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 2014, 125, 1407–1416. [Google Scholar] [CrossRef]
- Saunders, B.; Rodrigo, A.H.; Inzlicht, M. Mindful awareness of feelings increases neural performance monitoring. Cogn. Affect. Behav. Neurosci. 2016, 16, 93–105. [Google Scholar] [CrossRef]
- Vago, D.R.; Silbersweig, D.A. Self-awareness, self-regulation, and self-transcendence (S-ART): A framework for understanding the neurobiological mechanisms of mindfulness. Front. Hum. Neurosci. 2012, 6, 296. [Google Scholar] [CrossRef]
- Grossman, P. On measuring mindfulness in psychosomatic and psychological research. J. Psychosom. Res. 2008, 64, 405–408. [Google Scholar] [CrossRef]
- Chiesa, A.; Calati, R.; Serretti, A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin. Psychol. Rev. 2011, 31, 449–464. [Google Scholar] [CrossRef]
- Sauer, S.; Walach, H.; Schmidt, S.; Hinterberger, T.; Lynch, S.; Büssing, A.; Kohls, N. Assessment of mindfulness: Review on state of the art. Mindfulness 2013, 4, 3–17. [Google Scholar] [CrossRef]
- Holzel, B.K.; Carmody, J.; Vangel, M.; Congelton, C.; Yerramsetti, S.M.; Gard, T.; Lazar, S.W. Mindfulness practice leads to increase in regional brain gray matter density. Psychiatry Res. Neuroimaging 2011, 191, 36–43. [Google Scholar] [CrossRef]
- Coffey, K.A.; Hartman, M.; Fredrickson, B.L. Deconstructing mindfulness and constructing mental health: Understanding mindfulness and its mechanisms of action. Mindfulness 2010, 1, 235–253. [Google Scholar] [CrossRef]
- Grabovac, A.D.; Lau, M.A.; Willett, B.R. Mechanisms of mindfulness: A Buddhist psychological model. Mindfulness 2011, 2, 154–166. [Google Scholar] [CrossRef]
- Chambers, R.; Gullone, E.; Allen, N.B. Mindful emotion regulation: An integrative review. Clin. Psychol. Rev. 2009, 29, 560–572. [Google Scholar] [CrossRef]
- Lutz, A.; Jha, A.P.; Dunne, J.D.; Saron, C.D. Investigating the phenomenological matrix of mindfulness-related practices from a neurocognitive perspective. Am. Psychol. 2015, 70, 632–658. [Google Scholar] [CrossRef]
- Keng, S.-L.; Smoski, M.J.; Robins, C.J. Effects of mindfulness on psychological health: A review of empirical studies. Clin. Psychol. Rev. 2011, 31, 1041–1056. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.-Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef]
- Lutz, A.; Slagter, H.A.; Dunne, J.D.; Davidson, R.J. Attention regulation and monitoring in meditation. Trends Cogn. Sci. 2008, 12, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Fox, K.C.R.; Dixon, M.L.; Nijeboer, S.; Girn, M.; Floman, J.L.; Lifshitz, M.; Ellamil, M.; Sedlmeier, P.; Christoff, K. Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. Neurosci. Biobehav. Rev. 2016, 65, 208–228. [Google Scholar] [CrossRef] [Green Version]
- Manna, A.; Raffone, A.; Perrucci, M.G.; Nardo, D.; Ferretti, A.; Tartaro, A.; Londei, A.; Del Gratta, C.; Belardinelli, M.O.; Romani, G.L. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res. Bull. 2010, 82, 46–56. [Google Scholar] [CrossRef]
- Colzato, L.S.; Ozturk, A.; Hommel, B. Meditate to Create: The Impact of Focused-Attention and Open-Monitoring Training on Convergent and Divergent Thinking. Front. Psychol. 2012, 3, 116. [Google Scholar] [CrossRef] [Green Version]
- Perlman, D.M.; Salomons, T.V.; Davidson, R.J.; Lutz, A. Differential effects on pain intensity and unpleasantness of two meditation practices. Emotion 2010, 10, 65–71. [Google Scholar] [CrossRef]
- Lippelt, D.P.; Hommel, B.; Colzato, L.S. Focused attention, open monitoring and loving kindness meditation: Effects on attention, conflict monitoring, and creativity—A review. Front. Psychol. 2014, 5, 1083. [Google Scholar] [CrossRef]
- Cahn, B.R.; Delorme, A.; Polich, J. Occipital gamma activation during Vipassana meditation. Cogn. Process. 2010, 11, 39–56. [Google Scholar] [CrossRef]
- Britton, W.B.; Davis, J.H.; Loucks, E.B.; Peterson, B.; Cullen, B.H.; Reuter, L.; Rando, A.; Rahrig, H.; Lipsky, J.; Lindahl, J.R. Dismantling Mindfulness-Based Cognitive Therapy: Creation and validation of 8-week focused attention and open monitoring interventions within a 3-armed randomized controlled trial. Behav. Res. Ther. 2018, 101, 92–107. [Google Scholar] [CrossRef]
- Segal, Z.V.; Williams, M.; Teasdale, J. Mindfulness-Based Cognitive Therapy for Depression: Second Edition. Available online: https://www.guilford.com/books/Mindfulness-Based-Cognitive-Therapy-for-Depression/Segal-Williams-Teasdale/9781462537037 (accessed on 4 August 2019).
- Polak, E.L. Impact of Two Sessions of Mindfulness Training on Attention. Open Access Dissertations. p. 251. Available online: https://scholarlyrepository.miami.edu/oa_dissertations/251 (accessed on 6 August 2019).
- Goldberg, S.B.; Wielgosz, J.; Dahl, C.; Schuyler, B.; MacCoon, D.S.; Rosenkranz, M.; Lutz, A.; Sebranek, C.A.; Davidson, R.J. Does the Five Facet Mindfulness Questionnaire measure what we think it does? Construct validity evidence from an active controlled randomized clinical trial. Psychol. Assess. 2016, 28, 1009–1014. [Google Scholar] [CrossRef]
- Grossman, P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2011). Psychol. Assess. 2011, 23, 1034–1040. [Google Scholar] [CrossRef]
- Van Dam, N.T.; Earleywine, M.; Danoff-Burg, S. Differential item function across meditators and non-meditators on the Five Facet Mindfulness Questionnaire. Personal. Individ. Differ. 2009, 47, 516–521. [Google Scholar] [CrossRef]
- Baer, R.A.; Smith, G.T.; Hopkins, J.; Krietemeyer, J.; Toney, L. Using self-report assessment methods to explore facets of mindfulness. Assessment 2006, 13, 27–45. [Google Scholar] [CrossRef]
- Brown, K.W.; Goodman, R.J.; Inzlicht, M. Dispositional mindfulness and the attenuation of neural responses to emotional stimuli. Soc. Cogn. Affect. Neurosci. 2013, 8, 93–99. [Google Scholar] [CrossRef]
- Lin, Y.; Fisher, M.E.; Roberts, S.M.M.; Moser, J.S. Deconstructing the Emotion Regulatory Properties of Mindfulness: An Electrophysiological Investigation. Front. Hum. Neurosci. 2016, 10, 451. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Fisher, M.E.; Moser, J.S. Clarifying the relationship between mindfulness and executive attention: A combined behavioral and neurophysiological study. Soc. Cogn. Affect. Neurosci. 2018, 14, 205–215. [Google Scholar] [CrossRef]
- Bradley, M.M.; Codispoti, M.; Sabatinelli, D.; Lang, P.J. Emotion and motivation II: sex differences in picture processing. Emotion 2001, 1, 300–319. [Google Scholar] [CrossRef]
- Syrjänen, E.; Wiens, S. Gender moderates valence effects on the late positive potential to emotional distracters. Neurosci. Lett. 2013, 551, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Larson, M.J.; South, M.; Clayson, P. Sex differences in error-related performance monitoring. Neuroreport 2011, 22, 44–48. [Google Scholar] [CrossRef]
- Moran, T.P.; Taylor, D.; Moser, J.S. Sex moderates the relationship between worry and performance monitoring brain activity in undergraduates. Int. J. Psychophysiol. 2012, 85, 188–194. [Google Scholar] [CrossRef]
- Laurent, H.; Laurent, S.; Hertz, R.; Egan-Wright, D.; Granger, D.A. Sex-specific effects of mindfulness on romantic partners’ cortisol responses to conflict and relations with psychological adjustment. Psychoneuroendocrinology 2013, 38, 2905–2913. [Google Scholar] [CrossRef]
- De Vibe, M.; Solhaug, I.; Tyssen, R.; Friborg, O.; Rosenvinge, J.H.; Sørlie, T.; Bjørndal, A. Mindfulness training for stress management: a randomised controlled study of medical and psychology students. BMC Med. Educ. 2013, 13, 107. [Google Scholar] [CrossRef]
- Luders, E.; Thompson, P.M.; Kurth, F. Larger hippocampal dimensions in meditation practitioners: Differential effects in women and men. Front. Psychol. 2015, 6, 186. [Google Scholar] [CrossRef]
- Rojiani, R.; Santoyo, J.F.; Rahrig, H.; Roth, H.D.; Britton, W.B. Women Benefit More Than Men in Response to College-based Meditation Training. Front. Psychol. 2017, 8, 551. [Google Scholar] [CrossRef]
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and alternative medicine use among adults and children: United States, 2007. Natl. Health Stat. Rep. 2008, 1–23. Available online: https://stacks.cdc.gov/view/cdc/5266 (accessed on 10 December 2008).
- Hickman, S. 20-Minute Seated Meditation. [Audio File]. Available online: soundcloud.com/ucsdmindfulness/20-min-seated-meditation-by-steve-hickman?in=ucsdmindfulness/sets/seated-meditation (accessed on 5 September 2019).
- Lonsdale, C. How to Learn any Language in Six Months. [Video File]. Available online: https://www.youtube.com/watch?v=d0yGdNEWdn0 (accessed on 5 September 2019).
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Olvet, D.M.; Hajcak, G. The effect of trial-to-trial feedback on the error-related negativity and its relationship with anxiety. Cogn. Affect. Behav. Neurosci. 2009, 9, 427–433. [Google Scholar] [CrossRef]
- Britton, W.B.; Lindahl, J.R.; Cahn, B.R.; Davis, J.H.; Goldman, R.E. Awakening is not a metaphor: The effects of Buddhist meditation practices on basic wakefulness. Ann. N. Y. Acad. Sci. 2014, 1307, 64–81. [Google Scholar] [CrossRef]
- Hoddes, E.; Dement, W.; Zarcone, V. The development and use of the Stanford Sleepiness Scale (SSS). Psychophysiology 1972, 9, 150. [Google Scholar]
- Gratton, G.; Coles, M.G.; Donchin, E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef]
- Lin, Y.; Moran, T.P.; Schroder, H.S.; Moser, J.S. The role of hand of error and stimulus orientation in the relationship between worry and error-related brain activity: Implications for theory and practice. Psychophysiology 2015, 52, 1281–1292. [Google Scholar] [CrossRef]
- Moser, J.S.; Schroder, H.S.; Heeter, C.; Moran, T.P.; Lee, Y.-H. Mind your errors: evidence for a neural mechanism linking growth mind-set to adaptive posterror adjustments. Psychol. Sci. 2011, 22, 1484–1489. [Google Scholar] [CrossRef]
- Schroder, H.S.; Moran, T.P.; Infantolino, Z.P.; Moser, J.S. The relationship between depressive symptoms and error monitoring during response switching. Cogn. Affect. Behav. Neurosci. 2013, 13, 790–802. [Google Scholar] [CrossRef]
- Picton, T.W.; van Roon, P.; Armilio, M.L.; Berg, P.; Ille, N.; Scherg, M. The correction of ocular artifacts: A topographic perspective. Clin. Neurophysiol. 2000, 111, 53–65. [Google Scholar] [CrossRef]
- Luck, S.J.; Gaspelin, N. How to get statistically significant effects in any ERP experiment (and why you shouldn’t). Psychophysiology 2017, 54, 146–157. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Miles, J.; Shevlin, M. Applying Regression & Correlation: A Guide for Students and Researchers; Sage Publications: London, UK; Thousand Oaks, CA, USA, 2001; ISBN 978-0-7619-6229-8. [Google Scholar]
- Clayson, P.E.; Larson, M.J. Conflict adaptation and sequential trial effects: support for the conflict monitoring theory. Neuropsychologia 2011, 49, 1953–1961. [Google Scholar] [CrossRef]
- Klein, T.A.; Ullsperger, M.; Danielmeier, C. Error awareness and the insula: Links to neurological and psychiatric diseases. Front. Hum. Neurosci. 2013, 7, 14. [Google Scholar] [CrossRef]
- Jerath, R.; Barnes, V.A.; Crawford, M.W. Mind-body response and neurophysiological changes during stress and meditation: Central role of homeostasis. J. Biol. Regul. Homeost. Agents 2014, 28, 545–554. [Google Scholar]
- Jerath, R.; Edry, J.W.; Barnes, V.A.; Jerath, V. Physiology of long pranayamic breathing: Neural respiratory elements may provide a mechanism that explains how slow deep breathing shifts the autonomic nervous system. Med. Hypotheses 2006, 67, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Amihai, I.; Kozhevnikov, M. Arousal vs. Relaxation: A Comparison of the Neurophysiological and Cognitive Correlates of Vajrayana and Theravada Meditative Practices. PLoS ONE 2014, 9, e102990. [Google Scholar] [CrossRef]
- Bergomi, C.; Tschacher, W.; Kupper, Z. The assessment of mindfulness with self-report measures: Existing scales and open issues. Mindfulness 2013, 4, 191–202. [Google Scholar] [CrossRef]
- Grossman, P.; Van Dam, N.T. Mindfulness, by any other name…: Trials and tribulations of sati in western psychology and science. Contemp. Buddhism 2011, 12, 219–239. [Google Scholar] [CrossRef]
- Van Dam, N.T.; Hobkirk, A.L.; Danoff-Burg, S.; Earleywine, M. Mind Your Words: Positive and Negative Items Create Method Effects on the Five Facet Mindfulness Questionnaire. Assessment 2012, 19, 198–204. [Google Scholar] [CrossRef]
- Baer, R.A.; Smith, G.T.; Lykins, E.; Button, D.; Krietemeyer, J.; Sauer, S.; Walsh, E.; Duggan, D.; Williams, J.M.G. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment 2008, 15, 329–342. [Google Scholar] [CrossRef]
- Mallick, T.; Kulkarni, R. The effect of trataka, a yogic visual concentration practice, on critical flicker fusion. J. Altern. Complement. Med. 2010, 16, 1265–1267. [Google Scholar] [CrossRef]
- Zambrano-Vazquez, L.; Ziebell, P.; Bowles, A.E.; McGarrh, D.A.; Allen, J. The Interaction between Trait and State Worry on the Ern During a Worry Induction Task. Psychophysiology 2015, 52, S35. [Google Scholar]
- Endrass, T.; Reuter, B.; Kathmann, N. ERP correlates of conscious error recognition: Aware and unaware errors in an antisaccade task. Eur. J. Neurosci. 2007, 26, 1714–1720. [Google Scholar] [CrossRef]
- Holmes, A.J.; Pizzagalli, D.A. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch. Gen. Psychiatry 2008, 65, 179–188. [Google Scholar] [CrossRef]
- Schroder, H.S.; Moran, T.P.; Donnellan, M.B.; Moser, J.S. Mindset induction effects on cognitive control: A neurobehavioral investigation. Biol. Psychol. 2014, 103, 27–37. [Google Scholar] [CrossRef]
- Van Veen, V.; Carter, C.S. The timing of action-monitoring processes in the anterior cingulate cortex. J. Cogn. Neurosci. 2002, 14, 593–602. [Google Scholar] [CrossRef]
- Urigüen, J.A.; Garcia-Zapirain, B. EEG artifact removal-state-of-the-art and guidelines. J. Neural. Eng. 2015, 12, 031001. [Google Scholar] [CrossRef]
- Koenig, T.; Kottlow, M.; Stein, M.; Melie-García, L. Ragu: A Free Tool for the Analysis of EEG and MEG Event-Related Scalp Field Data Using Global Randomization Statistics. Available online: https://www.hindawi.com/journals/cin/2011/938925/ (accessed on 28 August 2019).
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef]
- Davidson, R.J.; Dahl, C.J. Outstanding Challenges in Scientific Research on Mindfulness and Meditation. Perspect. Psychol. Sci. 2018, 13, 62–65. [Google Scholar] [CrossRef]
- Hölzel, B.K.; Lazar, S.W.; Gard, T.; Schuman-Olivier, Z.; Vago, D.R.; Ott, U. How Does Mindfulness Meditation Work? Proposing Mechanisms of Action From a Conceptual and Neural Perspective. Perspect. Psychol. Sci. 2011, 6, 537–559. [Google Scholar] [CrossRef]
| Variable | Control N = 103 | Meditation N = 103 | ||||
|---|---|---|---|---|---|---|
| Range | M | SD | Range | M | SD | |
| FFMQ Overall | 2.23–4.49 | 3.21 | 0.43 | 2.21–4.23 | 3.19 | 0.43 |
| FFMQ-O | 11–37 | 25.45 | 5.14 | 17–40 | 26.52 | 5.22 |
| FFMQ-D | 9–36 | 26.21 | 5.39 | 12–38 | 26.14 | 5.68 |
| FFMQ- AA | 14–39 | 27.56 | 5.72 | 12–39 | 26.49 | 5.74 |
| FFMQ-NJ | 9–38 | 26 | 6.91 | 10–38 | 25.42 | 6.50 |
| FFMQ-NR | 11–34 | 19.93 | 4.50 | 12–30 | 19.67 | 3.92 |
| Audio Engagement | 1–7 | 4.23 | 1.41 | 1–7 | 4.17 | 1.60 |
| Audio Interest | 1–7 | 4.54 | 1.62 | 1–7 | 3.55 | 1.67 |
| Audio Emotion Reactivity | 2–7 | 4.76 | 0.99 | 1–7 | 4.49 | 1.07 |
| Audio Arousal | 1–6 | 3.14 | 1.53 | 1–6 | 2.79 | 1.52 |
| Audio Understanding | 1–7 | 5.43 | 1.36 | 1–7 | 5.43 | 1.60 |
| Audio Learning | 1–7 | 4.68 | 1.36 | 1–6 | 3.51 | 1.42 |
| Audio Sleepiness | 1–6 | 3.81 | 1.40 | 1–7 | 4.36 | 1.48 |
| Variable | Meditation N = 103 | Control N = 103 | ||||
|---|---|---|---|---|---|---|
| SD | M | Range | SD | M | Range | |
| Accuracy | 0.08 | 0.83 | 0.52–0.96 | 0.1 | 0.82 | 0.51–0.96 |
| Number of errors | 36.04 | 77.92 | 17.235 | 46.18 | 82.38 | 18–243 |
| Incongruent errors | 23.07 | 54.37 | 11–134 | 35.58 | 58.15 | 13–240 |
| Congruent errors | 18.48 | 23.55 | 0–105 | 19.94 | 24.23 | 1–107 |
| Error RT (ms) | 42.21 | 327.57 | 264.87–498.57 | 53.53 | 334.73 | 253.55–520.65 |
| Correct RT (ms) | 41.25 | 408.45 | 309.82–540.72 | 51.36 | 412.65 | 316.21–568.46 |
| Incongruent error RT (ms) | 42.74 | 332.68 | 266.12–509 | 53.73 | 340.78 | 257.69–534.46 |
| Incongruent correct RT (ms) | 45.38 | 438.87 | 322.27–601.92 | 54.95 | 441.3 | 332.88–598.06 |
| Congruent error RT (ms) | 50.91 | 313.44 | 249.08–511.41 | 59.98 | 313.85 | 233.88–574.64 |
| Congruent correct RT (ms) | 39.09 | 382.89 | 299.50–496.23 | 50.39 | 388.45 | 296.61–543.34 |
| PES (ms) | 33.87 | 26.22 | −210.7 | 30.76 | 26.16 | −180.66 |
| PEA (ms) | 0.13 | 0.84 | 0.23–1 | 0.15 | 0.82 | 0.24–1 |
| CRN amplitude (µV) | 2.44 | −0.23 | −14.73 | 2.97 | 0.25 | −23.96 |
| ERN amplitude (µV) | 3.51 | −5.34 | −19.04 | 3.67 | −4.98 | −21.32 |
| Δ ERN (µV) | 3.53 | −5.11 | −18.22 | 3.74 | −5.23 | −17.1 |
| Ce amplitude (µV) | 2.95 | −3.48 | −14.42 | 2.76 | −3.39 | −14.33 |
| Pe amplitude (µV) | 4.11 | 4.63 | −25.37 | 4.1 | 3.38 | −23.6 |
| Δ Pe (µV) | 4.19 | 8.1 | −28.91 | 4.7 | 6.77 | −25.47 |
| Control | 1. | 2. | 3. | 4. | 5. | 6. | 7. |
| 1. Δ ERN | -- | ||||||
| 2. Δ Pe | −0.19 | -- | |||||
| 3. Number of errors | 0.31 ** | −0.42 ** | -- | ||||
| 4. Error RT | 0.33 ** | −0.26 ** | 0.01 | -- | |||
| 5. Correct RT | 0.23 * | −0.08 | −0.41 ** | 0.71 ** | -- | ||
| 6. PES | 0.03 | 0.12 | −0.39 ** | 0.31 ** | 0.31 ** | -- | |
| 7. PEA | −0.36 ** | 0.41 ** | −0.86 ** | −0.19 | 0.23 * | 0.36 ** | -- |
| Meditation | 1. | 2. | 3. | 4. | 5. | 6. | 7. |
| 1. Δ ERN | -- | ||||||
| 2. Δ Pe | −0.20 * | -- | |||||
| 3. Number of errors | 0.26 ** | −0.37 ** | -- | ||||
| 4. Error RT | 0.30 ** | −0.29 ** | −0.01 | -- | |||
| 5. Correct RT | 0.31 ** | −0.10 | −0.32 ** | 0.77 ** | -- | ||
| 6. PES | −0.02 | 0.02 | −0.32 ** | 0.19 | 0.25 * | -- | |
| 7. PEA | −0.31 ** | 0.40 ** | −0.85 ** | −0.26 ** | 0.06 | 0.38 ** | -- |
| Control | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. |
| 1. Δ ERN | -- | |||||||
| 2. Δ Pe | −0.19 | -- | ||||||
| 3. FFMQ Overall | −0.02 | 0.01 | -- | |||||
| 4. FFMQ-O | 0.04 | 0.10 | 0.42 ** | -- | ||||
| 5. FFMQ-D | 0.02 | −0.11 | 0.75 ** | 0.27 ** | -- | |||
| 6. FFMQ-AA | −0.02 | −0.05 | 0.64 ** | −0.08 | 0.39 ** | -- | ||
| 7. FFMQ-NJ | −0.04 | 0.02 | 0.68 ** | −0.08 | 0.33 ** | 0.50 ** | -- | |
| 8. FFMQ-NR | −0.05 | 0.07 | 0.51 ** | 0.35 ** | 0.32 ** | 0.00 | 0.08 | -- |
| Meditation | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. |
| 1. Δ ERN | -- | |||||||
| 2. Δ Pe | −0.20 * | -- | ||||||
| 3. FFMQ Overall | 0.05 | 0.05 | -- | |||||
| 4. FFMQ-O | 0.15 | 0.09 | 0.46 ** | -- | ||||
| 5. FFMQ-D | 0.15 | −0.08 | 0.70 ** | 0.37 * | -- | |||
| 6. FFMQ-AA | 0.00 | 0.01 | 0.74 ** | 0.04 | 0.27 ** | -- | ||
| 7. FFMQ-NJ | −0.10 | 0.03 | 0.63 ** | −0.22 * | 0.26 ** | 0.61 ** | -- | |
| 8. FFMQ-NR | −0.03 | 0.14 | 0.56 ** | 0.42 ** | 0.23 * | 0.26 ** | 0.07 | -- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Eckerle, W.D.; Peng, L.W.; Moser, J.S. On Variation in Mindfulness Training: A Multimodal Study of Brief Open Monitoring Meditation on Error Monitoring. Brain Sci. 2019, 9, 226. https://doi.org/10.3390/brainsci9090226
Lin Y, Eckerle WD, Peng LW, Moser JS. On Variation in Mindfulness Training: A Multimodal Study of Brief Open Monitoring Meditation on Error Monitoring. Brain Sciences. 2019; 9(9):226. https://doi.org/10.3390/brainsci9090226
Chicago/Turabian StyleLin, Yanli, William D. Eckerle, Ling W. Peng, and Jason S. Moser. 2019. "On Variation in Mindfulness Training: A Multimodal Study of Brief Open Monitoring Meditation on Error Monitoring" Brain Sciences 9, no. 9: 226. https://doi.org/10.3390/brainsci9090226
APA StyleLin, Y., Eckerle, W. D., Peng, L. W., & Moser, J. S. (2019). On Variation in Mindfulness Training: A Multimodal Study of Brief Open Monitoring Meditation on Error Monitoring. Brain Sciences, 9(9), 226. https://doi.org/10.3390/brainsci9090226





