Effects of Sound-Pressure Change on the 40 Hz Auditory Steady-State Response and Change-Related Cerebral Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sound Stimuli
2.3. MEG Recording
2.4. Dipole Source Modeling
2.5. Data Analysis
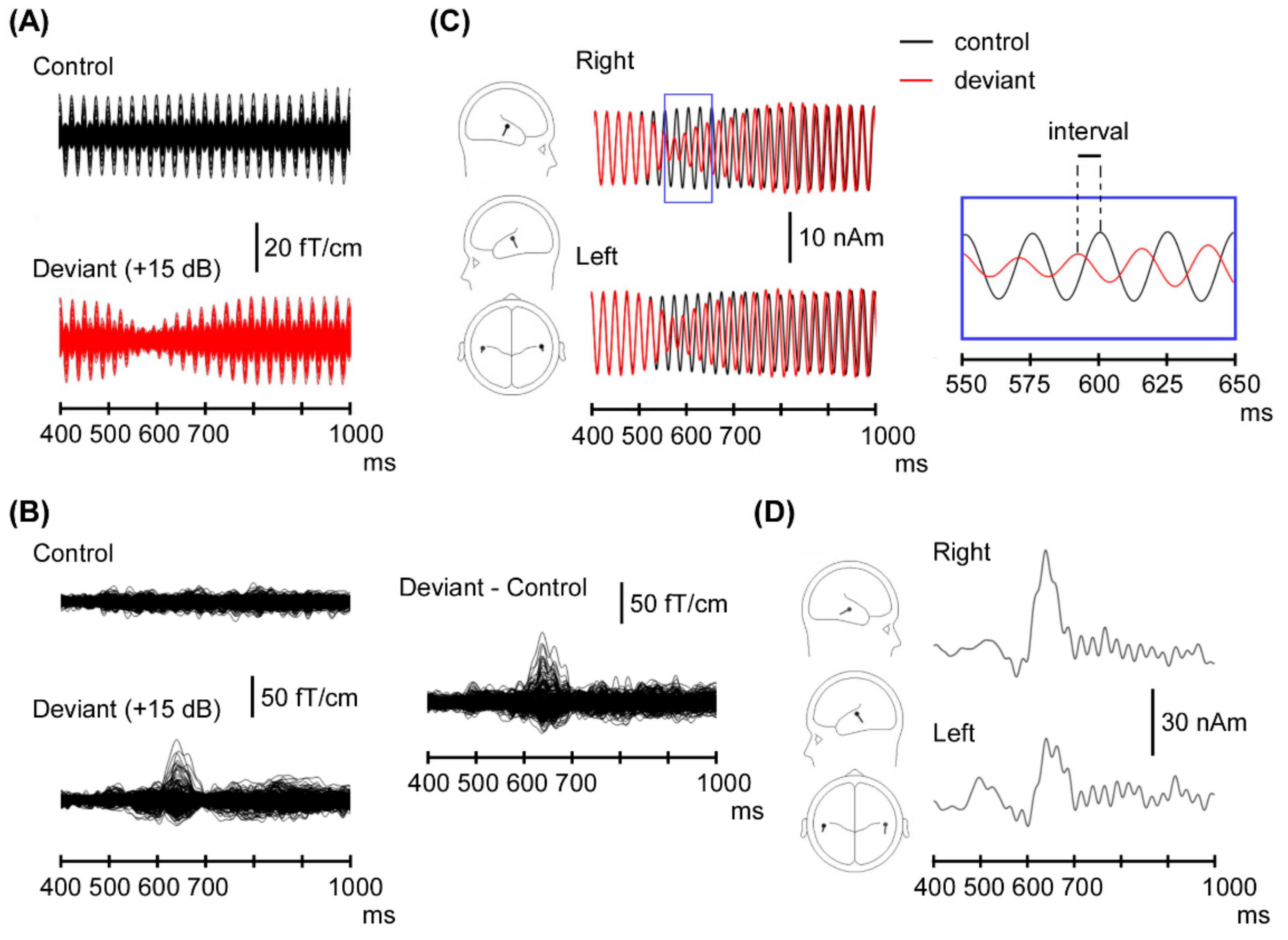
3. Results
3.1. ASSR Phase Deviation
3.2. Change-N1m Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inui, K.; Urakawa, T.; Yamashiro, K.; Otsuru, N.; Nishihara, M.; Takeshima, Y.; Keceli, S.; Kakigi, R. Non-linear laws of echoic memory and auditory change detection in humans. BMC Neurosci. 2010, 11, 80. [Google Scholar] [CrossRef]
- Yamashiro, K.; Inui, K.; Otsuru, N.; Kakigi, R. Change-related responses in the human auditory cortex: An MEG study. Psychophysiology 2011, 48, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Weise, A.; Schroger, E.; Feher, B.; Folyi, T.; Horvath, J. Auditory event-related potentials reflect dedicated change detection activity for higher-order acoustic transitions. Biol. Psychol. 2012, 91, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Weise, A.; Bendixen, A.; Muller, D.; Schroger, E. Which kind of transition is important for sound representation? An event-related potential study. Brain Res. 2012, 1464, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.C.; Mills, J.H.; Dubno, J.R. Electrophysiologic correlates of intensity discrimination in cortical evoked potentials of younger and older adults. Hear. Res. 2007, 228, 58–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soeta, Y.; Nakagawa, S. Temporal integration affects intensity change detection in human auditory cortex. Neuroreport 2010, 21, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, M.; Inui, K.; Motomura, E.; Otsuru, N.; Ushida, T.; Kakigi, R. Auditory N1 as a change-related automatic response. Neurosci. Res. 2011, 71, 145–148. [Google Scholar] [CrossRef]
- Otsuru, N.; Tsuruhara, A.; Motomura, E.; Tanii, H.; Nishihara, M.; Inui, K.; Kakigi, R. Effects of acute nicotine on auditory change-related cortical responses. Psychopharmacology 2012, 224, 327–335. [Google Scholar] [CrossRef]
- Soeta, Y.; Nakagawa, S. Auditory evoked responses in human auditory cortex to the variation of sound intensity in an ongoing tone. Hear. Res. 2012, 287, 67–75. [Google Scholar] [CrossRef]
- Kodaira, M.; Tsuruhara, A.; Motomura, E.; Tanii, H.; Inui, K.; Kakigi, R. Effects of acute nicotine on prepulse inhibition of auditory change-related cortical responses. Behav. Brain Res. 2013, 256, 27–35. [Google Scholar] [CrossRef]
- Akiyama, L.F.; Yamashiro, K.; Inui, K.; Kakigi, R. Automatic cortical responses to sound movement: A magnetoencephalography study. Neurosci. Lett. 2011, 488, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Ohoyama, K.; Motomura, E.; Inui, K.; Nishihara, M.; Otsuru, N.; Oi, M.; Kakigi, R.; Okada, M. Memory-based pre-attentive auditory N1 elicited by sound movement. Neurosci. Res. 2012, 73, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Otsuru, N.; Inui, K.; Kakigi, R. Change-related auditory P50: A MEG study. NeuroImage 2014, 86, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.J.; Longe, O.; Vaz Pato, M. Auditory evoked potentials to abrupt pitch and timbre change of complex tones: Electrophysiological evidence of ‘streaming’? Electroencephalogr. Clin. Neurophysiol. 1998, 108, 131–142. [Google Scholar] [CrossRef]
- Inui, K.; Urakawa, T.; Yamashiro, K.; Otsuru, N.; Takeshima, Y.; Nishihara, M.; Motomura, E.; Kida, T.; Kakigi, R. Echoic memory of a single pure tone indexed by change-related brain activity. BMC Neurosci. 2010, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, M.; Motomura, E.; Inui, K.; Ohoyama, K.; Tanii, H.; Konishi, Y.; Shiroyama, T.; Nishihara, M.; Kakigi, R.; Okada, M. Auditory change-related cerebral responses and personality traits. Neurosci. Res. 2016, 103, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.; Herdman, A.T.; Pantev, C. Stimulus induced desynchronization of human auditory 40-Hz steady-state responses. J. Neurophysiol. 2005, 94, 4082–4093. [Google Scholar] [CrossRef]
- Ross, B.; Herdman, A.T.; Pantev, C. Right hemispheric laterality of human 40 Hz auditory steady-state responses. Cereb. Cortex 2005, 15, 2029–2039. [Google Scholar] [CrossRef]
- Ross, B. A novel type of auditory responses: Temporal dynamics of 40-Hz steady-state responses induced by changes in sound localization. J. Neurophysiol. 2008, 100, 1265–1277. [Google Scholar] [CrossRef]
- Kuriki, S.; Kobayashi, Y.; Kobayashi, T.; Tanaka, K.; Uchikawa, Y. Steady-state MEG responses elicited by a sequence of amplitude-modulated short tones of different carrier frequencies. Hear. Res. 2013, 296, 25–35. [Google Scholar] [CrossRef]
- Ross, B.; Fujioka, T. 40-Hz oscillations underlying perceptual binding in young and older adults. Psychophysiology 2016, 53, 974–990. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.L.; Stecker, G.C.; Rinne, T.; Herron, T.J.; Cate, A.D.; Yund, E.W.; Liao, I.; Kang, X. Functional maps of human auditory cortex: Effects of acoustic features and attention. PLoS ONE 2009, 4, e5183. [Google Scholar] [CrossRef] [PubMed]
- Bohorquez, J.; Ozdamar, O. Generation of the 40-Hz auditory steady-state response (ASSR) explained using convolution. Clin. Neurophysiol. 2008, 119, 2598–2607. [Google Scholar] [CrossRef] [PubMed]
- Althen, H.; Grimm, S.; Escera, C. Fast detection of unexpected sound intensity decrements as revealed by human evoked potentials. PLoS ONE 2011, 6, e28522. [Google Scholar] [CrossRef] [PubMed]
- Tiitinen, H.; May, P.; Reinikainen, K.; Naatanen, R. Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature 1994, 372, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Takegata, R.; Heikkila, R.; Naatanen, R. Sound energy and the magnitude of change: Effects on mismatch negativity. Neuroreport 2011, 22, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Naatanen, R.; Pakarinen, S.; Rinne, T.; Takegata, R. The mismatch negativity (MMN): Towards the optimal paradigm. Clin. Neurophysiol. 2004, 115, 140–144. [Google Scholar] [CrossRef]
- Jankowiak, S.; Berti, S. Behavioral and event-related potential distraction effects with regularly occurring auditory deviants. Psychophysiology 2007, 44, 79–85. [Google Scholar] [CrossRef]
- Pakarinen, S.; Huotilainen, M.; Naatanen, R. The mismatch negativity (MMN) with no standard stimulus. Clin. Neurophysiol. 2010, 121, 1043–1050. [Google Scholar] [CrossRef]
- Tan, H.R.; Gross, J.; Uhlhaas, P.J. MEG-measured auditory steady-state oscillations show high test-retest reliability: A sensor and source-space analysis. NeuroImage 2015, 122, 417–426. [Google Scholar] [CrossRef]
- Kwon, J.S.; O’Donnell, B.F.; Wallenstein, G.V.; Greene, R.W.; Hirayasu, Y.; Nestor, P.G.; Hasselmo, M.E.; Potts, G.F.; Shenton, M.E.; McCarley, R.W. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch. Gen. Psychiatry 1999, 56, 1001–1005. [Google Scholar] [CrossRef]
- Onitsuka, T.; Oribe, N.; Nakamura, I.; Kanba, S. Review of neurophysiological findings in patients with schizophrenia. Psychiatry Clin. Neurosci. 2013, 67, 461–470. [Google Scholar] [CrossRef]
- O’Donnell, B.F.; Vohs, J.L.; Krishnan, G.P.; Rass, O.; Hetrick, W.P.; Morzorati, S.L. Chapter 6-The auditory steady-state response (ASSR): A translational biomarker for schizophrenia. Suppl. Clin. Neurophysiol. 2013, 62, 101–112. [Google Scholar]
- Koerner, T.K.; Zhang, Y. Effects of background noise on inter-trial phase coherence and auditory N1-P2 responses to speech stimuli. Hear. Res. 2015, 328, 113–119. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motomura, E.; Inui, K.; Kawano, Y.; Nishihara, M.; Okada, M. Effects of Sound-Pressure Change on the 40 Hz Auditory Steady-State Response and Change-Related Cerebral Response. Brain Sci. 2019, 9, 203. https://doi.org/10.3390/brainsci9080203
Motomura E, Inui K, Kawano Y, Nishihara M, Okada M. Effects of Sound-Pressure Change on the 40 Hz Auditory Steady-State Response and Change-Related Cerebral Response. Brain Sciences. 2019; 9(8):203. https://doi.org/10.3390/brainsci9080203
Chicago/Turabian StyleMotomura, Eishi, Koji Inui, Yasuhiro Kawano, Makoto Nishihara, and Motohiro Okada. 2019. "Effects of Sound-Pressure Change on the 40 Hz Auditory Steady-State Response and Change-Related Cerebral Response" Brain Sciences 9, no. 8: 203. https://doi.org/10.3390/brainsci9080203
APA StyleMotomura, E., Inui, K., Kawano, Y., Nishihara, M., & Okada, M. (2019). Effects of Sound-Pressure Change on the 40 Hz Auditory Steady-State Response and Change-Related Cerebral Response. Brain Sciences, 9(8), 203. https://doi.org/10.3390/brainsci9080203




