Gamma Knife Radiosurgery for Trigeminal Neuralgia: A Comparison of Dose Protocols
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Population
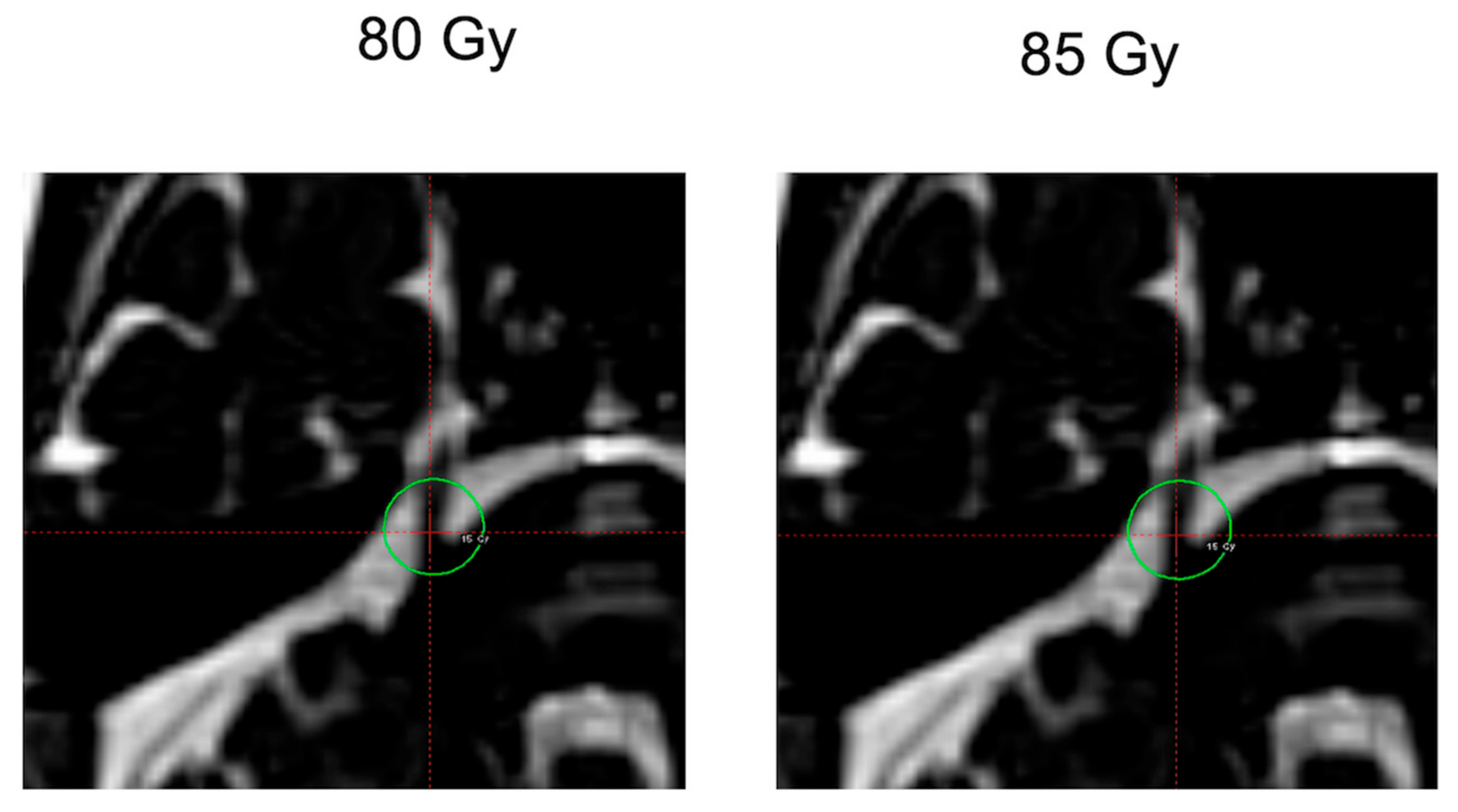
2.2. GKR Treatment Plan
2.3. Statistics
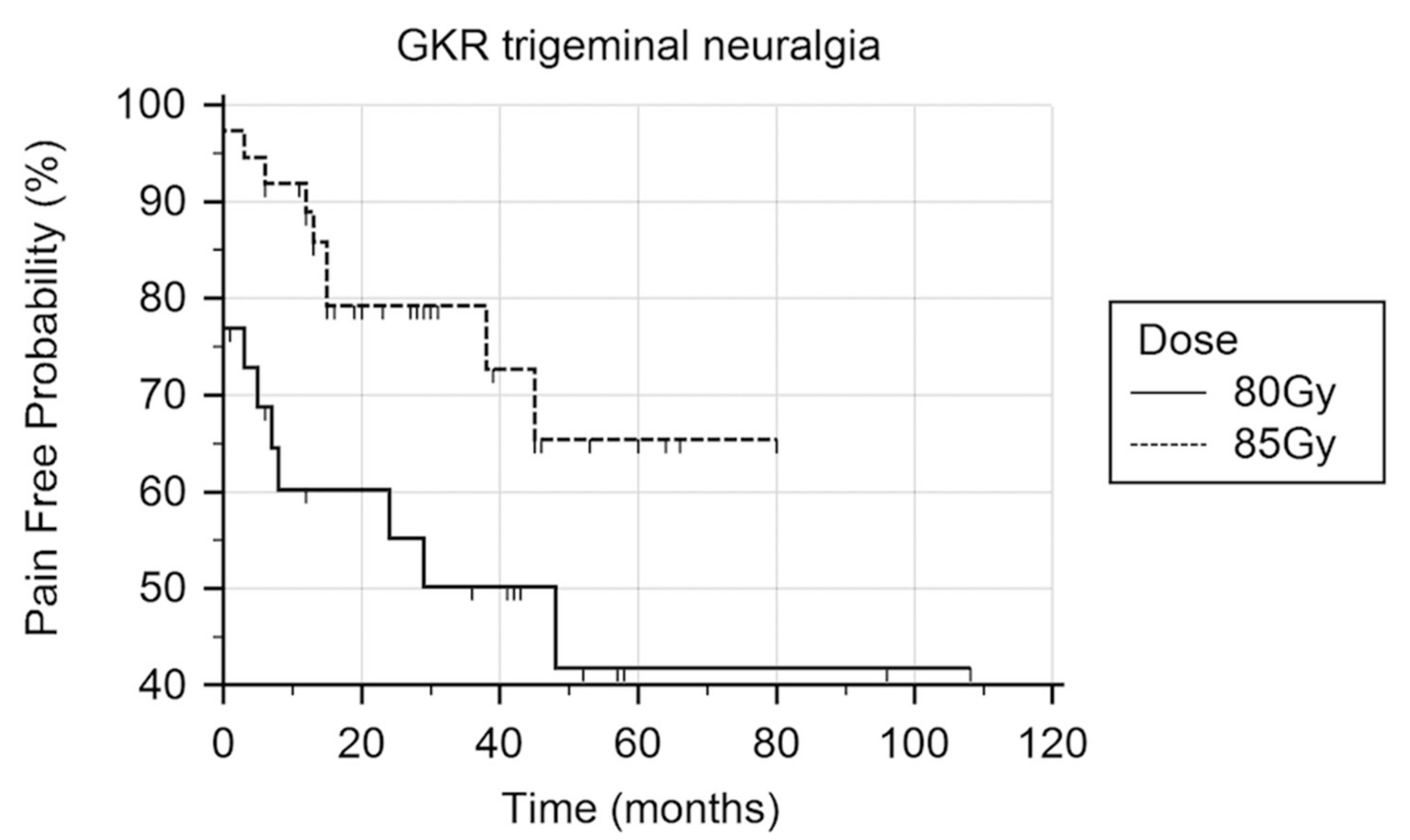
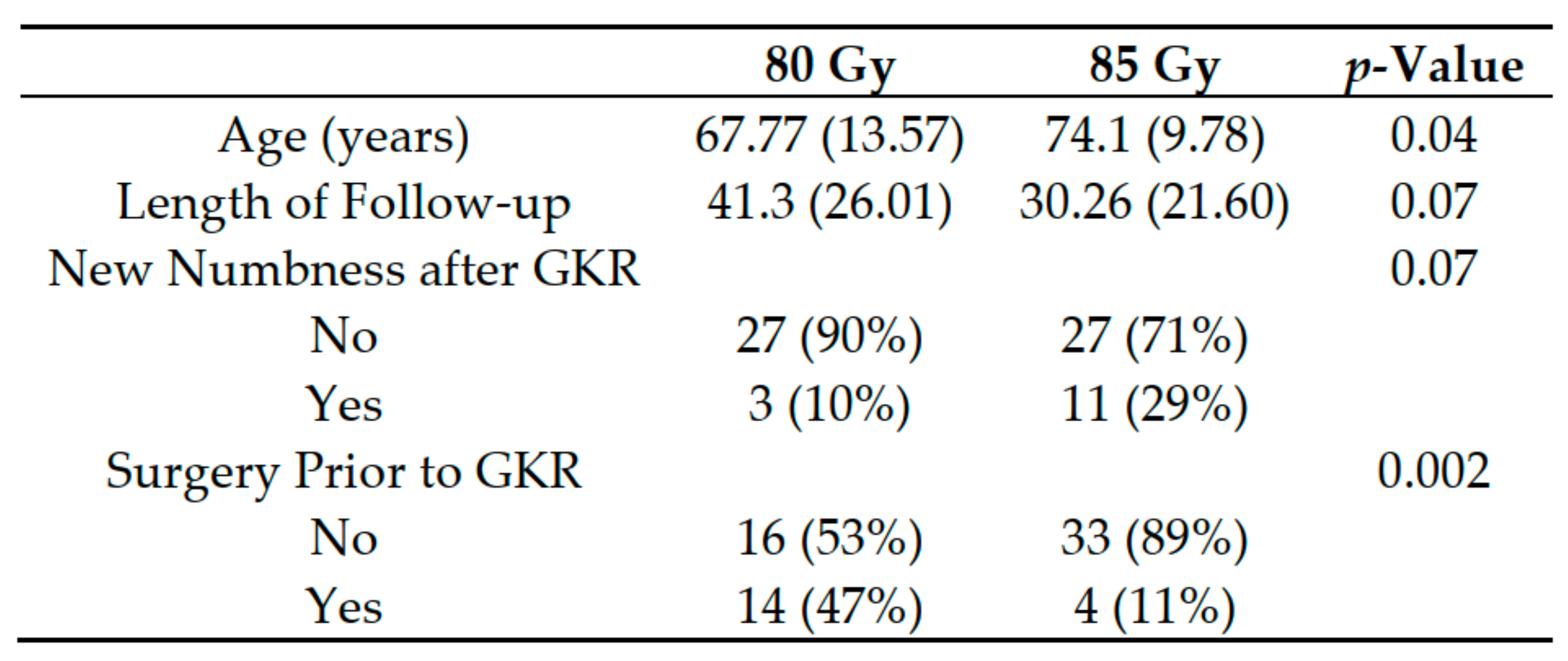
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Niranjan, A.; Maitz, A.H.; Lunsford, A.; Gerszten, P.C.; Flickinger, J.C.; Kondziolka, D.; Lunsford, L.D. Radiosurgery techniques and current devices. Prog. Neurol. Surg. 2007, 20, 50–67. [Google Scholar] [PubMed]
- Régis, J.; Tuleasca, C. Fifteen years of Gamma Knife surgery for trigeminal neuralgia in the Journal of Neurosurgery. History of a revolution in functional neurosurgery. J. Neurosurg. 2011, 115, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Mousavi, S.H.; Faraji, A.H.; Akpinar, B.; Monaco, E.A.; Flickinger, J.C.; Niranjan, A.; Lunsford, L.D. Stereotactic Radiosurgery as Initial Surgical Management for Elderly Patients with Trigeminal Neuralgia. Stereotact. Funct. Neurosurg. 2017, 95, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.L.; Shetter, A.G.; Fiedler, J.A.; Smith, K.A.; Han, P.P.; Speiser, B.L. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of The Barrow Neurological Institute. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1013–1019. [Google Scholar] [CrossRef]
- Kondziolka, D.; Lunsford, L.D.; Flickinger, J.C.; Young, R.F.; Vermeulen, S.; Duma, C.M.; Jacques, D.B.; Rand, R.W.; Regis, J.; Peragut, J.C.; et al. Stereotactic radiosurgery for trigeminal neuralgia: A multiinstitutional study using the gamma unit. J. Neurosurg. 1996, 84, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Greathouse, H.E.; Girvigian, M.R.; Miller, M.J.; Liu, A.; Rahimian, J. Prognostic factors for radiosurgery treatment of trigeminal neuralgia. Neurosurgery 2008, 62, A53–A60. [Google Scholar] [CrossRef] [PubMed]
- Fountas, K.N.; Smith, J.R.; Lee, G.P.; Jenkins, P.D.; Cantrell, R.R.; Sheils, W.C. Gamma Knife stereotactic radiosurgical treatment of idiopathic trigeminal neuralgia: Long-term outcome and complications. Neurosurg. Focus 2007, 23, E8. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E.; Phuong, L.K.; Foote, R.L.; Stafford, S.L.; Gorman, D.A. High-dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunction. Neurosurgery 2001, 49, 58–62. [Google Scholar] [PubMed]
- Sheehan, J.; Pan, H.C.; Stroila, M.; Steiner, L. Gamma knife surgery for trigeminal neuralgia: Outcomes and prognostic factors. J. Neurosurg. 2005, 102, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Tawk, R.G.; Duffy-Fronckowiak, M.; Scott, B.E.; Alberico, R.A.; Diaz, A.Z.; Podgorsak, M.B.; Plunkett, R.J.; Fenstermaker, R.A. Stereotactic gamma knife surgery for trigeminal neuralgia: Detailed analysis of treatment response. J. Neurosurg. 2005, 102, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Régis, J.; Metellus, P.; Hayashi, M.; Roussel, P.; Donnet, A.; Bille-Turc, F. Prospective controlled trial of gamma knife surgery for essential trigeminal neuralgia. J. Neurosurg. 2006, 104, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, P.; Zhang, S.; Gong, F.; Yang, S.; Wang, W. Effect of radiation dose on the outcomes of gamma knife treatment for trigeminal neuralgia: A multi-factor analysis. Neurol. India 2014, 62, 400–405. [Google Scholar] [PubMed]
- Kim, Y.H.; Kim, D.G.; Kim, J.W.; Han, J.H.; Chung, H.T.; Paek, S.H. Is it effective to raise the irradiation dose from 80 to 85 Gy in gamma knife radiosurgery for trigeminal neuralgia? Stereotact. Funct. Neurosurg. 2010, 88, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Alpert, T.E.; Chung, C.T.; Mitchell, L.T.; Hodge, C.J.; Montgomery, C.T.; Bogart, J.A.; Kim, D.Y.; Bassano, D.A.; Hahn, S.S. Gamma knife surgery for trigeminal neuralgia: improved initial response with two isocenters and increasing dose. J. Neurosurg. 2005, 102, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Longhi, M.; Rizzo, P.; Nicolato, A.; Foroni, R.; Reggio, M.; Gerosa, M. Gamma knife radiosurgery for trigeminal neuralgia: results and potentially predictive parameters—Part I: Idiopathic trigeminal neuralgia. Neurosurgery 2007, 61, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Serizawa, T.; Nagano, O.; Ono, J. Comparison of the results of 2 targeting methods in Gamma Knife surgery for trigeminal neuralgia. J. Neurosurg. 2008, 109, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.A.; Gorgulho, A.A.; Bezrukiy, N.; McArthur, D.; Agazaryan, N.; Selch, M.T.; De Salles, A.A. Dedicated linear accelerator radiosurgery for trigeminal neuralgia: A single-center experience in 179 patients with varied dose prescriptions and treatment plans. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Morbidini-Gaffney, S.; Chung, C.T.; Alpert, T.E.; Newman, N.; Hahn, S.S.; Shah, H.; Mitchell, L.; Bassano, D.; Darbar, A.; Bajwa, S.A.; et al. Doses greater than 85 Gy and two isocenters in Gamma Knife surgery for trigeminal neuralgia: Updated results. J. Neurosurg. 2006, 105, 107–111. [Google Scholar] [CrossRef]
- Kotecha, R.; Kotecha, R.; Modugula, S.; Murphy, E.S.; Jones, M.; Kotecha, R.; Reddy, C.A.; Suh, J.H.; Barnett, G.H.; Neyman, G.; et al. Trigeminal Neuralgia Treated with Stereotactic Radiosurgery: The Effect of Dose Escalation on Pain Control and Treatment Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 142–148. [Google Scholar] [CrossRef]
- Massager, N.; Murata, N.; Tamura, M.; Devriendt, D.; Levivier, M.; Régis, J. Influence of nerve radiation dose in the incidence of trigeminal dysfunction after trigeminal neuralgia radiosurgery. Neurosurgery 2007, 60, 681–687. [Google Scholar] [CrossRef]
- Villavicencio, A.T.; Lim, M.; Burneikiene, S.; Romanelli, P.; Adler, J.R.; McNeely, L.; Chang, S.D.; Fariselli, L.; McIntyre, M.; Bower, R.; et al. Cyberknife radiosurgery for trigeminal neuralgia treatment: A preliminary multicenter experience. Neurosurgery 2008, 62, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Shivazad, A.; Kryscio, R.J.; St Clair, W.; Bush, H.M. Long-term outcome of high-dose γ knife surgery in treatment of trigeminal neuralgia. J. Neurosurg. 2013, 119, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Pollock, B.E.; Phuong, L.K.; Gorman, D.A.; Foote, R.L.; Stafford, S.L. Stereotactic radiosurgery for idiopathic trigeminal neuralgia. J. Neurosurg. 2002, 97, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Taich, Z.J.; Goetsch, S.J.; Monaco, E.; Carter, B.S.; Ott, K.; Alksne, J.F.; Chen, C.C. Stereotactic Radiosurgery Treatment of Trigeminal Neuralgia: Clinical Outcomes and Prognostic Factors. World Neurosurg 2016, 90, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Hwang, S.K.; Kang, D.H.; Park, J.; Hwang, J.H.; Sung, J.K. The retrogasserian zone versus dorsal root entry zone: comparison of two targeting techniques of gamma knife radiosurgery for trigeminal neuralgia. Acta Neurochir. 2010, 152, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Pintea, B.; Kinfe, T.M.; Surber, G.; Hamm, K.; Boström, J.P. LINAC stereotactic radiosurgery for trigeminal neuralgia-retrospective two-institutional examination of treatment outcomes. Radiat. Oncol. 2018, 13, 153. [Google Scholar] [CrossRef]
- Xu, Z.; Schlesinger, D.; Moldovan, K.; Przybylowski, C.; Sun, X.; Lee, C.C.; Yen, C.P.; Sheehan, J. Impact of target location on the response of trigeminal neuralgia to stereotactic radiosurgery. J. Neurosurg. 2014, 120, 716–724. [Google Scholar] [CrossRef]
- Sharim, J.; Lo, W.L.; Kim, W.; Chivukula, S.; Tenn, S.; Kaprealian, T.; Pouratian, N. Radiosurgical target distance from the root entry zone in the treatment of trigeminal neuralgia. Pract. Radiat. Oncol. 2017, 7, 221–227. [Google Scholar] [CrossRef]
- Régis, J. High–dose trigeminal neuralgia radiosurgery associated with increased risk of trigeminal nerve dysfunction. Neurosurgery 2002, 50, 1401–1402. [Google Scholar]
- Tuleasca, C.; Régis, J.; Sahgal, A.; De Salles, A.; Hayashi, M.; Ma, L.; Martínez–Álvarez, R.; Paddick, I.; Ryu, S.; Slotman, B.J.; et al. Stereotactic radiosurgery for trigeminal neuralgia: A systematic review International Stereotactic Radiosurgery Society practice guidelines. J. Neurosurg. 2019, 130, 733–757. [Google Scholar] [CrossRef]
- Flickinger, J.C.; Pollock, B.E.; Kondziolka, D.; Phuong, L.K.; Foote, R.L.; Stafford, S.L.; Lunsford, L.D. Does increased nerve length within the treatment volume improve trigeminal neuralgia radiosurgery? A prospective double-blind, randomized study. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 449–454. [Google Scholar] [CrossRef]
- Zhao, H.; Shen, Y.; Yao, D.; Xiong, N.; Abdelmaksoud, A.; Wang, H. Outcomes of Two-Isocenter Gamma Knife Radiosurgery for Patients with Typical Trigeminal Neuralgia: Pain Response and Quality of Life. World Neurosurg. 2018, 109, e531–e538. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Tyburczy, A.; Ye, J.C.; Fatterpekar, G.; Silverman, J.S.; Kondziolka, D. The relationship of dose to nerve volume in predicting pain recurrence after stereotactic radiosurgery in trigeminal neuralgia. J. Neurosurg. 2018, 128, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Debono, B.; Lotterie, J.A.; Sol, J.C.; Bousquet, P.; Duthil, P.; Monfraix, S.; Lazorthes, Y.; Sabatier, J.; Latorzeff, I. Dedicated Linear Accelerator Radiosurgery for Classic Trigeminal Neuralgia: A Single–Center Experience with Long-Term Follow-Up. World Neurosurg. 2019, 121, e775–e785. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.C.; Hamlyn, P.J.; Zakrzewska, J.M. Stereotactic radiosurgery for primary trigeminal neuralgia: state of the evidence and recommendations for future reports. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Régis, J.; Carron, R.; Park, M. Is radiosurgery a neuromodulation therapy? A 2009 Fabrikant award lecture. J. Neurooncol. 2010, 98, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Nugent, G.R. Technique and results of 800 percutaneous radiofrequency thermocoagulations for trigeminal neuralgia. Appl. Neurophysiol. 1982, 45, 504–507. [Google Scholar] [PubMed]
- Pollock, B.E. Radiosurgery for trigeminal neuralgia: Is sensory disturbance required for pain relief? J. Neurosurg. 2006, 105, 103–106. [Google Scholar] [CrossRef]
- Kondziolka, D.; Lacomis, D.; Niranjan, A.; Mori, Y.; Maesawa, S.; Fellows, W.; Lunsford, L.D. Histological effects of trigeminal nerve radiosurgery in a primate model: Implications for trigeminal neuralgia radiosurgery. Neurosurgery 2000, 46, 971–976. [Google Scholar]
- Zhao, Z.F.; Yang, L.Z.; Jiang, C.L.; Zheng, Y.R.; Zhang, J.W. Gamma Knife irradiation–induced histopathological changes in the trigeminal nerves of rhesus monkeys. J. Neurosurg. 2010, 113, 39–44. [Google Scholar] [CrossRef]
| Study | Max Dose to Nerve | Dose Related Pain Freedom | Facial Numbness Dose Related | Radiosurgery Target |
|---|---|---|---|---|
| Kondziolka et al. [5] | ≤65 Gy ≥70 Gy | Yes ≥ 70 Gy p = 0.02 | No relationship | REZ |
| Pollock et al. [8] | 70 Gy 90 Gy | No difference | Yes. Numbness and dysesthesia | REZ |
| Alpert et al. [14] | ≤80 Gy 85 Gy ≥90 Gy | Yes, with escalating doses p < 0.001 | No relationship | REZ ±second shot 3–4 mm more distal |
| Sheehan et al. [9] | 50–90 Gy | No difference | No relationship | REZ |
| Tawk et al. [10] | 70, 80, or 90 Gy | No difference | Trend to dose relationship | REZ |
| Morbidini-Gaffney et al. [18] | <80 Gy 85 Gy >85 Gy | Yes < 85 Gy p < 0.001 | REZ ±second shot 2–4 mm more distal | |
| Régis et al. [11] | 70–90 Gy | No difference | No relationship | 7.5 mm anterior to pons |
| Fountas et al. [7] | 75–85 Gy | No difference | REZ | |
| Longhi et al. [15] | 75–95 Gy >80 Gy | Yes > 80 Gy p = 0.008 | >90 Gy increased numbness | REZ |
| Chen et al. * [6] | 85 or 90 Gy | No difference | Cisternal nerve segment | |
| Matsuda et al. [16] | 80 or 90 Gy | No difference | Trend to more numbness in 90 Gy RG target | 80 Gy REZ 90 Gy RG target |
| Kim et al. [13] | 75 or 85 Gy | No difference | No relationship | REZ |
| Smith et al. * [17] | 70 or 90 Gy | Yes, at one year | Trend to dose relationship | REZ |
| Zhang et al. [12] | 75 or 90 Gy | No difference | No dose relationship | Cisternal portion of nerve with one or two isocenters |
| Kotecha et al. [19] | ≤82, 83–86, or ≥90 Gy | Improved > 82 Gy | No dose relationship prescription doses ≥ 83 Gy | REZ |
| Massager et al. [20] | 70–85 Gy, 90 Gy, or 90 Gy with shielding | No, trend to better pain freedom in higher dose | Yes. Numbness related with higher dose | Anterior cisternal nerve segment |
| Villavicencio et al. * [21] | Range of 50–80 Gy, median 75 Gy | Yes, related with longer nerve segment treated | Yes | REZ |
| Study | Radiosurgery Target and Max Dose to Nerve | Pain Freedom | Facial Numbness Target Related |
|---|---|---|---|
| Matsuda et al. [16] | 80 Gy REZ 90 Gy retrogasserian target | No difference | Trend to more numbness in the anterior target patients |
| Park et al. [25] | 80–90 Gy REZ or retrogasserian target | No difference | No relationship |
| Xu et al. [27] | 80 Gy REZ or retrogasserian target | Similar initial pain relief. REZ more durable | More facial numbness in the REZ target patients |
| Rashid et al. [26] | 90 Gy REZ or retrogasserian target | REZ target better pain control | No relationship |
| Study | Max Dose to Nerve | Pain Freedom | Facial Numbness | Radiosurgery Target |
|---|---|---|---|---|
| Flickinger et al. [31] | 75 Gy one or two isocenters | No difference | Increased numbness with two isocenters | REZ ±second shot 2–4 mm distal to brainstem |
| Pollock et al. [23] | 70–90 Gy one or two isocenters | Trend for longer length of treated nerve | No relation with nerve volume treated | Single anterior target ±second shot along nerve |
| Alpert et al. [14] | ≤80, 85, or ≥90 Gy one or two isocenters | Improved with escalating dose, two-shot treatment received higher dose | No relation with nerve volume treated | REZ ±second shot 3–4 mm more distal |
| Morbidini-Gaffney et al. [18] | <80, 85, or >85 Gy one or two isocenters | Improved in ≥85 Gy dose and number of isocenters treated | 11% mild numbness, unknown relation to number of isocenters | REZ ±second shot 2–4 mm more distal |
| Zhang et al. Neurol India, [12] | 75–90 Gy, one or two isocenters | No difference detected in dose delivered or number of isocenters | Numbness or paresthesia increased with two isocenter treatment | Cisternal portion of trigeminal nerve |
| Zhao et al. [32] | 88 Gy and two isocenters | 32% numbness 3.6% bothersome numbness | Two adjacent 4 mm shots commencing at the REZ | |
| Wolf et al. [33] | 80–90 Gy initial GKR, 65–70 Gy repeat GKR, 80 Gy shorter-nerve treatment, 85 Gy longer-nerve treatment | No relationship to nerve length or volume treated More durable in higher dose to smaller volume | Numbness in 23.6%, bothersome in 3.6% | REZ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boling, W.; Song, M.; Shih, W.; Karlsson, B. Gamma Knife Radiosurgery for Trigeminal Neuralgia: A Comparison of Dose Protocols. Brain Sci. 2019, 9, 134. https://doi.org/10.3390/brainsci9060134
Boling W, Song M, Shih W, Karlsson B. Gamma Knife Radiosurgery for Trigeminal Neuralgia: A Comparison of Dose Protocols. Brain Sciences. 2019; 9(6):134. https://doi.org/10.3390/brainsci9060134
Chicago/Turabian StyleBoling, Warren, Minwoo Song, Wendy Shih, and Bengt Karlsson. 2019. "Gamma Knife Radiosurgery for Trigeminal Neuralgia: A Comparison of Dose Protocols" Brain Sciences 9, no. 6: 134. https://doi.org/10.3390/brainsci9060134
APA StyleBoling, W., Song, M., Shih, W., & Karlsson, B. (2019). Gamma Knife Radiosurgery for Trigeminal Neuralgia: A Comparison of Dose Protocols. Brain Sciences, 9(6), 134. https://doi.org/10.3390/brainsci9060134




