The Endocannabinoid System as a Potential Mechanism through which Exercise Influences Episodic Memory Function
Abstract
1. Introduction
2. Effects of Exercise on Memory
3. The Endocannabinoid System
4. The Endocannabinoid System and Memory Function
5. Exercise and the Endocannabinoid System
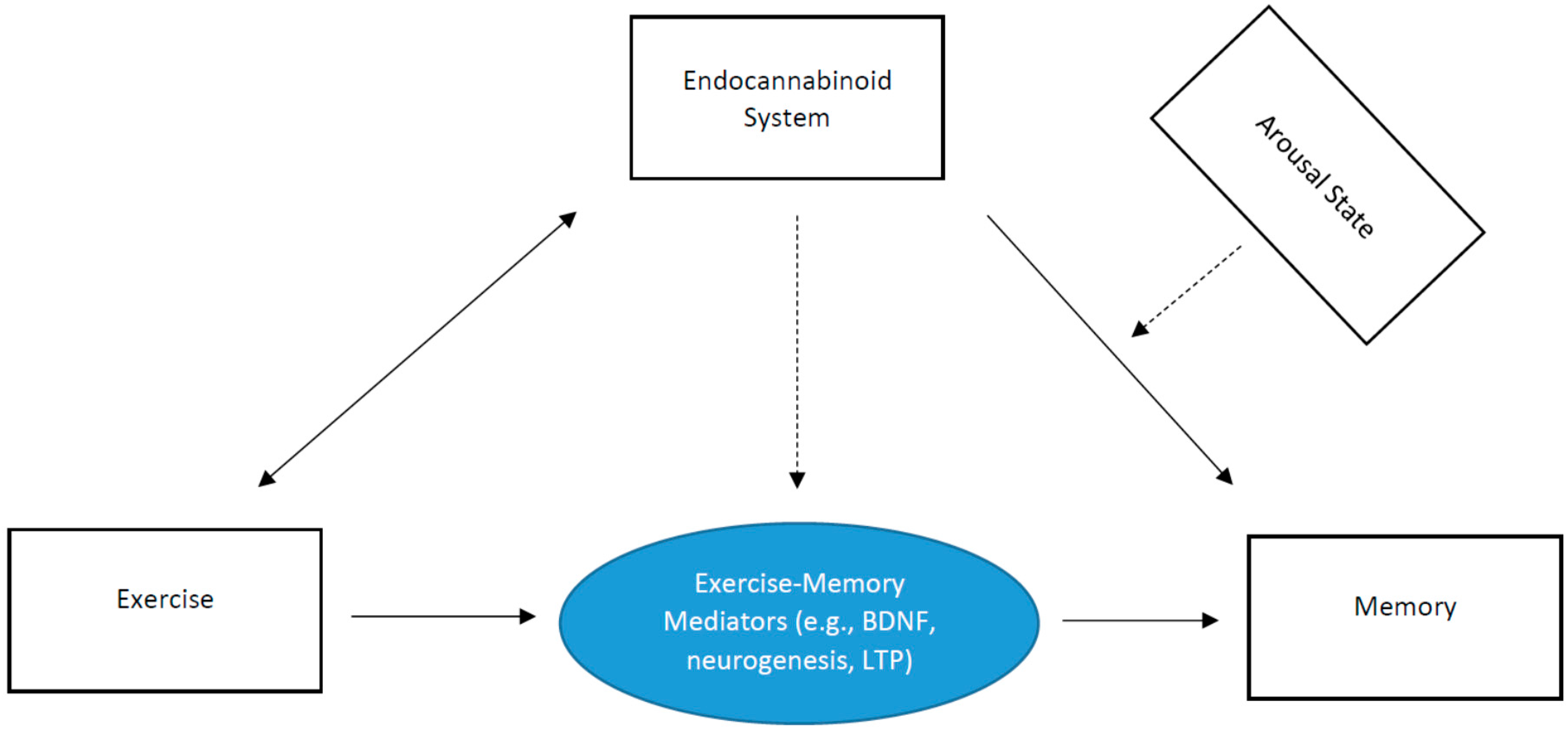
6. Hypothetical Model
7. Model Evaluation
8. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Loprinzi, P.D.; Frith, E.; Edwards, M.K.; Sng, E.; Ashpole, N. The Effects of Exercise on Memory Function Among Young to Middle-Aged Adults: Systematic Review and Recommendations for Future Research. Am. J. Health Promot. 2018, 32, 691–704. [Google Scholar] [CrossRef]
- Iv, J.T.H.; Frith, E.; Sng, E.; Loprinzi, P.D. Experimental Effects of Acute Exercise on Episodic Memory Function: Considerations for the Timing of Exercise. Psychol. Rep. 2018, 33294118786688. [Google Scholar]
- Johnson, L.; Loprinzi, P.D. The effects of acute exercise on episodic memory function among young University students: Moderation considerations by biological sex. Health Promot. Perspect. 2019. [Google Scholar]
- Loprinzi, P.D.; Blough, J.; Crawford, L.; Ryu, S.; Zou, L.; Li, H. The Temporal Effects of Acute Exercise on Episodic Memory Function: Systematic Review with Meta-Analysis. Brain Sci. 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K. Resistance exercise and episodic memory function: a systematic review. Clin. Physiol. Funct. Imaging 2018, 38, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Ponce, P.; Loprinzi, P.D. A bi-directional model of exercise and episodic memory function. Med. Hypotheses 2018, 117, 3–6. [Google Scholar] [CrossRef]
- Siddiqui, A.; Loprinzi, P.D. Experimental Investigation of the Time Course Effects of Acute Exercise on False Episodic Memory. J. Clin. Med. 2018, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Sng, E.; Frith, E.; Loprinzi, P.D. Experimental effects of acute exercise on episodic memory acquisition: Decomposition of multi-trial gains and losses. Physiol. Behav. 2018, 186, 82–84. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Scott, T.M.; Ikuta, T.; Addoh, O.; Tucker, K.L. Association of physical activity on changes in cognitive function: Boston Puerto Rican Health Study. Physician Sportsmed. 2018, 47, 227–231. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Edwards, M.K.; Frith, E. Potential avenues for exercise to activate episodic memory-related pathways: a narrative review. Eur. J. Neurosci. 2017, 46, 2067–2077. [Google Scholar] [CrossRef]
- Frith, E.; Loprinzi, P.D. Physical activity and individual cognitive funcion parameters: unique exercise-induced mechansims. J. Cognit. Behav. Psychother. Res. 2018, 7, 92–106. [Google Scholar] [CrossRef]
- El-Sayes, J.; Harasym, D.; Turco, C.V.; Locke, M.B.; Nelson, A.J. Exercise-Induced Neuroplasticity: A Mechanistic Model and Prospects for Promoting Plasticity. Neuroscientist 2019, 25, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P. The role of astrocytes on the effects of exercise on episodic memory function. Physiol. Int. 2019, 106, 21–28. [Google Scholar] [CrossRef]
- Loprinzi, P.D. IGF-1 in exercise-induced enhancement of episodic memory. Acta Physiol. 2018, 226, e13154. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin. Physiol. Funct. Imaging 2019, 39, 9–14. [Google Scholar] [CrossRef]
- Loprinzi, P.D. Does brain-derived neurotrophic factor mediate the effects of exercise on memory? Physician Sportsmed. 2019, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.; Ponce, P.; Frith, E. Hypothesized mechanisms through which acute exercise influences episodic memory. Physiol. Int. 2018, 105, 285–297. [Google Scholar] [CrossRef]
- Poo, M.-M.; Pignatelli, M.; Ryan, T.J.; Tonegawa, S.; Bonhoeffer, T.; Martin, K.C.; Rudenko, A.; Tsai, L.-H.; Tsien, R.W.; Fishell, G.; et al. What is memory? The present state of the engram. BMC Boil. 2016, 14, 1133. [Google Scholar] [CrossRef]
- Nakai, T.; Nagai, T.; Tanaka, M.; Itoh, N.; Asai, N.; Enomoto, A.; Asai, M.; Yamada, S.; Saifullah, A.B.; Sokabe, M.; et al. Girdin Phosphorylation Is Crucial for Synaptic Plasticity and Memory: A Potential Role in the Interaction of BDNF/TrkB/Akt Signaling with NMDA Receptor. J. Neurosci. 2014, 34, 14995–15008. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Kruk-Slomka, M.; Dzik, A.; Budzynska, B.; Biala, G. Endocannabinoid System: The Direct and Indirect Involvement in the Memory and Learning Processes-a Short Review. Mol. Neurobiol. 2017, 54, 8332–8347. [Google Scholar] [CrossRef] [PubMed]
- Hampson, R.E.; Deadwyler, S.A. Role of Cannabinoid Receptors in Memory Storage. Neurobiol. Dis. 1998, 5, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Riedel, G.; Davies, S.N. Cannabinoid Function in Learning, Memory and Plasticity. Cannabinoids 2005, 168, 445–477. [Google Scholar]
- Miller, L.L.; Branconnier, R.J. Cannabis: Effects on memory and the cholinergic limbic system. Psychol. Bull. 1983, 93, 441–456. [Google Scholar] [CrossRef]
- Campbell, K.A.; Foster, T.C.; Hampson, R.E.; Deadwyler, S.A. Effects of delta 9-tetrahydrocannabinol on sensory-evoked discharges of granule cells in the dentate gyrus of behaving rats. J. Pharmacol. Exp. Ther. 1986, 239, 941–945. [Google Scholar] [PubMed]
- Campbell, K.A.; Foster, T.C.; Hampson, R.E.; Deadwyler, S.A. delta 9-Tetrahydrocannabinol differentially affects sensory-evoked potentials in the rat dentate gyrus. J. Pharmacol. Exp. Ther. 1986, 239, 936–940. [Google Scholar] [PubMed]
- Deadwyler, S.A.; Hampson, R.E.; Childers, S.R. Functional significance of cannabinoid receptors in brain. In Cannabinoid Receptors; Pertwee, R.G., Ed.; Academic Press: London, UK, 1995; pp. 205–231. [Google Scholar]
- Wise, L.E.; Long, K.A.; Abdullah, R.A.; Long, J.Z.; Cravatt, B.F.; Lichtman, A.H. Dual Fatty Acid Amide Hydrolase and Monoacylglycerol Lipase Blockade Produces THC-Like Morris Water Maze Deficits in Mice. ACS Chem. Neurosci. 2012, 3, 369–378. [Google Scholar] [CrossRef]
- Abush, H.; Akirav, I. Cannabinoids modulate hippocampal memory and plasticity. Hippocampus 2010, 20, 1126–1138. [Google Scholar] [CrossRef]
- Takahashi, R.N.; Pamplona, F.A.; Fernandes, M.S. The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci. Lett. 2005, 380, 270–275. [Google Scholar] [CrossRef]
- Kruk-Slomka, M.; Biala, G. CB1 receptors in the formation of the different phases of memory-related processes in the inhibitory avoidance test in mice. Behav. Brain 2016, 301, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Johnson, M.R.; Melvin, L.S.; Milne, G.M. Nonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor model. Mol. Pharmacol. 1988, 33, 297–302. [Google Scholar] [PubMed]
- Twitchell, W.; Brown, S.; Mackie, K. Cannabinoids Inhibit N- and P/Q-Type Calcium Channels in Cultured Rat Hippocampal Neurons. J. Neurophysiol. 1997, 78, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Piser, T.M.; Seybold, V.S.; Thayer, S.A. Cannabinoid Receptor Agonists Inhibit Glutamatergic Synaptic Transmission in Rat Hippocampal Cultures. J. Neurosci. 1996, 16, 4322–4334. [Google Scholar] [CrossRef]
- Stella, N.; Schweitzer, P.; Piomelli, D. A second endogenous cannabinoid that modulates long-term potentiation. Nat. Cell Boil. 1997, 388, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Nowicky, A.V.; Teyler, T.J.; Vardaris, R.M. The modulation of long-term potentiation by delta-9-tetrahydrocannabinol in the rat hippocampus, in vitro. Brain Res. Bull. 1987, 19, 663–672. [Google Scholar] [CrossRef]
- Ruehle, S.; Rey, A.A.; Remmers, F.; Lutz, B. The endocannabinoid system in anxiety, fear memory and habituation. J. Psychopharmacol 2012, 26, 23–39. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Bains, J.; Marsicano, G. CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacolo. 2018, 43, 4–20. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A.; Albayram, O.; Draffehn, A.; Michel, K.; Piyanova, A.; Oppenheimer, H.; Dvir-Ginzberg, M.; Rácz, I.; Ulas, T.; Imbeault, S.; et al. A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat. Med. 2017, 23, 782–787. [Google Scholar] [CrossRef]
- Caballero-Florán, R.N.; Conde-Rojas, I.; Chávez, A.O.; Cortes-Calleja, H.; Lopez-Santiago, L.F.; Isom, L.L.; Aceves, J.; Erlij, D.; Florán, B. Cannabinoid-induced depression of synaptic transmission is switched to stimulation when dopaminergic tone is increased in the globus pallidus of the rodent. Neuropharmacology 2016, 110, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Ratano, P.; Palmery, M.; Trezza, V.; Campolongo, P. Cannabinoid Modulation of Memory Consolidation in Rats: Beyond the Role of Cannabinoid Receptor Subtype 1. Front. Pharmacol. 2017, 8, 45. [Google Scholar] [CrossRef]
- Kim, J.; Li, Y. Chronic activation of CB2 cannabinoid receptors in the hippocampus increases excitatory synaptic transmission. J. Physiol. 2015, 593, 871–886. [Google Scholar] [CrossRef]
- Ratano, P.; Petrella, C.; Forti, F.; Passeri, P.P.; Morena, M.; Palmery, M.; Trezza, V.; Severini, C.; Campolongo, P. Pharmacological inhibition of 2-arachidonoilglycerol hydrolysis enhances memory consolidation in rats through CB2 receptor activation and mTOR signaling modulation. Neuropharmacology 2018, 138, 210–218. [Google Scholar] [CrossRef]
- Çakır, M.; Tekin, S.; Doğanyiğit, Z.; Erden, Y.; Soytürk, M.; Çiğremiş, Y.; Sandal, S. Cannabinoid type 2 receptor agonist JWH-133, attenuates Okadaic acid induced spatial memory impairment and neurodegeneration in rats. Life Sci. 2019, 217, 25–33. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J. CB2 Cannabinoid Receptor Knockout in Mice Impairs Contextual Long-Term Memory and Enhances Spatial Working Memory. Neural Plast. 2016, 2016, 9817089. [Google Scholar] [CrossRef]
- Ahn, K.; Johnson, D.S.; Cravatt, B.F. Fatty acid amide hydrolase as a potential therapeutic target for the treatment of pain and CNS disorders. Expert Opin. Drug Discov. 2009, 4, 763–784. [Google Scholar] [CrossRef]
- Rivera, P.; Fernández-Arjona, M.D.M.; Silva-Peña, D.; Blanco, E.; Vargas, A.; López-Ávalos, M.D.; Grondona, J.M.; Serrano, A.; Pavón, F.J.; De Fonseca, F.R.; et al. Pharmacological blockade of fatty acid amide hydrolase (FAAH) by URB597 improves memory and changes the phenotype of hippocampal microglia despite ethanol exposure. Biochem. Pharmacol. 2018, 157, 244–257. [Google Scholar] [CrossRef]
- Morena, M.; Roozendaal, B.; Trezza, V.; Ratano, P.; Peloso, A.; Hauer, D.; Atsak, P.; Trabace, L.; Cuomo, V.; McGaugh, J.L.; et al. Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proc. Acad. Sci. 2014, 111, 18333–18338. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Cowley, T.R.; Blau, C.W.; Dempsey, C.N.; Noonan, J.; Gowran, A.; Tanveer, R.; Olango, W.M.; Finn, D.P.; Campbell, V.; et al. The fatty acid amide hydrolase inhibitor URB597 exerts anti-inflammatory effects in hippocampus of aged rats and restores an age-related deficit in long-term potentiation. J. Neuroinflammation 2012, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K. Exercise and Emotional Memory: a Systematic Review. J. Cogn. Enhanc. 2018, 3, 94–103. [Google Scholar] [CrossRef]
- Wade, B.; Loprinzi, P.D. The Experimental Effects of Acute Exercise on Long-Term Emotional Memory. J. Clin. Med. 2018, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Thompson, Z.; Argueta, D.; Garland, T., Jr.; DiPatrizio, N. Circulating levels of endocannabinoids respond acutely to voluntary exercise, are altered in mice selectively bred for high voluntary wheel running, and differ between the sexes. Physiol. Behav. 2017, 170, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541. [Google Scholar] [CrossRef] [PubMed]
- De Chiara, V.; Errico, F.; Musella, A.; Rossi, S.; Mataluni, G.; Sacchetti, L.; Siracusano, A.; Castelli, M.; Cavasinni, F.; Bernardi, G. Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology 2010, 35, 374–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise activates the endocannabinoid system. NeuroReport 2003, 14, 2209–2211. [Google Scholar] [CrossRef]
- Hill, M.N.; McLaughlin, R.J.; Bingham, B.; Shrestha, L.; Lee, T.T.Y.; Gray, J.M.; Hillard, C.J.; Gorzalka, B.B.; Viau, V. Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Acad. Sci. 2010, 107, 9406–9411. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Foster, A.D.; Seillier, A.; Giuffrida, A.; Gerdeman, G.L. Exercise-induced endocannabinoid signaling is modulated by intensity. Eur. J. Appl. Physiol. 2013, 113, 869–875. [Google Scholar] [CrossRef]
- Fuss, J.; Steinle, J.; Bindila, L.; Auer, M.K.; Kirchherr, H.; Lutz, B.; Gass, P. A runner’s high depends on cannabinoid receptors in mice. Proc. Acad. Sci. 2015, 112, 13105–13108. [Google Scholar] [CrossRef] [PubMed]
- Dubreucq, S.; Durand, A.; Matias, I.; Benard, G.; Richard, E.; Soria-Gómez, E.; Glangetas, C.; Groc, L.; Wadleigh, A.; Massa, F.; et al. Ventral Tegmental Area Cannabinoid Type-1 Receptors Control Voluntary Exercise Performance. Boil. Psychiatry 2013, 73, 895–903. [Google Scholar] [CrossRef]
- Chaouloff, F.; Dubreucq, S.; Bellocchio, L.; Marsicano, G. Endocannabinoids and Motor Behavior: CB1 Receptors Also Control Running Activity. Physiology 2011, 26, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Fuss, J.; Gass, P. Endocannabinoids and voluntary activity in mice: Runner’s high and long-term consequences in emotional behaviors. Exp. Neurol. 2010, 224, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.A.; Bick-Sander, A.; Fabel, K.; Leal-Galicia, P.; Tauber, S.; Ramírez-Rodríguez, G.; Müller, A.; Melnik, A.; Waltinger, T.P.; Ullrich, O.; et al. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun. Signal. 2010, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Titterness, A.K.; Morrish, A.C.; Carrier, E.; Lee, T.; Gil-Mohapel, J.; Gorzalka, B.; Hillard, C.; Christie, B. Endogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampus. Hippocampus 2010, 20, 513–523. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Bastos, C.P.; Pereira, G.S.; Moreira, F.A.; Massensini, A.R. A role for the endocannabinoid system in exercise-induced spatial memory enhancement in mice. Hippocampus 2014, 24, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.M.; Bringard, A.; Logrieco, M.G.; Lauer, E.; Imobersteg, N.; Ferretti, G.; Thomas, A.; Schwartz, S.; Igloi, K. Acute physical exercise of moderate intensity improves memory consolidation in humans via BDNF and endocannabinoid signaling. bioRxiv 2019. [Google Scholar] [CrossRef]
- Smallwood, N.; Spriggs, M.J.; Thompson, C.S.; Wu, C.C.; Hamm, J.P.; Moreau, D.; Kirk, I.J. Influence of Physical Activity on Human Sensory Long-Term Potentiation. J. Int. Neuropsychol. Soc. 2015, 21, 831–840. [Google Scholar] [CrossRef]
- Sierra, A.; Encinas, J.M.; Maletic-Savatic, M. Adult Human Neurogenesis: From Microscopy to Magnetic Resonance Imaging. Front. Neurosci. 2011, 5, 47. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loprinzi, P.D.; Zou, L.; Li, H. The Endocannabinoid System as a Potential Mechanism through which Exercise Influences Episodic Memory Function. Brain Sci. 2019, 9, 112. https://doi.org/10.3390/brainsci9050112
Loprinzi PD, Zou L, Li H. The Endocannabinoid System as a Potential Mechanism through which Exercise Influences Episodic Memory Function. Brain Sciences. 2019; 9(5):112. https://doi.org/10.3390/brainsci9050112
Chicago/Turabian StyleLoprinzi, Paul D., Liye Zou, and Hong Li. 2019. "The Endocannabinoid System as a Potential Mechanism through which Exercise Influences Episodic Memory Function" Brain Sciences 9, no. 5: 112. https://doi.org/10.3390/brainsci9050112
APA StyleLoprinzi, P. D., Zou, L., & Li, H. (2019). The Endocannabinoid System as a Potential Mechanism through which Exercise Influences Episodic Memory Function. Brain Sciences, 9(5), 112. https://doi.org/10.3390/brainsci9050112




