Author Contributions
Conceptualization, M.Z.B. and M.K.; methodology, M.Z.B. and M.K.; software, M.Z.B.; validation, M.Z.B. and M.K.; formal analysis, M.Z.B. and M.K.; investigation, M.Z.B.; resources, M.Z.B. and M.K.; writing–original draft preparation, M.Z.B.; writing–review and editing, M.Z.B. and M.K.; visualization, M.Z.B.; supervision, M.K.; project administration, M.K.; funding acquisition, M.Z.B. and M.K.
Figure 1.
A subject is using AutoCAD and wearing EEG headset in real experimental setting.
Figure 1.
A subject is using AutoCAD and wearing EEG headset in real experimental setting.
Figure 2.
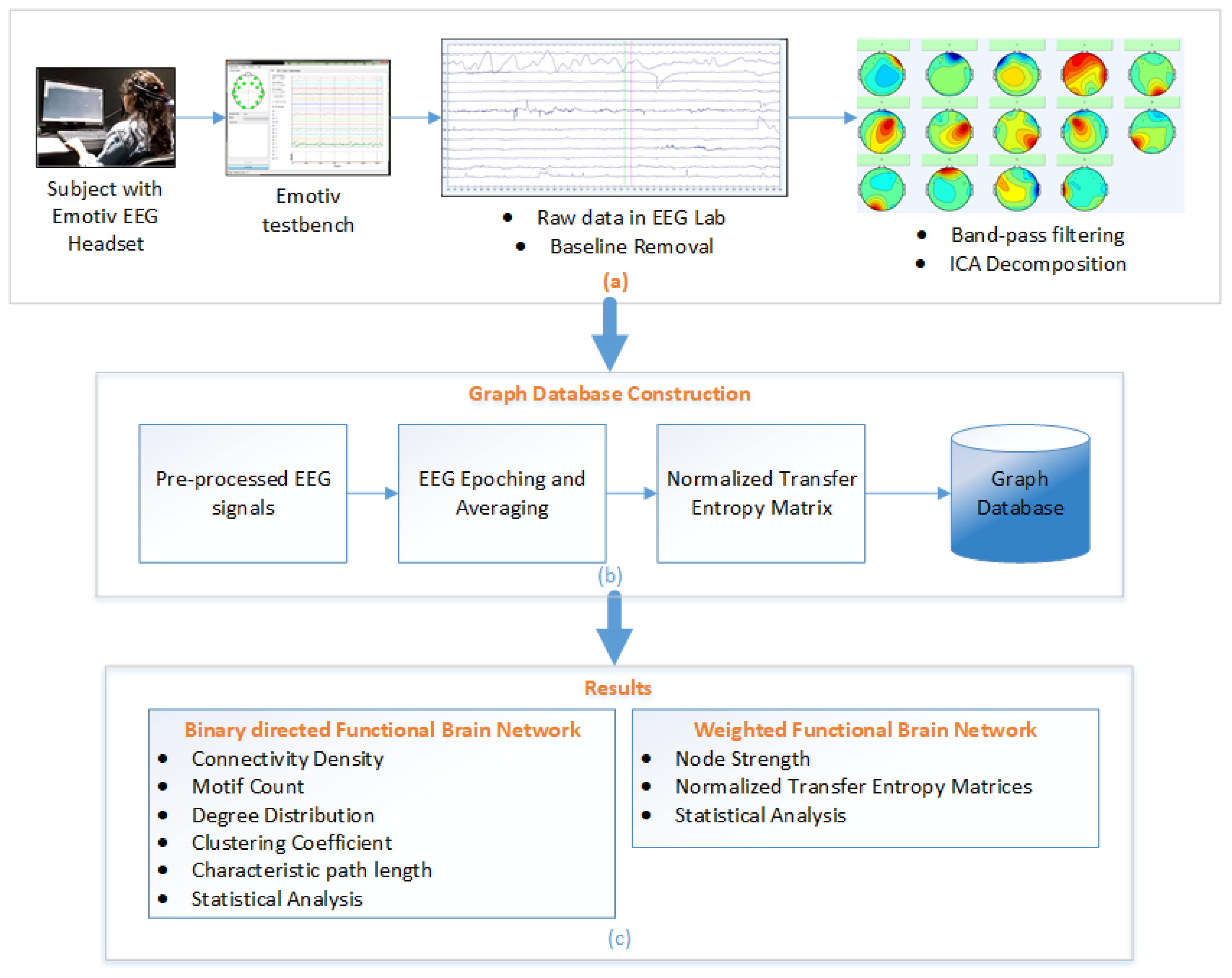
Normalized Transfer Entropy Framework (a) EEG data acquisition and pre-processing, (b) Transfer Entropy calculation and graph database construction, and (c) Result and analysis of binary and weighted FBNs.
Figure 2.
Normalized Transfer Entropy Framework (a) EEG data acquisition and pre-processing, (b) Transfer Entropy calculation and graph database construction, and (c) Result and analysis of binary and weighted FBNs.
Figure 3.
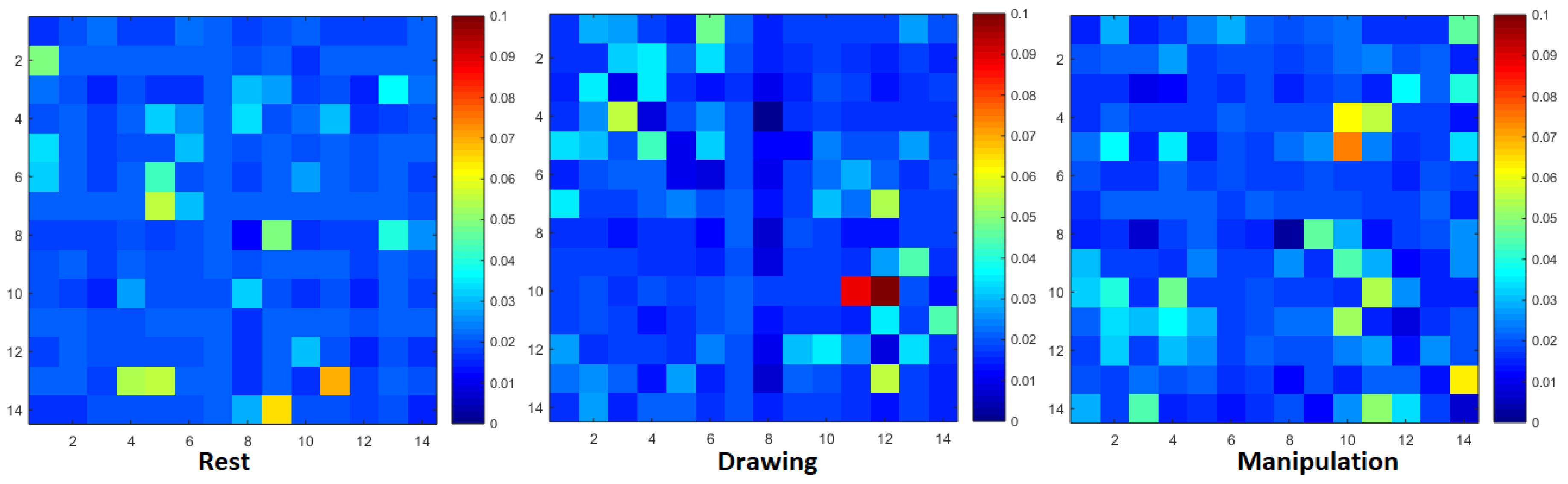
Normalized transfer entropy matrix during rest, drawing and manipulation states of novice user.
Figure 3.
Normalized transfer entropy matrix during rest, drawing and manipulation states of novice user.
Figure 4.
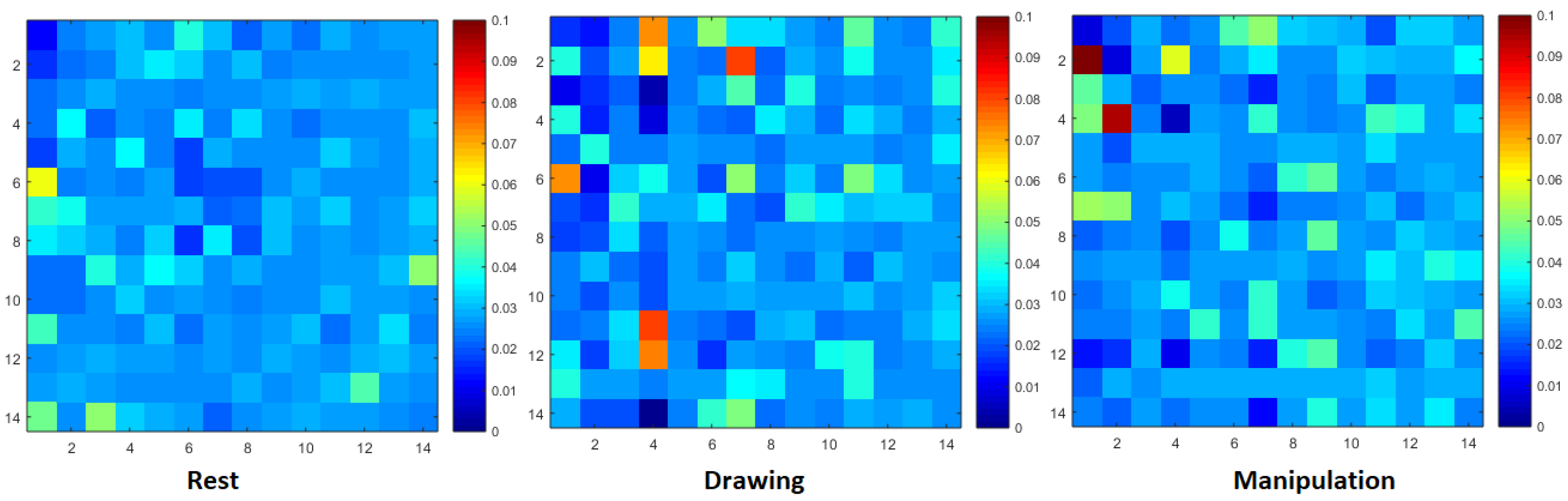
Normalized transfer entropy matrix during rest, drawing and manipulation states of expert user.
Figure 4.
Normalized transfer entropy matrix during rest, drawing and manipulation states of expert user.
Figure 5.
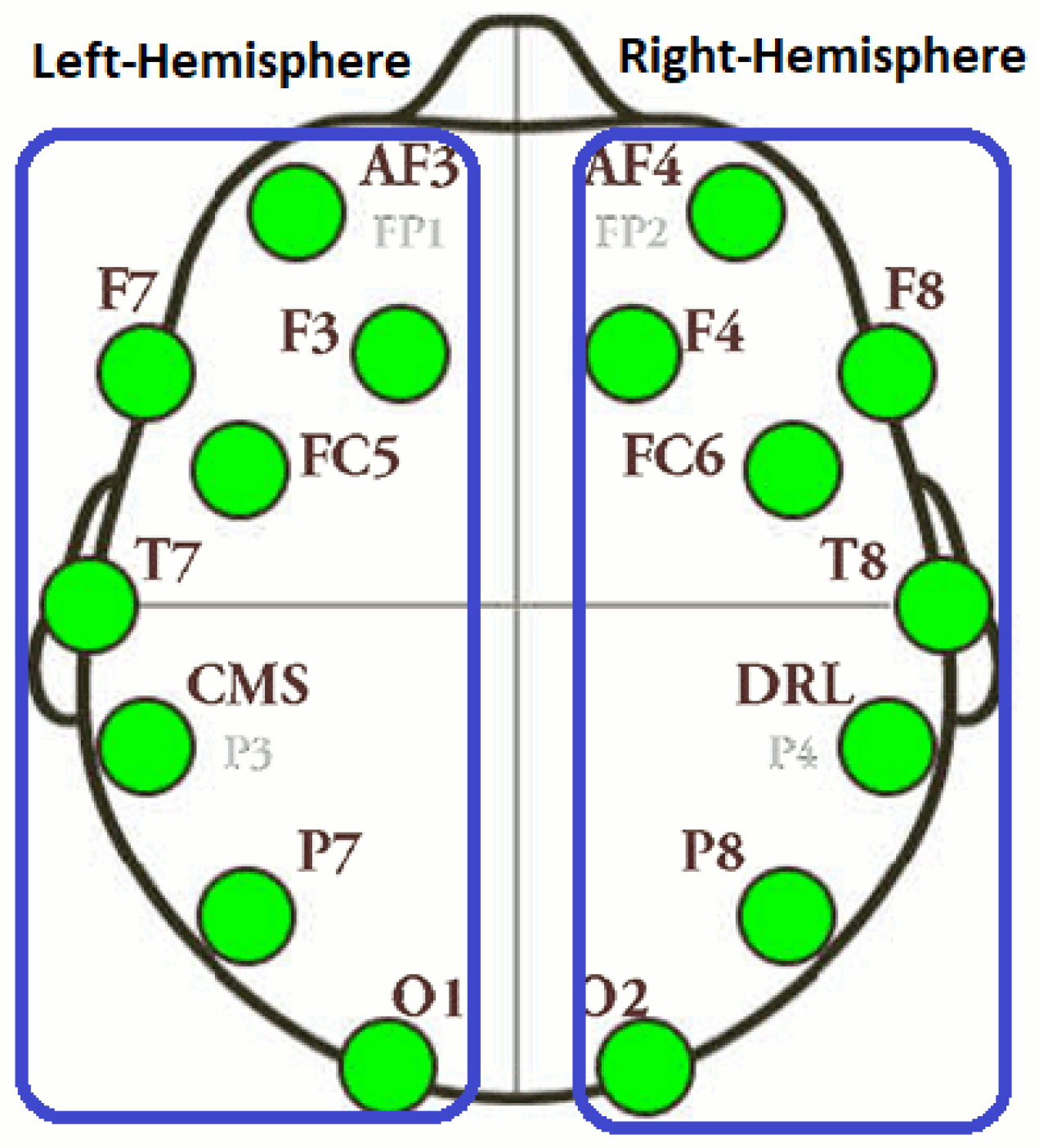
Electrodes layout of LH and RH.
Figure 5.
Electrodes layout of LH and RH.
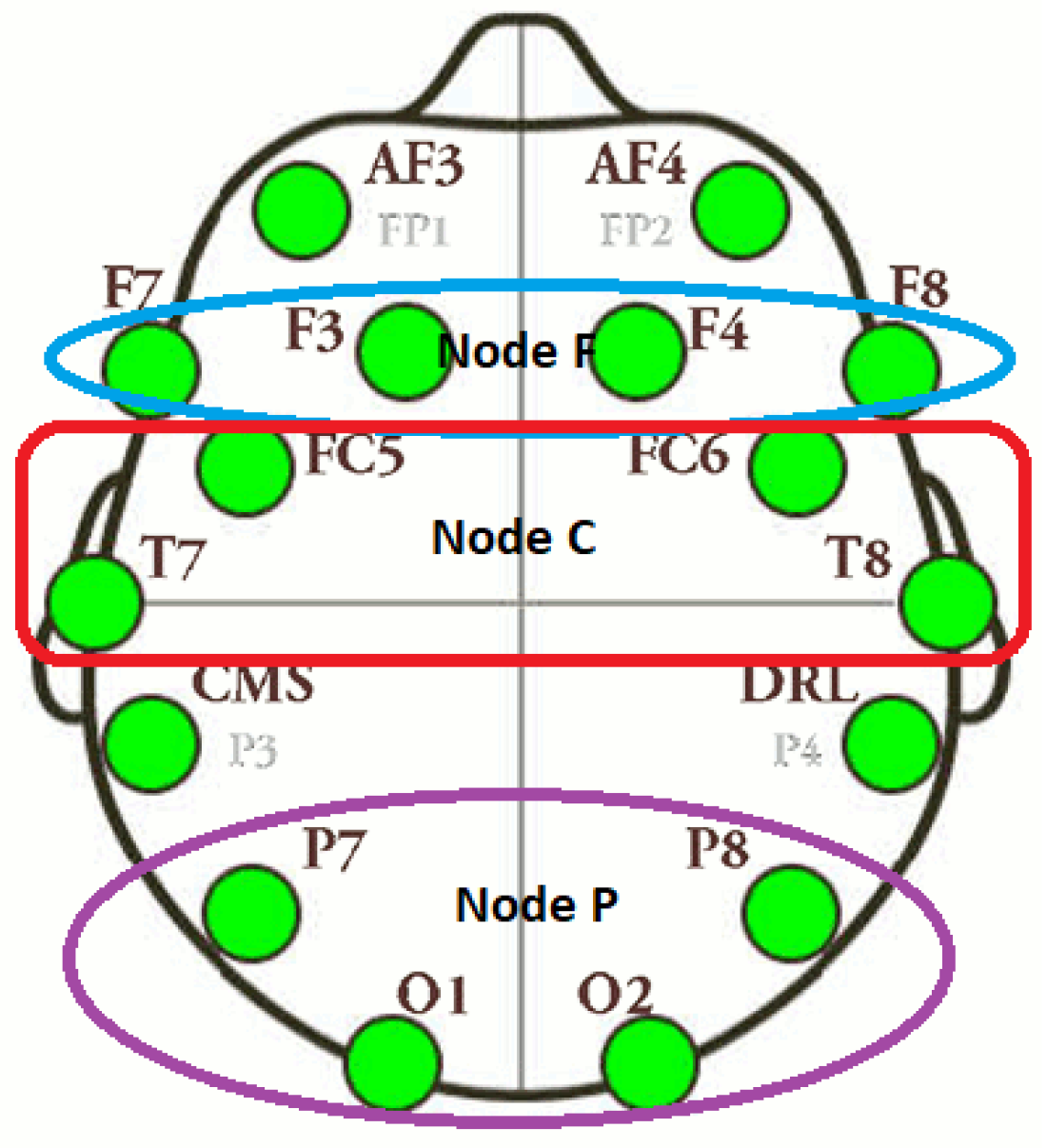
Figure 6.
Electrodes layout of F, C and P nodes.
Figure 6.
Electrodes layout of F, C and P nodes.
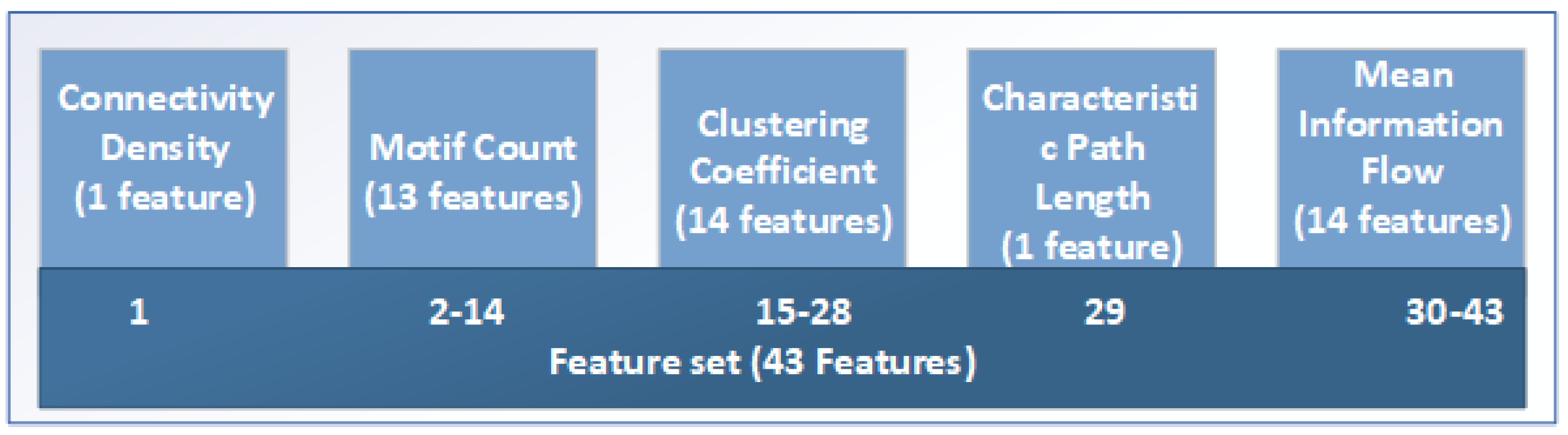
Figure 7.
The complete feature set.
Figure 7.
The complete feature set.
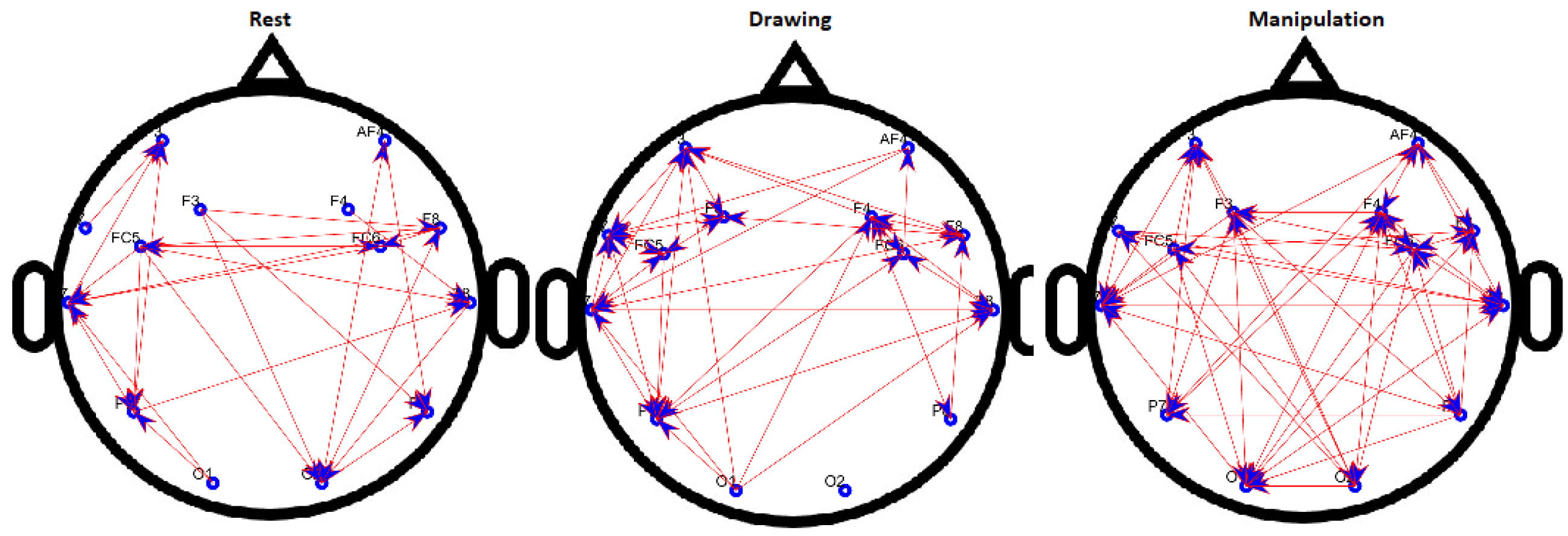
Figure 8.
Binary directed functional brain network during rest, drawing and manipulation states of novice user.
Figure 8.
Binary directed functional brain network during rest, drawing and manipulation states of novice user.
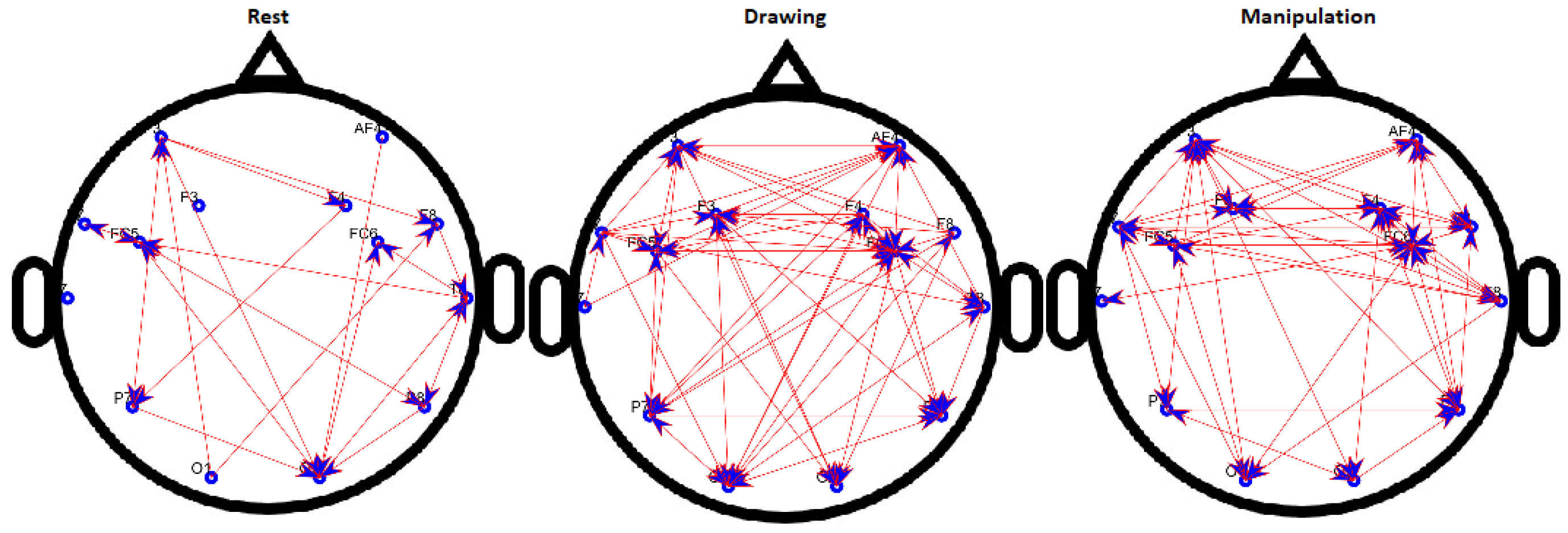
Figure 9.
Binary directed functional brain network during rest, drawing and manipulation states of expert user.
Figure 9.
Binary directed functional brain network during rest, drawing and manipulation states of expert user.
Figure 10.
Comparison of connectivity density of all users brain activity during rest, drawing and manipulation states.
Figure 10.
Comparison of connectivity density of all users brain activity during rest, drawing and manipulation states.
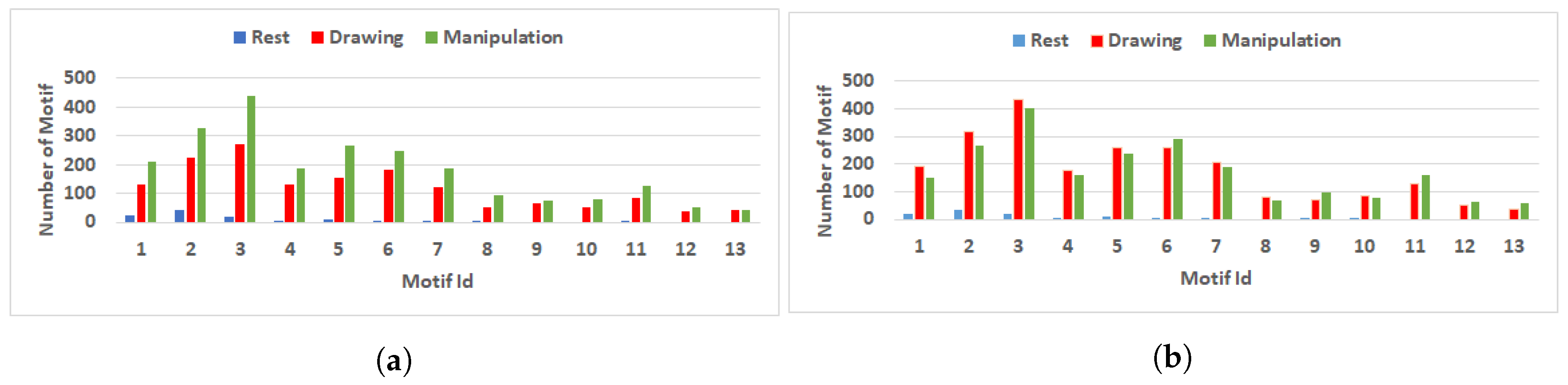
Figure 11.
Comparison of number of motif with three nodes during rest, drawing and manipulation states. (a) User 3 (Novice user). (b) User 8 (Expert user).
Figure 11.
Comparison of number of motif with three nodes during rest, drawing and manipulation states. (a) User 3 (Novice user). (b) User 8 (Expert user).
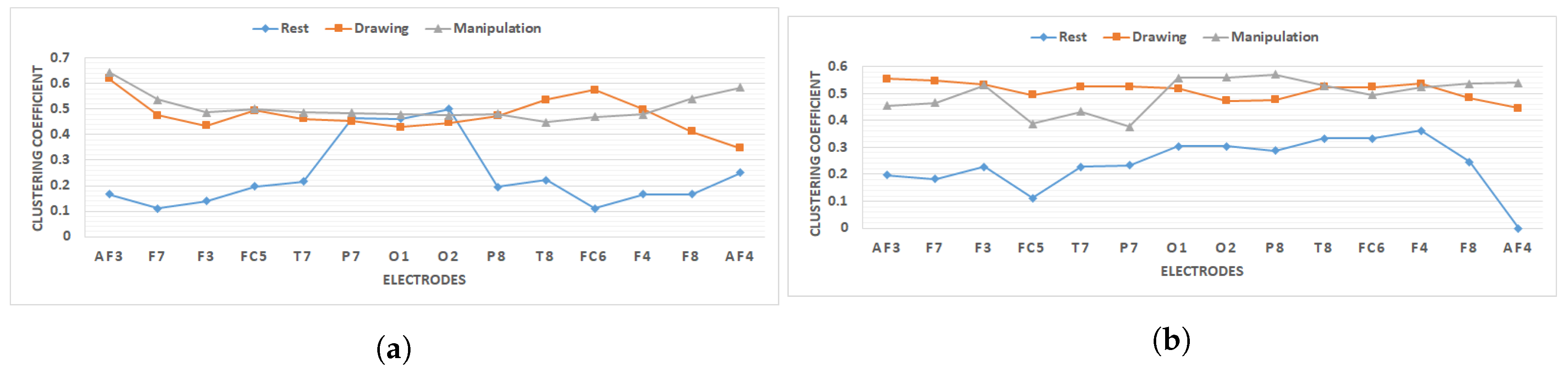
Figure 12.
Clustering coefficient of all electrodes for novice and expert users during rest, drawing and manipulation states. (a) User 3 (A representative novice user). (b) User 8 (A representative expert user).
Figure 12.
Clustering coefficient of all electrodes for novice and expert users during rest, drawing and manipulation states. (a) User 3 (A representative novice user). (b) User 8 (A representative expert user).
Figure 13.
Comparison of node strength across electrodes during rest, drawing and manipulation states. (a) User 3 (Novice). (b) User 8 (Expert).
Figure 13.
Comparison of node strength across electrodes during rest, drawing and manipulation states. (a) User 3 (Novice). (b) User 8 (Expert).
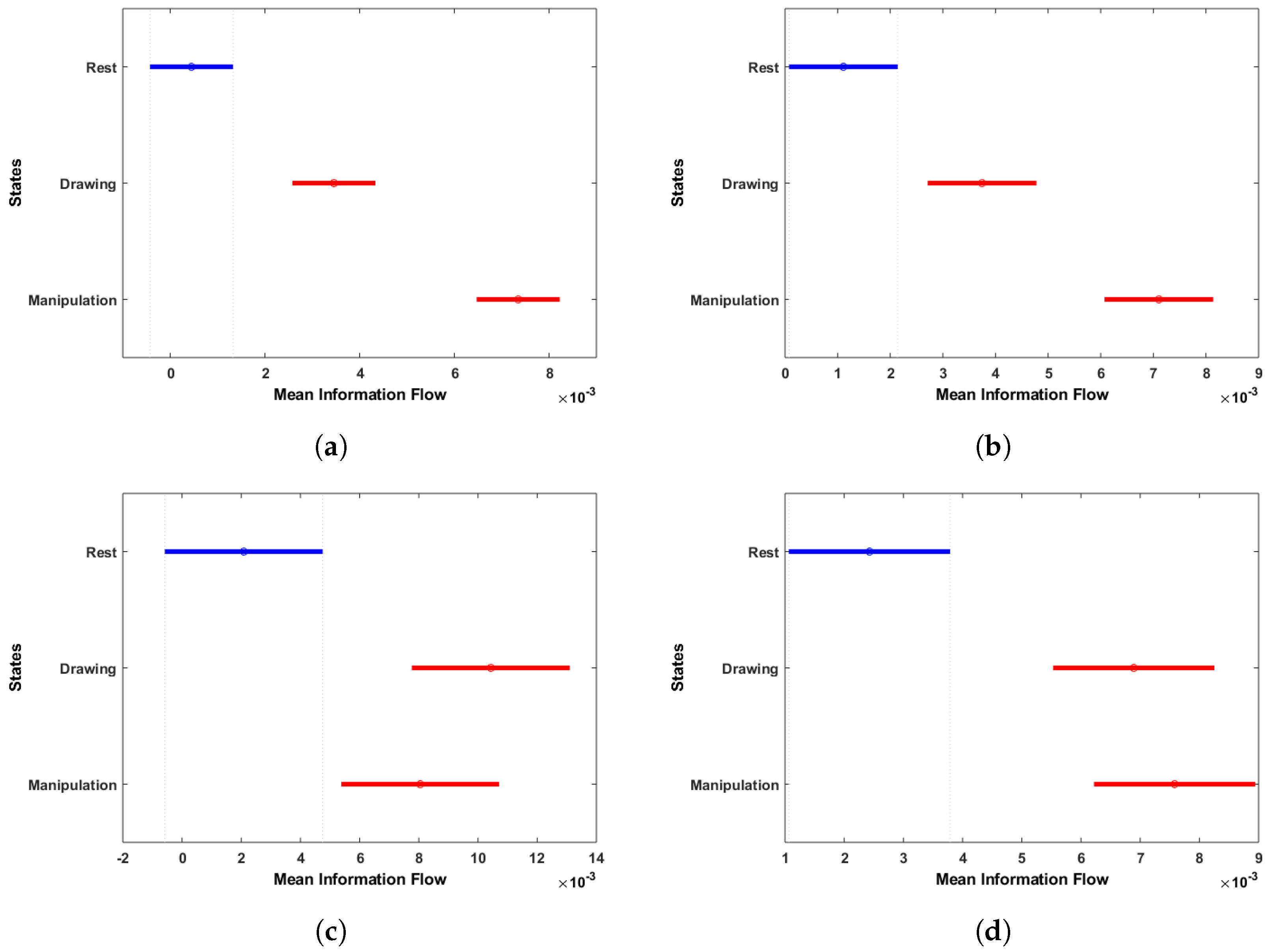
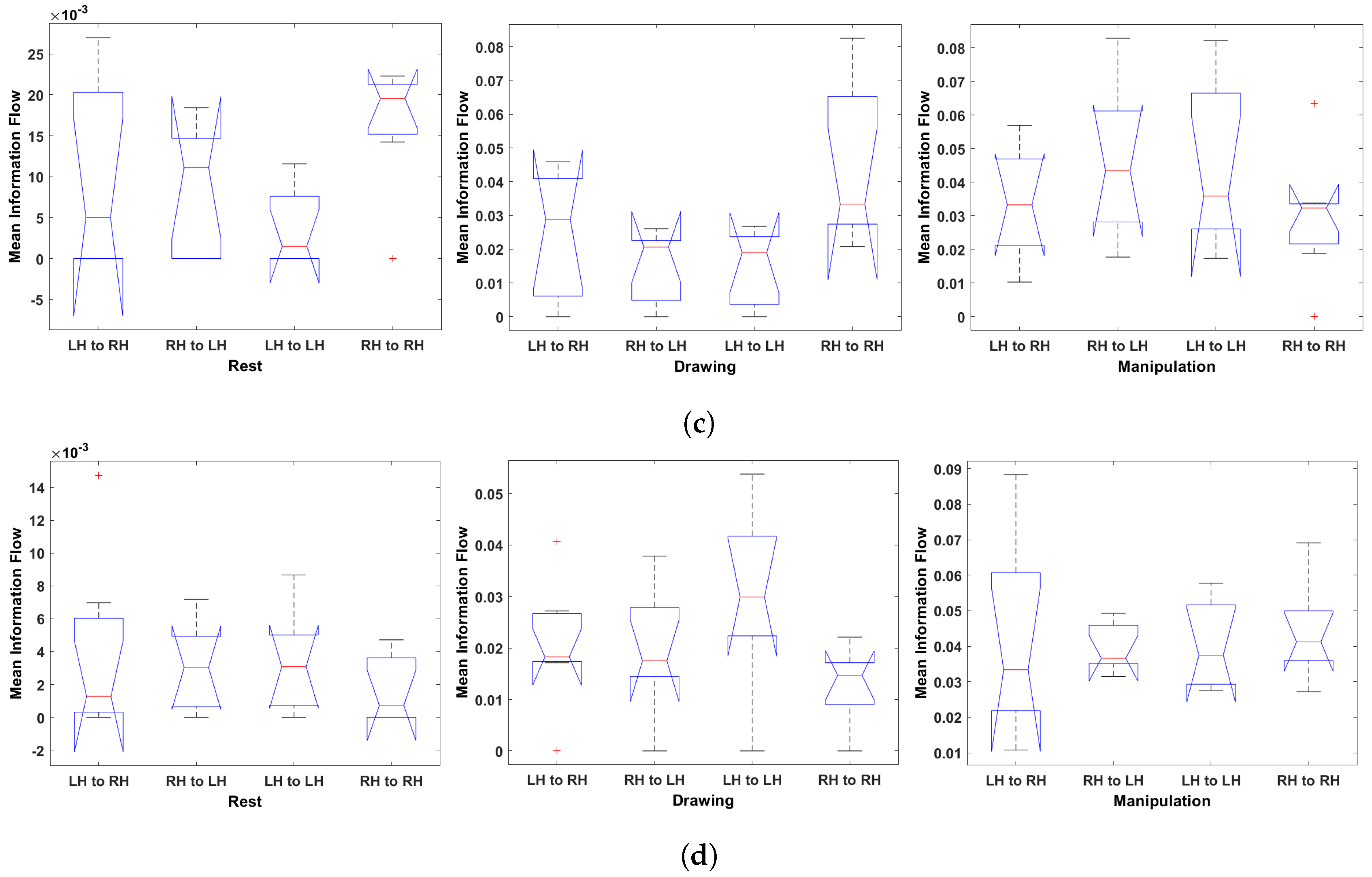
Figure 14.
Multi-comparison of ANOVA on mean information flow of each electrode to all other electrodes during rest, drawing and manipulation states. (a) User 3 (Novice). (b) User 6 (Novice). (c) User 7 (Expert). (d) User 8 (Expert).
Figure 14.
Multi-comparison of ANOVA on mean information flow of each electrode to all other electrodes during rest, drawing and manipulation states. (a) User 3 (Novice). (b) User 6 (Novice). (c) User 7 (Expert). (d) User 8 (Expert).
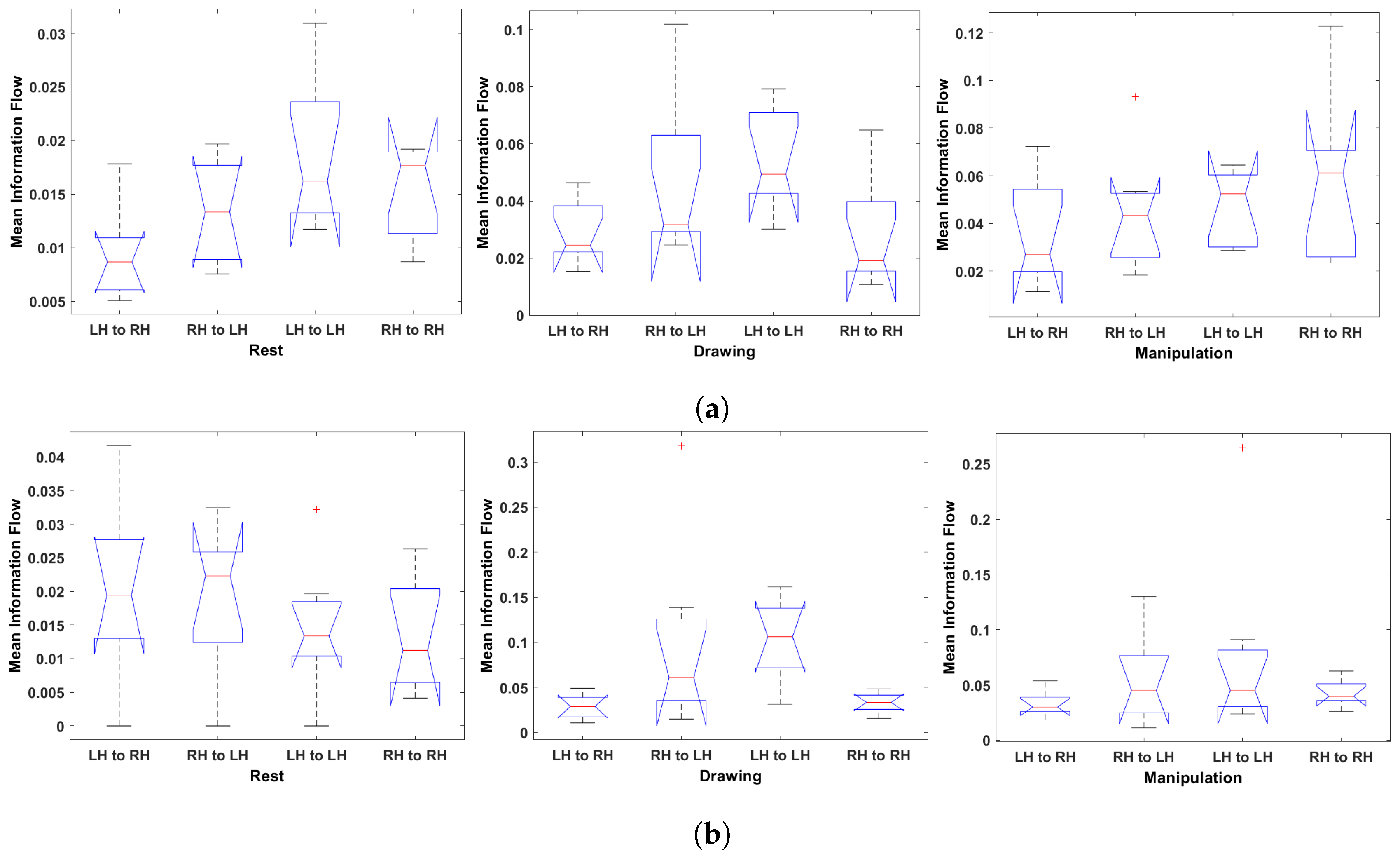
Figure 15.
One-way ANOVA of Hemisphere-wise mean information flow during rest, drawing and manipulation states. (a) User 7 (Expert). (b) User 8 (Expert). (c) User 3 (Novice). (d) User 6 (Novice).
Figure 15.
One-way ANOVA of Hemisphere-wise mean information flow during rest, drawing and manipulation states. (a) User 7 (Expert). (b) User 8 (Expert). (c) User 3 (Novice). (d) User 6 (Novice).
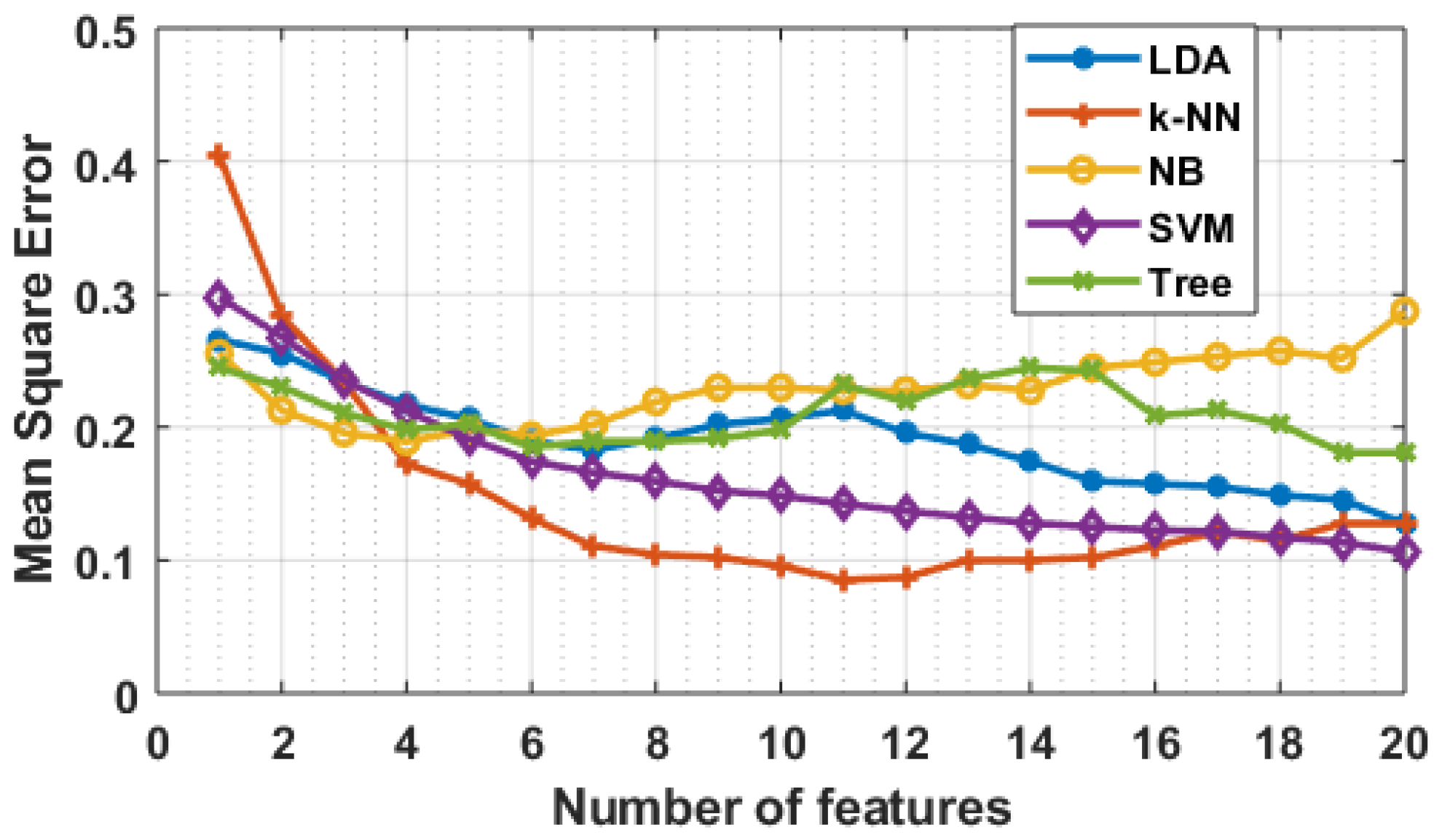
Figure 16.
Mean square error with respect to features selected.
Figure 16.
Mean square error with respect to features selected.
Table 1.
Time taken by each participant to complete the task.
Table 1.
Time taken by each participant to complete the task.
| Expertise | Users | Drawing (s) | Manipulation (s) |
|---|
| Expert | User 1 | 38 | 72 |
| Novice | User 2 | 81 | 125 |
| Novice | User 3 | 137 | 93 |
| Novice | User 4 | 54 | 72 |
| Novice | User 5 | 43 | 118 |
| Novice | User 6 | 61 | 98 |
| Expert | User 7 | 120 | 101 |
| Expert | User 8 | 58 | 113 |
Table 2.
Statistical validation of clustering coefficients for typical novice and expert users.
Table 2.
Statistical validation of clustering coefficients for typical novice and expert users.
| Users | States | Mean Difference | 95% CI | DF | t | p |
|---|
| User 3 (Novice) | Drawing | Rest | 0.156 * | (0.0763, 0.2357) | 20 | 4.0838 | 0.0006 |
| Manipulation | Drawing | 0.031 | (−0.0169, 0.0789) | 24 | 1.3362 | 0.097 |
| Manipulation | Rest | 0.187 * | (0.1102, 0.2638) | 17 | 5.1374 | 0.00001 |
| User 8 (Expert) | Drawing | Rest | 0.273 * | (0.2148, 0.3312) | 15 | 10 | 0.00001 |
| Manipulation | Drawing | −0.015 | (−0.0988, 0.0688) | 17 | −0.7895 | 0.3551 |
| Manipulation | Rest | 0.258 * | (0.1935, 0.3225) | 22 | 8.2958 | 0.00001 |
Table 3.
Small-worldness of binary directed FBNs during rest, drawing and manipulation states for 2 typical users.
Table 3.
Small-worldness of binary directed FBNs during rest, drawing and manipulation states for 2 typical users.
| Users | Cognitive State | | | | | |
|---|
| User 3 (Novice) | Rest | 0.9119 | 0.8676 | 1.1939 | 1.1934 | 1.0506 |
| Drawing | 0.6675 | 0.5358 | 1.5255 | 1.5043 | 1.2286 |
| Manipulation | 0.7714 | 0.6582 | 1.3878 | 1.3879 | 1.0809 |
| User 8 (Expert) | Rest | 0.8214 | 0.6998 | 1.3469 | 1.3470 | 1.1739 |
| Drawing | 0.4777 | 0.4126 | 1.7092 | 1.6537 | 1.1202 |
| Manipulation | 0.6607 | 0.5267 | 1.5408 | 1.5256 | 1.2420 |
Table 4.
Result of pairwise mean difference using the t-test for hemispheres information flow during rest, drawing and manipulation states.
Table 4.
Result of pairwise mean difference using the t-test for hemispheres information flow during rest, drawing and manipulation states.
| States | Novice User 3 | Novice User 6 |
|---|
| Drawing | Rest | Mean Diff. | CI | Mean Diff. | CI |
|---|
| LH-RH | LH-RH | 0.017 * | 0.005 | 0.029 | 0.016 | −0.003 | 0.034 |
| RH-LH | RH-LH | 0.017 * | 0.006 | 0.028 | 0.005 | −0.004 | 0.019 |
| LH-LH | LH-LH | 0.027 * | 0.011 | 0.043 | 0.012 * | 0.001 | 0.022 |
| RH-RH | RH-RH | 0.012 * | 0.005 | 0.018 | 0.030 * | 0.007 | 0.052 |
| Manipulation | Drawing | | | | | | |
| LH-RH | LH-RH | 0.022 | −0.004 | 0.048 | 0.008 | −0.014 | 0.029 |
| RH-LH | RH-LH | 0.020 * | 0.008 | 0.032 | 0.031 * | 0.001 | 0.052 |
| LH-LH | LH-LH | 0.010 | −0.007 | 0.028 | 0.029 * | 0.006 | 0.052 |
| RH-RH | RH-RH | 0.031 * | 0.018 | 0.044 | −0.016 | −0.042 | 0.009 |
| Manipulation | Rest | | | | | | |
| LH-RH | LH-RH | 0.039 * | 0.014 | 0.064 | 0.024 * | 0.002 | 0.041 |
| RH-LH | RH-LH | 0.037 * | 0.01 | 0.043 | 0.038 | 0.017 | 0.059 |
| LH-LH | LH-LH | 0.037 * | 0.026 | 0.048 | 0.041 * | 0.018 | 0.064 |
| RH-RH | RH-RH | 0.043 * | 0.030 | 0.055 | 0.014 | −0.004 | 0.032 |
| | | Expert User 7 | Expert User 8 |
| Drawing | Rest | Mean Diff. | CI | Mean Diff. | CI |
| LH-RH | LH-RH | 0.008 | −0.008 | 0.024 | 0.020 * | 0.009 | 0.030 |
| RH-LH | RH-LH | 0.080 | −0.017 | 0.177 | 0.034 * | 0.008 | 0.060 |
| LH-LH | LH-LH | 0.088 * | 0.046 | 0.130 | 0.035 * | 0.018 | 0.052 |
| RH-RH | RH-RH | 0.020 * | 0.008 | 0.031 | 0.013 | −0.005 | 0.031 |
| Manipulation | Drawing | | | | | | |
| LH-RH | LH-RH | 0.005 | −0.010 | 0.019 | 0.006 | −0.016 | 0.028 |
| RH-LH | RH-LH | −0.042 | −0.141 | 0.057 | −0.003 | −0.035 | 0.028 |
| LH-LH | LH-LH | −0.025 | −0.108 | 0.057 | −0.007 | −0.027 | 0.013 |
| RH-RH | RH-RH | 0.009 | −0.005 | 0.023 | 0.029 | −0.005 | 0.063 |
| Manipulation | Rest | | | | | | |
| LH-RH | LH-RH | 0.013 | −0.002 | 0.027 | 0.026 * | 0.004 | 0.047 |
| RH-LH | RH-LH | 0.038 | −0.000 | 0.076 | 0.031 * | 0.008 | 0.054 |
| LH-LH | LH-LH | 0.063 | −0.017 | 0.142 | 0.028 * | 0.013 | 0.043 |
| RH-RH | RH-RH | 0.029 * | 0.016 | 0.041 | 0.042 * | 0.009 | 0.075 |
Table 5.
Information flow among F, C and P nodes during rest, drawing and manipulation states.
Table 5.
Information flow among F, C and P nodes during rest, drawing and manipulation states.
| Subjects | States | F-C | C-F | F-P | P-F | P-C | C-P | Max | Min |
|---|
| User 3 (Novice) | Rest | 0.0061 | 0.0121 | 0.0111 | 0.0037 | 0.0048 | 0.0058 | C-F | P-F |
| Drawing | 0.0611 | 0.0502 | 0.0709 | 0.0224 | 0.0330 | 0.0637 | F-P | P-F |
| Manipulation | 0.1097 | 0.0919 | 0.1350 | 0.1091 | 0.0987 | 0.1139 | F-P | C-F |
| User 6 (Novice) | Rest | 0.0212 | 0.0162 | 0.0135 | 0.0313 | 0.0372 | 0.0242 | P-C | F-P |
| Drawing | 0.0741 | 0.0555 | 0.0434 | 0.0386 | 0.0964 | 0.0730 | P-C | P-F |
| Manipulation | 0.1080 | 0.0797 | 0.0557 | 0.0579 | 0.0431 | 0.0636 | F-C | P-C |
| User 8 (Expert) | Rest | 0.0465 | 0.0325 | 0.0175 | 0.0254 | 0.0359 | 0.0446 | F-C | F-P |
| Drawing | 0.0736 | 0.0702 | 0.1154 | 0.1516 | 0.0622 | 0.0606 | P-F | C-P |
| Manipulation | 0.1792 | 0.1215 | 0.1027 | 0.1478 | 0.0526 | 0.1020 | F-C | P-C |
| User 7 (Expert) | Rest | 0.0242 | 0.0134 | 0.0356 | 0.0406 | 0.0606 | 0.0689 | C-P | C-F |
| Drawing | 0.2260 | 0.0752 | 0.1570 | 0.0739 | 0.0735 | 0.1027 | F-C | P-C |
| Manipulation | 0.1631 | 0.0577 | 0.0952 | 0.0642 | 0.0782 | 0.0590 | F-C | C-F |
Table 6.
Classification results using all features.
Table 6.
Classification results using all features.
| Classifiers | Acc | Sen | Spec | F-Measure |
|---|
| LDA | 88% | 0.94 | 0.80 | 0.91 |
| KNN | 71% | 0.75 | 0.65 | 0.76 |
| NB | 69% | 0.80 | 0.53 | 0.76 |
| SVM | 82% | 0.87 | 0.74 | 0.85 |
| Tree | 83% | 0.88 | 0.75 | 0.86 |
Table 7.
Classification results after feature selection by SFS.
Table 7.
Classification results after feature selection by SFS.
| Classifiers | Acc | Sen | Spec | F-Measure | Features |
|---|
| LDA | 88% | 0.95 | 0.76 | 0.90 | 40.00 |
| KNN | 95% | 0.96 | 0.92 | 0.96 | 11.00 |
| NB | 78% | 0.93 | 0.56 | 0.84 | 4.00 |
| SVM | 88% | 0.95 | 0.76 | 0.90 | 20.00 |
| Tree | 89% | 0.90 | 0.88 | 0.91 | 16.00 |
Table 8.
Time taken by the algorithm to select features using various classifiers.
Table 8.
Time taken by the algorithm to select features using various classifiers.
| Classifiers | Acc | Feature Selected | Time in s |
|---|
| LDA | 80% | 12 32 35 37 41 | 7.96 |
| KNN | 92% | 23 29 32 35 41 | 7.30 |
| NB | 78% | 29 30 32 35 41 | 7.52 |
| SVM | 81% | 19 20 29 32 38 | 818.47 |
| Tree | 86% | 9 17 22 29 35 | 7.71 |