Reduced Apoptotic Injury by Phenothiazine in Ischemic Stroke through the NOX-Akt/PKC Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Focal Cerebral Ischemia
2.3. Phenothiazine Administration
2.4. NOX Inhibitor Administration
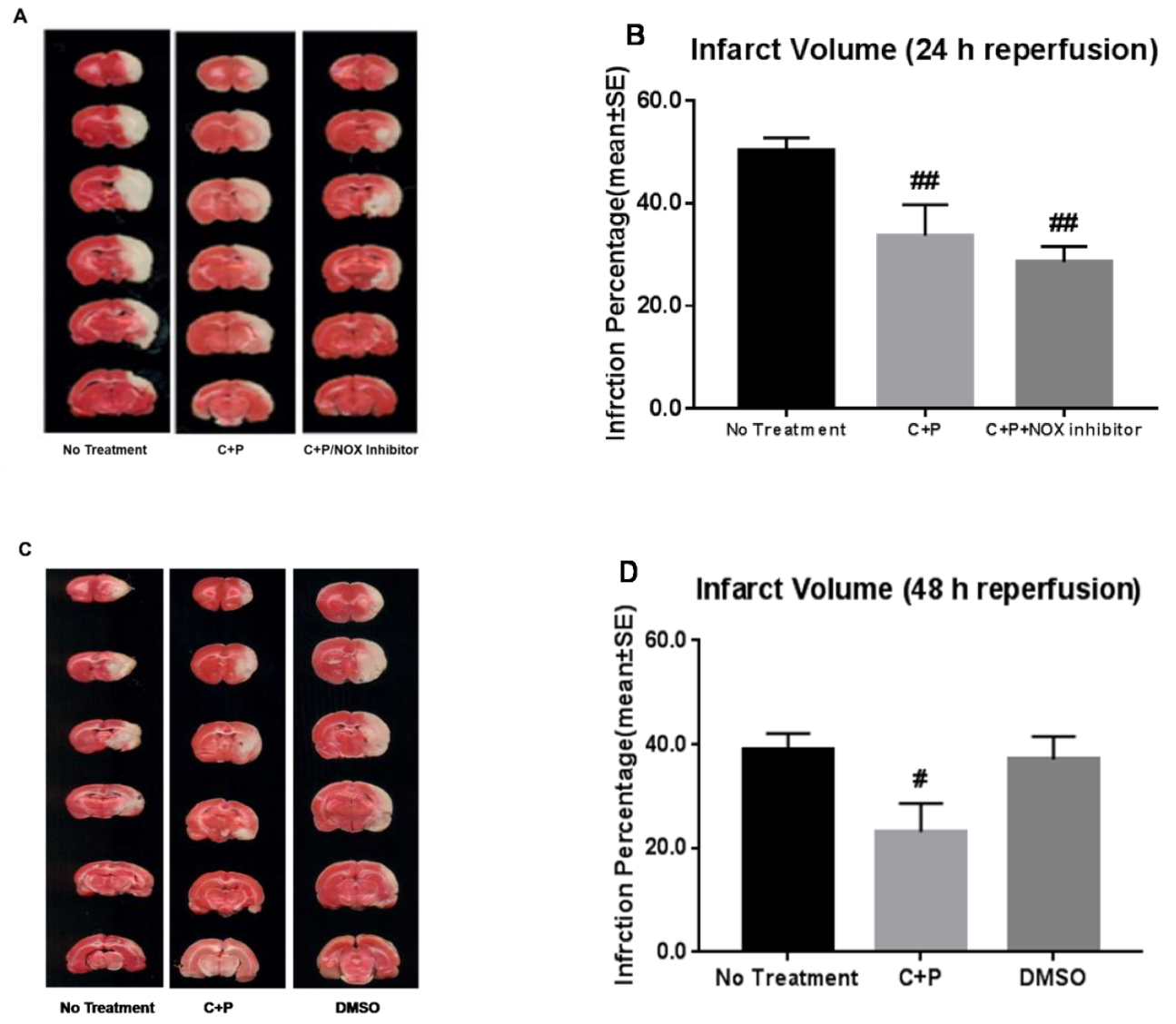
2.5. Cerebral Infarct Volume
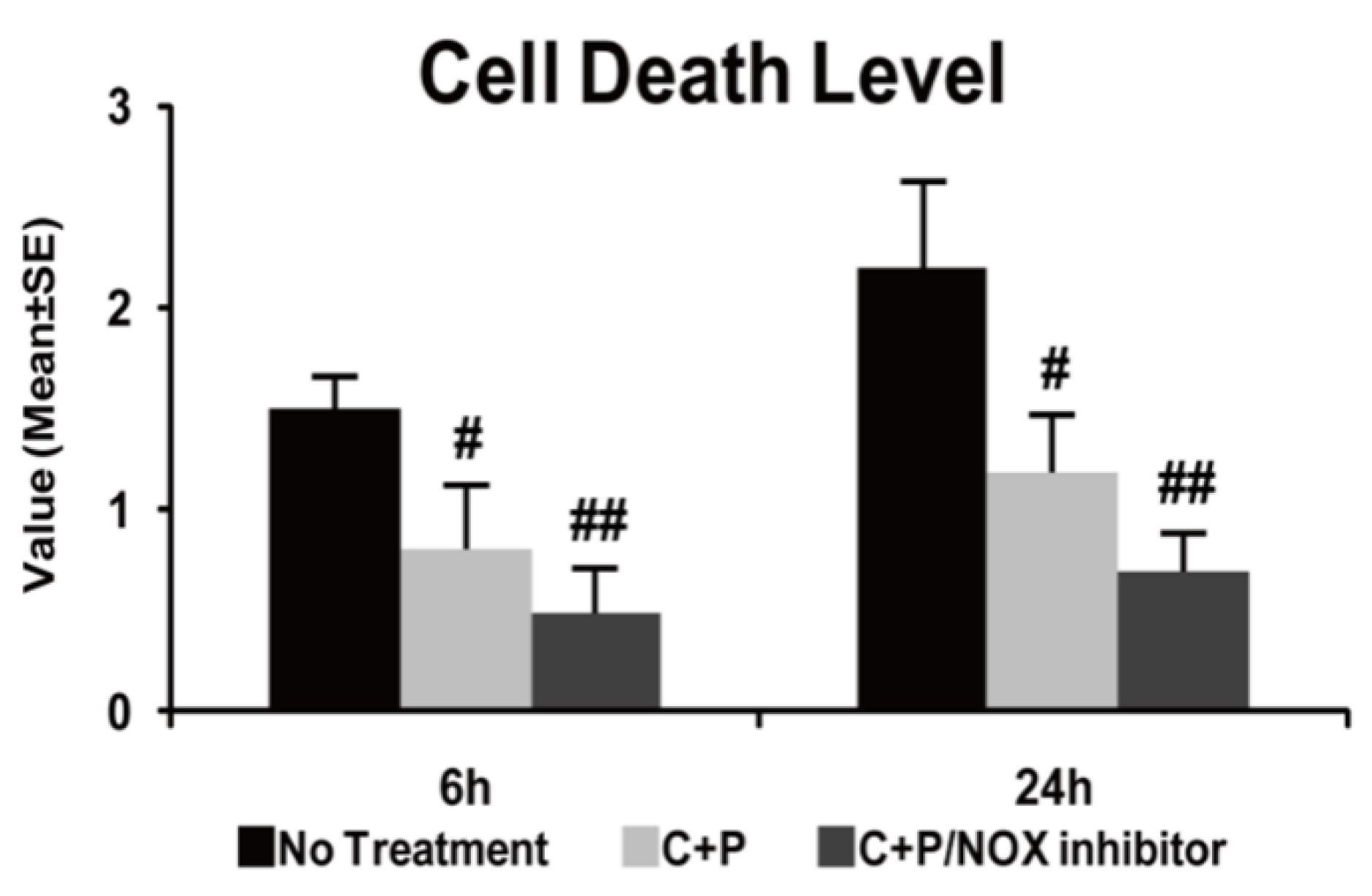
2.6. Apoptotic Cell Death Assay
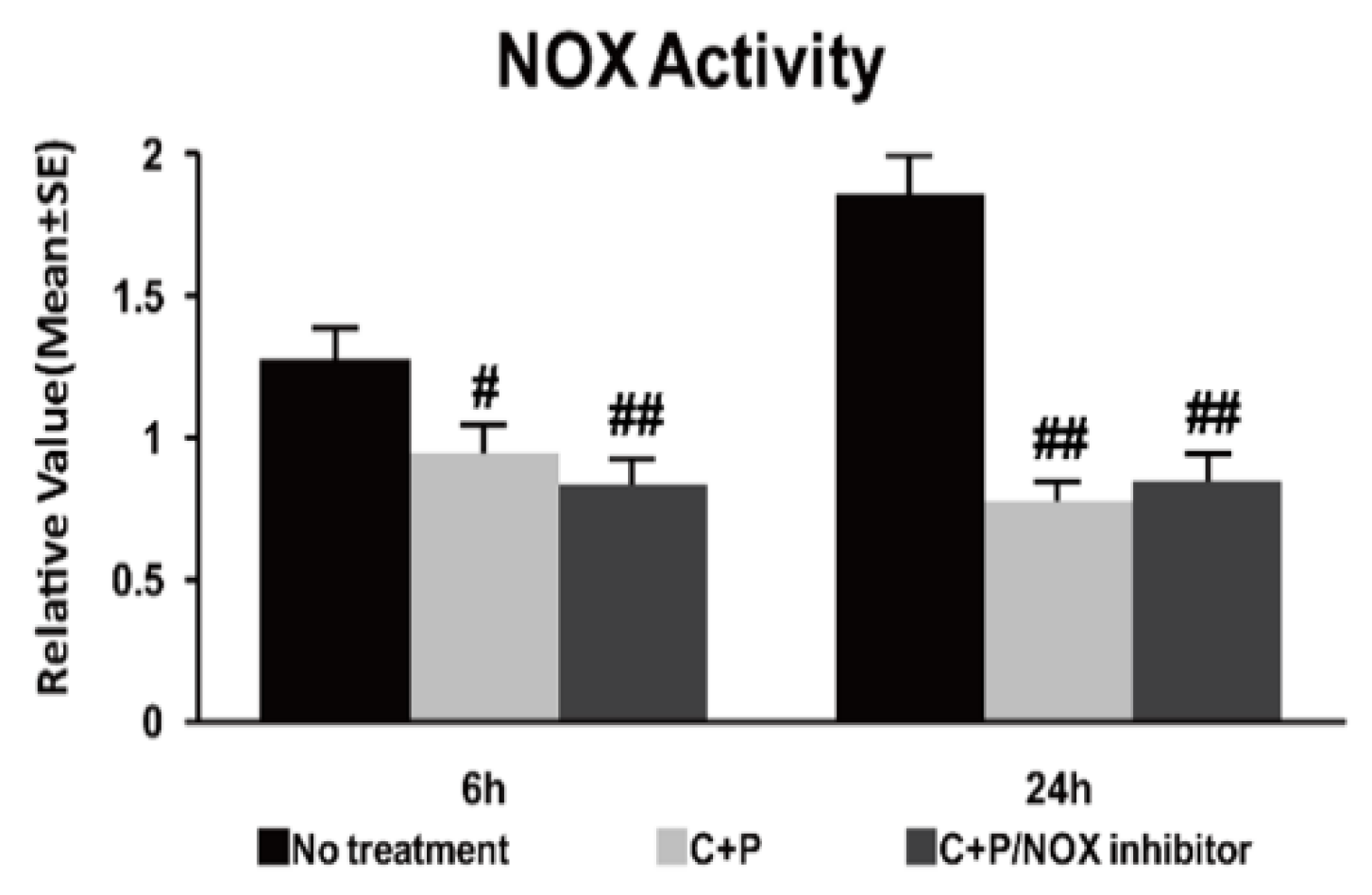
2.7. NOX Activity Assay
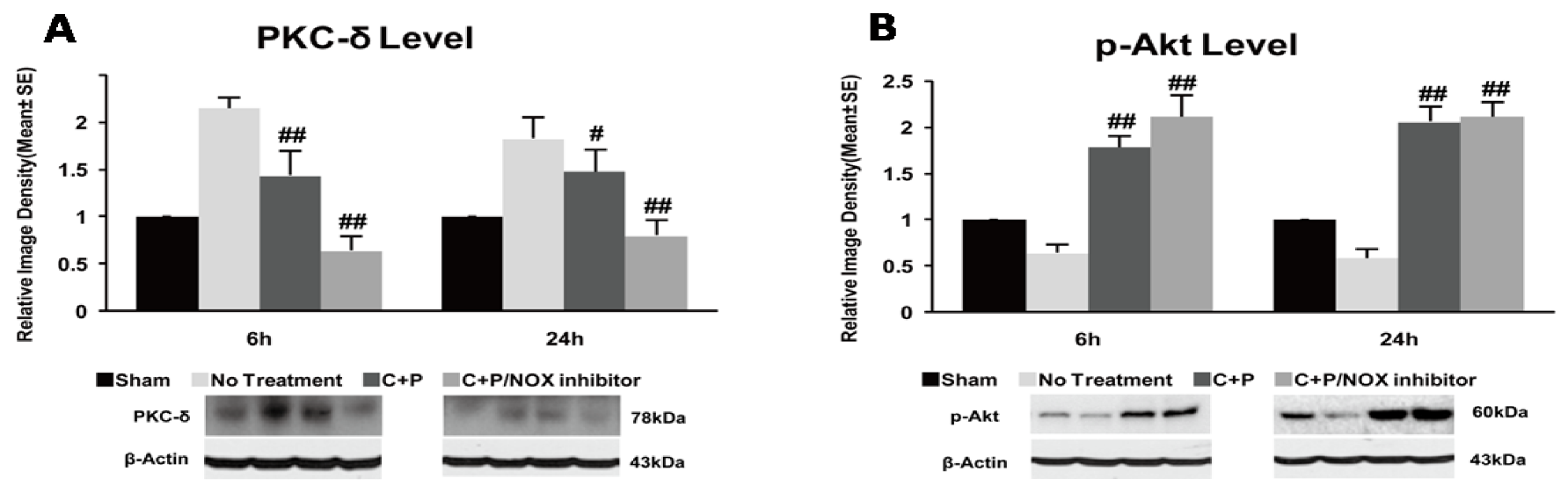
2.8. Western Blotting
2.9. Statistical Analysis (SPSS Software, Version 19, SPSS Inc.)
3. Results
3.1. Physiological Parameters.
3.2. Cerebral Infarct Volume
3.3. Cell Death
3.4. NOX Activity
3.5. PKC-δ and p-Akt Protein
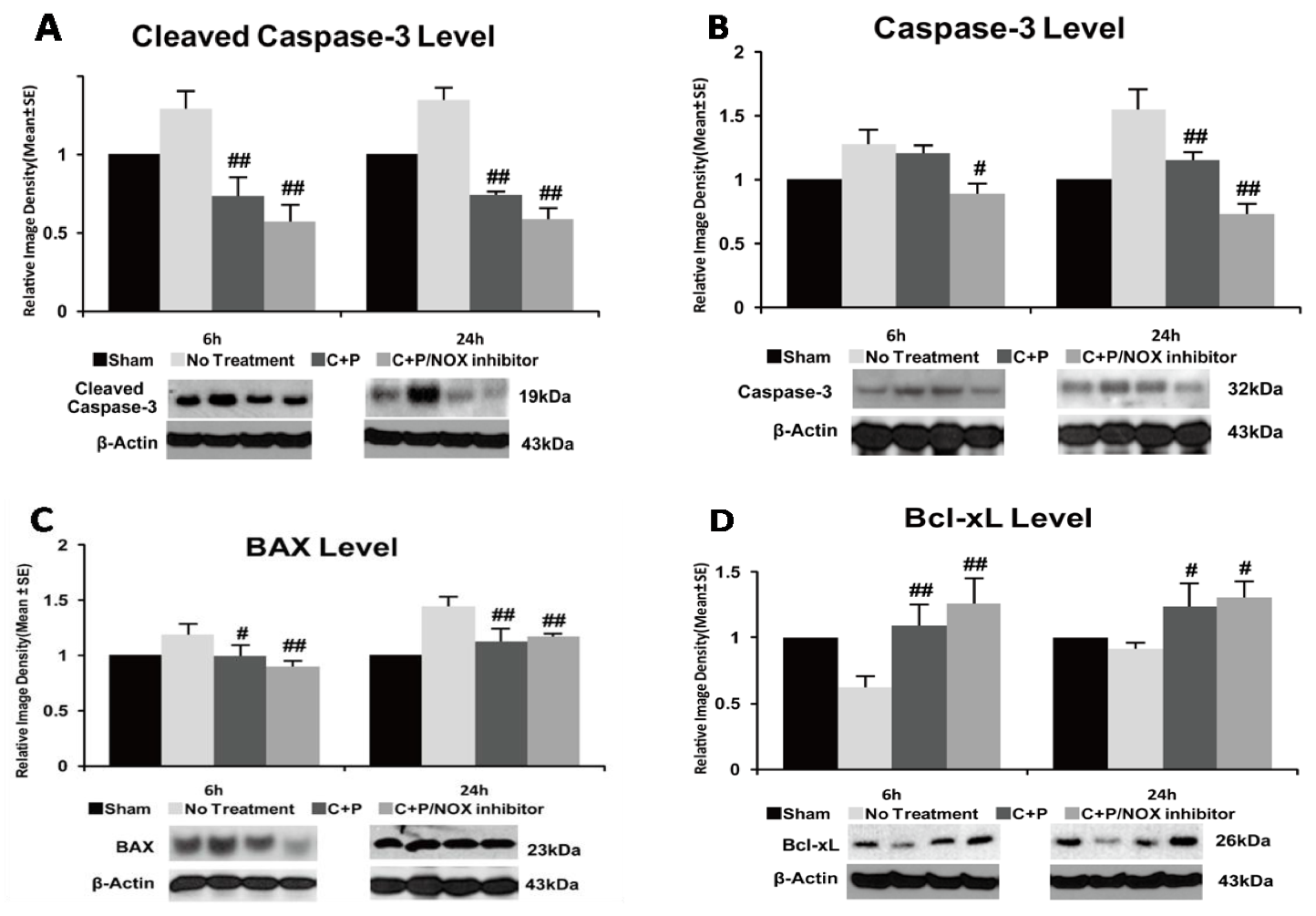
3.6. Caspase-3 Activity and Bax Protein
3.7. Bcl-XL Protein
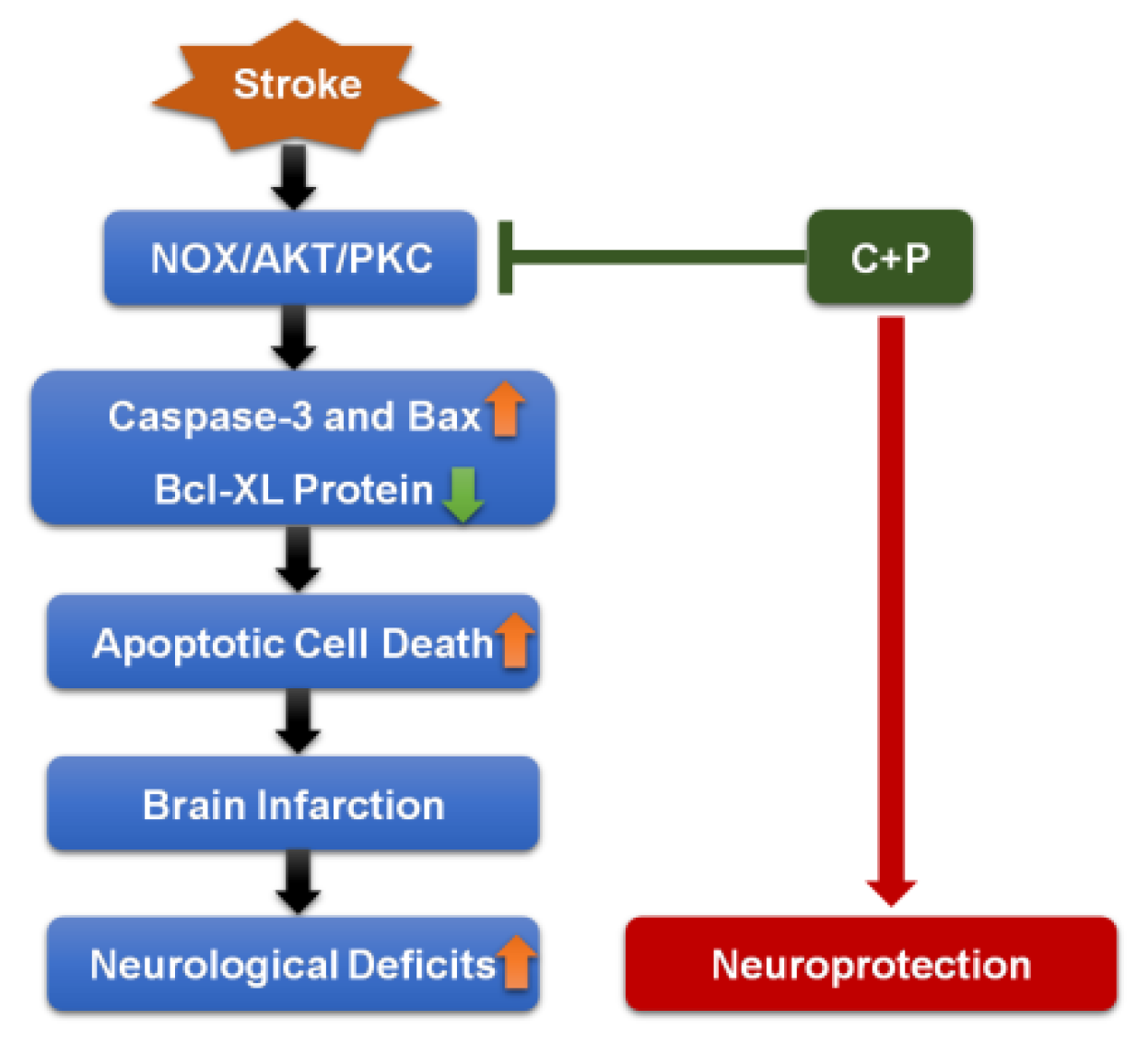
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stone, C.R.; Ding, Y. The cerebral circulation: The centrality of its function, the catastrophe of its failure. Brain Circ. 2017, 3, 43–44. [Google Scholar] [PubMed]
- Li, W.A.; Geng, X.; Ding, Y. Stroke is a global epidemic: New developments in clinical and translational cerebrovascular diseases research. Neurol. Res. 2017, 39, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, K.; Elmadhoun, O.; Ji, X.; Duan, Y.; Shi, J.; He, X.; Liu, X.; Wu, D.; Che, R.; et al. Synergistically induced hypothermia and enhanced neuroprotection by pharmacological and physical approaches in stroke. Aging Dis. 2018, 9, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Sader, A.A.; Barbieri-Neto, J.; Sader, S.L.; Mazzetto, S.A.; Alves, P., Jr.; Vanni, J.C. The protective action of chlorpromazine on the spinal cord of rabbits submitted to ischemia and reperfusion is dose-dependent. J. Cardiovasc. Surg. 2002, 43, 827–831. [Google Scholar]
- Chandra, A.; Geng, X.; Ding, Y. Brain and disease: An insight into new developments in the pathogenesis and novel therapies for neurological disorders. Neurol. Res. 2018, 40, 419–420. [Google Scholar] [CrossRef]
- Gonzalez-Munoz, G.C.; Arce, M.P.; Lopez, B.; Perez, C.; Villarroya, M.; Lopez, M.G.; Garcia, A.G.; Conde, S.; Rodriguez-Franco, M.I. Old phenothiazine and dibenzothiadiazepine derivatives for tomorrow’s neuroprotective therapies against neurodegenerative diseases. Eur. J. Med. Chem. 2010, 45, 6152–6158. [Google Scholar] [CrossRef]
- Stavrovskaya, I.G.; Narayanan, M.V.; Zhang, W.; Krasnikov, B.F.; Heemskerk, J.; Young, S.S.; Blass, J.P.; Brown, A.M.; Beal, M.F.; Friedlander, R.M.; et al. Clinically approved heterocyclics act on a mitochondrial target and reduce stroke-induced pathology. J. Exp. Med. 2004, 200, 211–222. [Google Scholar] [CrossRef]
- An, H.; Duan, Y.; Wu, D.; Yip, J.; Elmadhoun, O.; Wright, J.C.; Shi, W.; Liu, K.; He, X.; Shi, J.; et al. Phenothiazines enhance mild hypothermia-induced neuroprotection via pi3k/akt regulation in experimental stroke. Sci. Rep. 2017, 7, 7469. [Google Scholar] [CrossRef]
- Zhao, E.Y.; Efendizade, A.; Cai, L.; Ding, Y. The role of akt (protein kinase b) and protein kinase c in ischemia-reperfusion injury. Neurol. Res. 2016, 38, 301–308. [Google Scholar] [CrossRef]
- Bellacosa, A.; Testa, J.R.; Staal, S.P.; Tsichlis, P.N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an sh2-like region. Science 1991, 254, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Q.; Chong, Z.Z.; Maiese, K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J. Neurosci. Res. 2003, 74, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Li, F.; Maiese, K. Activating akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol. Histopathol. 2005, 20, 299–315. [Google Scholar] [PubMed]
- Li, Y.; Tennekoon, G.I.; Birnbaum, M.; Marchionni, M.A.; Rutkowski, J.L. Neuregulin signaling through a pi3k/akt/bad pathway in schwann cell survival. Mol. Cell. Neurosci. 2001, 17, 761–767. [Google Scholar] [CrossRef]
- Lee, S.H.; Chun, W.; Kong, P.J.; Han, J.A.; Cho, B.P.; Kwon, O.Y.; Lee, H.J.; Kim, S.S. Sustained activation of akt by melatonin contributes to the protection against kainic acid-induced neuronal death in hippocampus. J. Pineal Res. 2006, 40, 79–85. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, X.; Chang, S.; Wang, Y.; Xu, Y.; Ran, S.; Huang, Z.; Li, P.; Li, J.; Zhang, L.; et al. Totarol prevents neuronal injury in vitro and ameliorates brain ischemic stroke: Potential roles of akt activation and ho-1 induction. Toxicol. Appl. Pharmacol. 2015, 289, 142–154. [Google Scholar] [CrossRef]
- Jin, X.F.; Wang, S.; Shen, M.; Wen, X.; Han, X.R.; Wu, J.C.; Tang, G.Z.; Wu, D.M.; Lu, J.; Zheng, Y.L. Effects of rehabilitation training on apoptosis of nerve cells and the recovery of neural and motor functions in rats with ischemic stroke through the pi3k/akt and nrf2/are signaling pathways. Brain Res. Bull. 2017, 134, 236–245. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Chai, S.; Wang, X. Progesterone alleviates hypoxic-ischemic brain injury via the akt/gsk-3beta signaling pathway. Exp. Ther. Med. 2014, 8, 1241–1246. [Google Scholar] [CrossRef]
- Bright, R.; Mochly-Rosen, D. The role of protein kinase c in cerebral ischemic and reperfusion injury. Stroke 2005, 36, 2781–2790. [Google Scholar] [CrossRef]
- Budas, G.R.; Churchill, E.N.; Mochly-Rosen, D. Cardioprotective mechanisms of pkc isozyme-selective activators and inhibitors in the treatment of ischemia-reperfusion injury. Pharmacol. Res. 2007, 55, 523–536. [Google Scholar] [CrossRef]
- Churchill, E.N.; Szweda, L.I. Translocation of δpkc to mitochondria during cardiac reperfusion enhances superoxide anion production and induces loss in mitochondrial function. Arch. Biochem. Biophys. 2005, 439, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Raval, A.P.; Dave, K.R.; Prado, R.; Katz, L.M.; Busto, R.; Sick, T.J.; Ginsberg, M.D.; Mochly-Rosen, D.; Perez-Pinzon, M.A. Protein kinase c delta cleavage initiates an aberrant signal transduction pathway after cardiac arrest and oxygen glucose deprivation. J. Cereb. Blood Flow Metab. 2005, 25, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.W.; Gresia, V.L.; Stradecki, H.M.; Alekseyenko, A.; Dezfulian, C.; Neumann, J.T.; Dave, K.R.; Perez-Pinzon, M.A. Protein kinase c delta modulates endothelial nitric oxide synthase after cardiac arrest. J. Cereb. Blood Flow Metab. 2014, 34, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Stevenson, J.; Geng, X.; Peng, C.; Ji, X.; Xin, R.; Rastogi, R.; Sy, C.; Rafols, J.A.; Ding, Y. Combining normobaric oxygen with ethanol or hypothermia prevents brain damage from thromboembolic stroke via pkc-akt-nox modulation. Mol. Neurobiol. 2017, 54, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The nox family of ros-generating nadph oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Radermacher, K.A.; Wingler, K.; Langhauser, F.; Altenhofer, S.; Kleikers, P.; Hermans, J.J.; Hrabe de Angelis, M.; Kleinschnitz, C.; Schmidt, H.H. Neuroprotection after stroke by targeting nox4 as a source of oxidative stress. Antioxid. Redox Signal. 2013, 18, 1418–1427. [Google Scholar] [CrossRef]
- Dang, S.; Liu, X.; Fu, P.; Gong, W.; Yan, F.; Han, P.; Ding, Y.; Ji, X.; Luo, Y. Neuroprotection by local intra-arterial infusion of erythropoietin after focal cerebral ischemia in rats. Neurol. Res. 2011, 33, 520–528. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Geng, X.; Asmaro, K.; Peng, C.; Sullivan, J.M.; Ding, J.Y.; Ji, X.; Ding, Y. Neuroprotective effect of acute ethanol administration in a rat with transient cerebral ischemia. Stroke 2012, 43, 205–210. [Google Scholar] [CrossRef]
- Geng, X.; Li, F.; Yip, J.; Peng, C.; Elmadhoun, O.; Shen, J.; Ji, X.; Ding, Y. Neuroprotection by chlorpromazine and promethazine in severe transient and permanent ischemic stroke. Mol. Neurobiol. 2017, 54, 8140–8150. [Google Scholar] [CrossRef]
- Tang, X.N.; Cairns, B.; Cairns, N.; Yenari, M.A. Apocynin improves outcome in experimental stroke with a narrow dose range. Neuroscience 2008, 154, 556–562. [Google Scholar] [CrossRef]
- Holdenrieder, S.; Stieber, P.; Bodenmuller, H.; Fertig, G.; Furst, H.; Schmeller, N.; Untch, M.; Seidel, D. Nucleosomes in serum as a marker for cell death. Clin. Chem. Lab. Med. 2001, 39, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Peng, C.; Ding, J.Y.; Asmaro, K.; Sullivan, J.M.; Guthikonda, M.; Ding, Y. Acute administration of ethanol reduces apoptosis following ischemic stroke in rats. Neurosci. Res. 2013, 76, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Kochanski, R.; Peng, C.; Higashida, T.; Geng, X.; Huttemann, M.; Guthikonda, M.; Ding, Y. Neuroprotection conferred by post-ischemia ethanol therapy in experimental stroke: An inhibitory effect on hyperglycolysis and nadph oxidase activation. J. Neurochem. 2013, 126, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Geng, X.; Hussain, M.; Liu, Z.; Gao, Z.; Liu, S.; Du, H.; Ji, X.; Ding, Y. Weight loss: Indication of brain damage and effect of combined normobaric oxygen and ethanol therapy after stroke. Neurol. Res. 2015, 37, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Yenari, M.; Kitagawa, K.; Lyden, P.; Perez-Pinzon, M. Metabolic downregulation: A key to successful neuroprotection? Stroke 2008, 39, 2910–2917. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.V.; Zhang, W.; Stavrovskaya, I.G.; Kristal, B.S.; Friedlander, R.M. Promethazine: A novel application as a neuroprotectant that reduces ischemia-mediated injury by inhibiting mitochondrial dysfunction. Clin. Neurosurg. 2004, 51, 102–107. [Google Scholar] [PubMed]
- Liu, S.; Geng, X.; Forreider, B.; Xiao, Y.; Kong, Q.; Ding, Y.; Ji, X. Enhanced beneficial effects of mild hypothermia by phenothiazine drugs in stroke therapy. Neurol. Res. 2015, 37, 454–460. [Google Scholar] [CrossRef]
- Forreider, B.; Pozivilko, D.; Kawaji, Q.; Geng, X.; Ding, Y. Hibernation-like neuroprotection in stroke by attenuating brain metabolic dysfunction. Prog. Neurobiol. 2017, 157, 174–187. [Google Scholar] [CrossRef]
- MacMillan, V. Effects of promethazine on the energy metabolism of normoxic and hypoxic rat brain. Stroke 1982, 13, 464–469. [Google Scholar] [CrossRef]
- Zager, E.L.; Ames, A., 3rd. Reduction of cellular energy requirements. Screening for agents that may protect against cns ischemia. J. Neurosurg. 1988, 69, 568–579. [Google Scholar] [CrossRef]
- Krausz, Y.; Eylon, L.; Cerasi, E. Calcium-binding proteins and insulin release. Differential effects of phenothiazines on first-and second-phase secretion and on islet camp response to glucose. Acta Endocrinol. 1987, 116, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S. The effect of chlorpromazine on the glucose metabolism in different parts of the goat brain. Acta Physiol. Scand. 1961, 53, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.S.; Liu, Y.; Bradley, R.J. Dopamine receptor antagonists modulate glucose uptake in rat pheochromocytoma (pc12) cells. Neurosci. Lett. 1999, 274, 151–154. [Google Scholar] [CrossRef]
- Chou, W.H.; Messing, R.O. Protein kinase c isozymes in stroke. Trends Cardiovasc. Med. 2005, 15, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, M.A.; Alessi, D.R. Pkb/akt: A key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 2001, 114, 2903–2910. [Google Scholar]
- Nishibori, M.; Itoh, Y.; Oishi, R.; Saeki, K. Mechanism of the central hyperglycemic action of histamine in mice. J. Pharm. Exp. 1987, 241, 582–586. [Google Scholar]
- Nagai, K.; Frohman, L.A. Neurotensin hyperglycemia: Evidence for histamine mediation and the assessment of a possible physiologic role. Diabetes 1978, 27, 577–582. [Google Scholar] [CrossRef]
- Wu, C.; Fujihara, H.; Yao, J.; Qi, S.; Li, H.; Shimoji, K.; Baba, H. Different expression patterns of bcl-2, bcl-xl, and bax proteins after sublethal forebrain ischemia in c57black/crj6 mouse striatum. Stroke 2003, 34, 1803–1808. [Google Scholar] [CrossRef]
- Korsmeyer, S.J. Regulators of cell death. Trends Genet. 1995, 11, 101–105. [Google Scholar] [CrossRef]
- Mayer, B.; Oberbauer, R. Mitochondrial regulation of apoptosis. News Physiol. Sci. 2003, 18, 89–94. [Google Scholar] [CrossRef]
- Lazou, A.; Iliodromitis, E.K.; Cieslak, D.; Voskarides, K.; Mousikos, S.; Bofilis, E.; Kremastinos, D.T. Ischemic but not mechanical preconditioning attenuates ischemia/reperfusion induced myocardial apoptosis in anaesthetized rabbits: The role of bcl-2 family proteins and erk1/2. Apoptosis 2006, 11, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Parmar, S.; Li, X.; Peng, C.; Ji, X.; Chakraborty, T.; Li, W.A.; Du, H.; Tan, X.; Ling, F.; et al. Reduced apoptosis by combining normobaric oxygenation with ethanol in transient ischemic stroke. Brain Res. 2013, 1531, 17–24. [Google Scholar] [CrossRef] [PubMed]
| Sham Control | No Treatment | C+P | C+P/NOX Inhibitor | |
|---|---|---|---|---|
| MAP (mmHg) | ||||
| Pre MCAO | 86.6 ± 2.5 | 85.8 ± 2.5 | 86.5 ± 2.9 | 84.2 ± 2.1 |
| Onset of reperfusion | 88.6 ± 2.8 | 87.9 ± 2.8 | 86.3 ± 3.6 | 89.1 ± 3.3 |
| After reperfusion | 83.2 ± 3.0 | 86.5 ± 2.8 | 84.5 ± 4.4 | 86.2 ± 3.7 |
| pO2 (mmHg) | ||||
| Pre MCAO | 134.8 ± 5.6 | 135.5 ± 5.0 | 133.6 ± 5.3 | 134.9 ± 5.7 |
| Onset of reperfusion | 131.3 ± 6.7 | 132.7 ± 5.8 | 133.1 ± 6.3 | 131.5 ± 6.7 |
| After reperfusion | 134.1 ± 8.8 | 140.1 ± 7.3 | 136.9 ± 6.2 | 139.1 ± 7.7 |
| pCO2 (mmHg) | ||||
| Pre MCAO | 45.0 ± 1.4 | 46.2 ± 1.9 | 46.8 ± 2.9 | 45.5 ± 1.6 |
| Onset of reperfusion | 43.6 ± 3.2 | 44.4 ± 2.5 | 43.2 ± 2.7 | 43.9 ± 2.7 |
| After reperfusion | 44.6 ± 5.0 | 45.6 ± 2.7 | 46.7 ± 3.3 | 48.1 ± 4.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Y.; Elkin, K.B.; Peng, C.; Shen, J.; Li, F.; Guan, L.; Ji, Y.; Wei, W.; Geng, X.; Ding, Y. Reduced Apoptotic Injury by Phenothiazine in Ischemic Stroke through the NOX-Akt/PKC Pathway. Brain Sci. 2019, 9, 378. https://doi.org/10.3390/brainsci9120378
Tong Y, Elkin KB, Peng C, Shen J, Li F, Guan L, Ji Y, Wei W, Geng X, Ding Y. Reduced Apoptotic Injury by Phenothiazine in Ischemic Stroke through the NOX-Akt/PKC Pathway. Brain Sciences. 2019; 9(12):378. https://doi.org/10.3390/brainsci9120378
Chicago/Turabian StyleTong, Yanna, Kenneth B. Elkin, Changya Peng, Jiamei Shen, Fengwu Li, Longfei Guan, Yu Ji, Wenjing Wei, Xiaokun Geng, and Yuchuan Ding. 2019. "Reduced Apoptotic Injury by Phenothiazine in Ischemic Stroke through the NOX-Akt/PKC Pathway" Brain Sciences 9, no. 12: 378. https://doi.org/10.3390/brainsci9120378
APA StyleTong, Y., Elkin, K. B., Peng, C., Shen, J., Li, F., Guan, L., Ji, Y., Wei, W., Geng, X., & Ding, Y. (2019). Reduced Apoptotic Injury by Phenothiazine in Ischemic Stroke through the NOX-Akt/PKC Pathway. Brain Sciences, 9(12), 378. https://doi.org/10.3390/brainsci9120378





