Characteristics of the Uncinate Fasciculus and Cingulum in Patients with Mild Cognitive Impairment: Diffusion Tensor Tractography Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI Acquisition
2.3. Neuropsychological Tests
2.4. Statistical Analysis
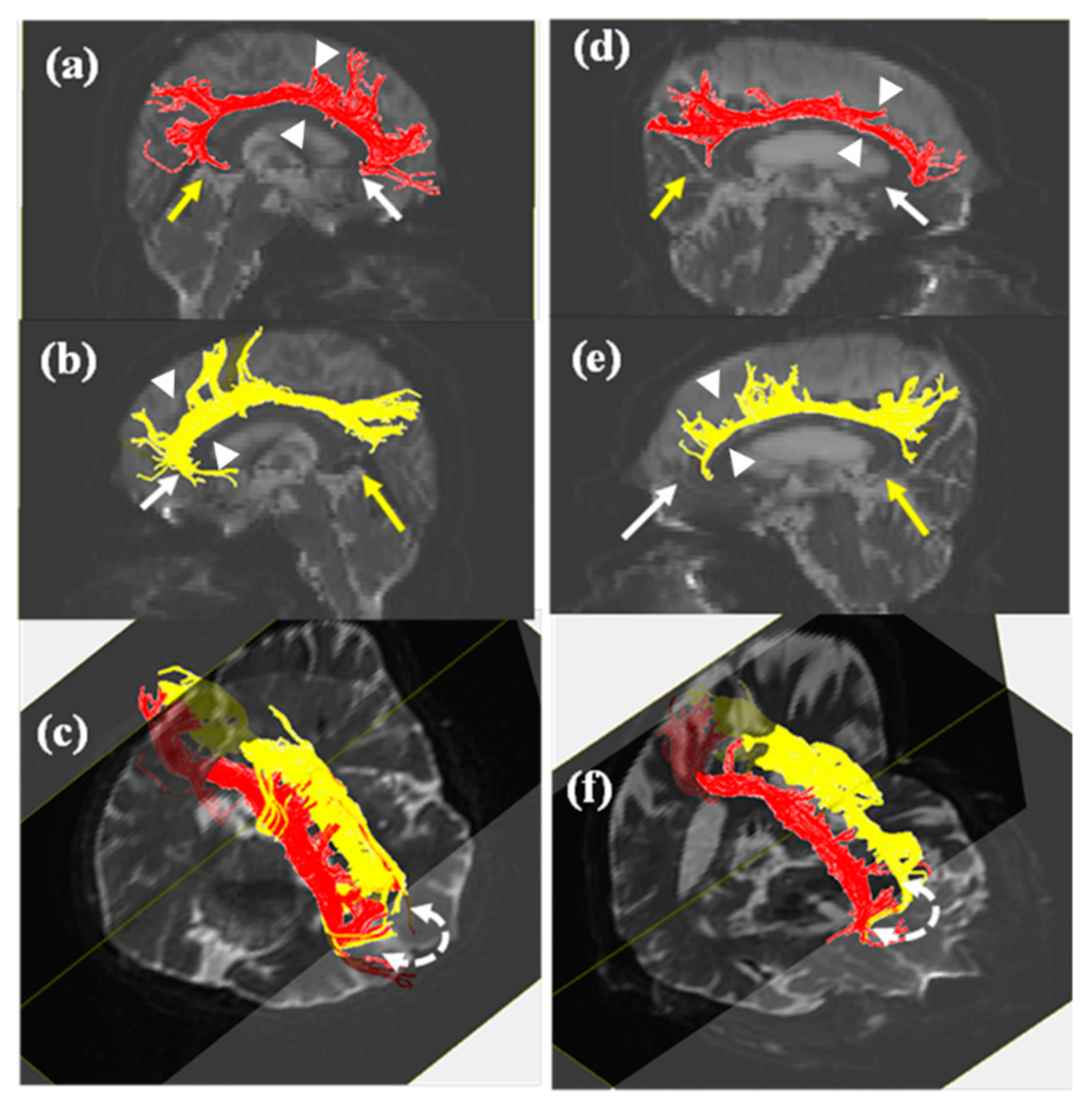
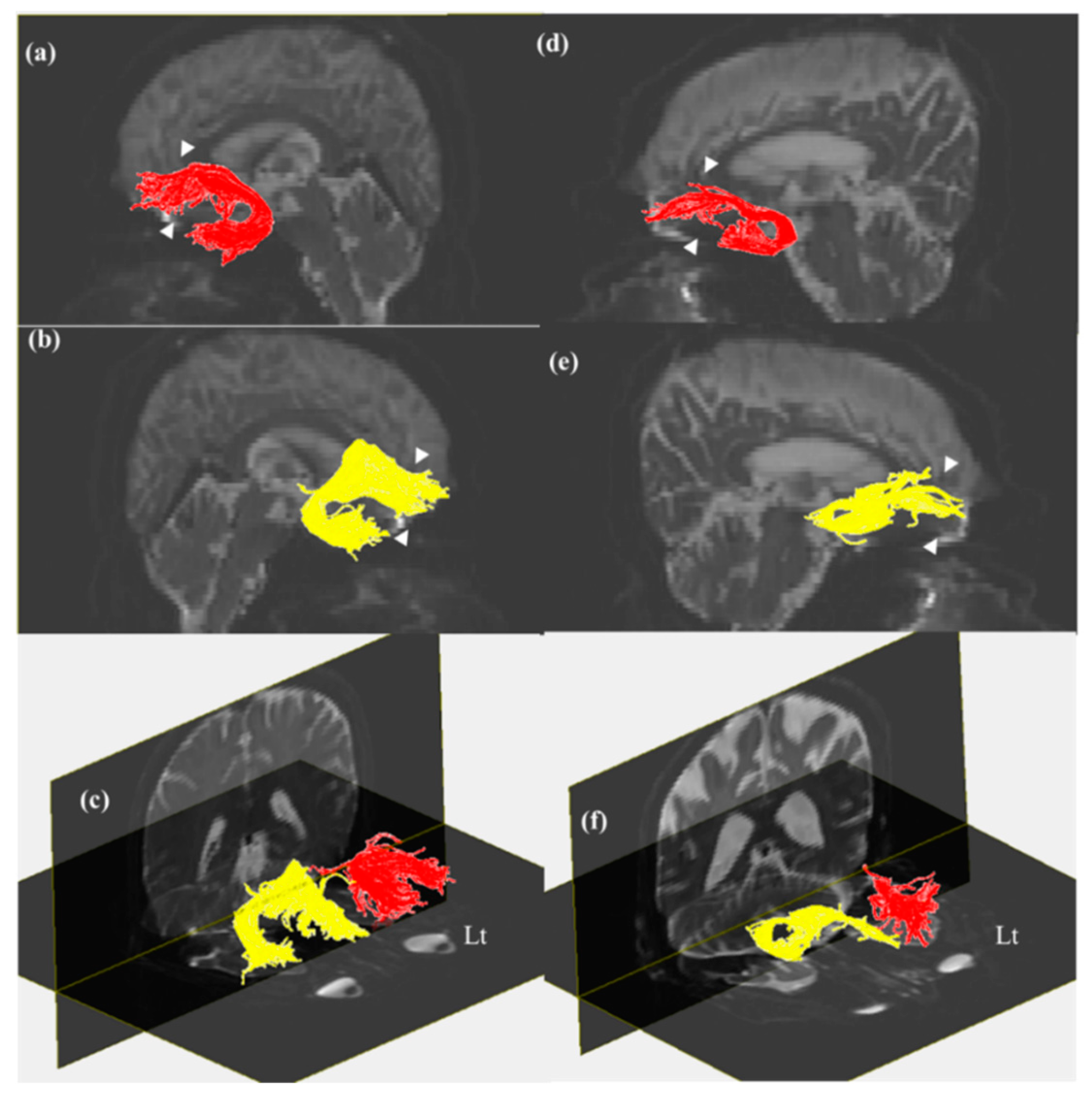
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morikawa, M.; Kiuchi, K.; Taoka, T.; Nagauchi, K.; Kichikawa, K.; Kishimoto, T. Uncinate fasciculus-correlated cognition in Alzheimer’s disease: A diffusion tensor imaging study by tractography. Psychogeriatrics 2010, 10, 15–20. [Google Scholar] [CrossRef]
- Zhang, A.; Leow, A.; Ajilore, O.; Lamar, M.; Yang, S.; Joseph, J.; Medina, J.; Zhan, L.; Kumar, A. Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology 2012, 37, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Papagno, C.; Miracapillo, C.; Casarotti, A.; Romero Lauro, L.J.; Castellano, A.; Falini, A.; Casaceli, G.; Fava, E.; Bello, L. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain 2011, 134, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Weis, C.N.; Belleau, E.L.; Pedersen, W.S.; Miskovich, T.A.; Larson, C.L. Structural Connectivity of the Posterior Cingulum Is Related to Reexperiencing Symptoms in Posttraumatic Stress Disorder. Chronic Stress 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Baddeley, C.; Jones, D.K.; Steventon, J.; Westacott, L.; Aggleton, J.P.; O’Sullivan, M.J. Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. J. Neurosci. 2012, 32, 17612–17619. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Kim, S.H.; Lee, H. Do Traumatic axonal injury of the cingulum in patients with mild traumatic brain injury: A diffusion tensor tractography study. Neural Regen. Res. 2019, 14, 1556–1561. [Google Scholar]
- Hong, J.H.; Jang, S.H. Neural pathway from nucleus basalis of Meynert passing through the cingulum in the human brain. Brain Res. 2010, 1346, 190–194. [Google Scholar] [CrossRef]
- Takahashi, S.; Yonezawa, H.; Takahashi, J.; Kudo, M.; Inoue, T.; Tohgi, H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurosci. Lett. 2002, 332, 45–48. [Google Scholar] [CrossRef]
- Kamagata, K.; Motoi, Y.; Abe, O.; Shimoji, K.; Hori, M.; Nakanishi, A.; Sano, T.; Kuwatsuru, R.; Aoki, S.; Hattori, N. White matter alteration of the cingulum in Parkinson disease with and without dementia: Evaluation by diffusion tensor tract-specific analysis. Am. J. Neuroradiol. 2012, 33, 890–895. [Google Scholar] [CrossRef]
- Lin, Y.C.; Shih, Y.C.; Tseng, W.Y.I.; Chu, Y.H.; Wu, M.T.; Chen, T.F.; Tang, P.F.; Chiu, M.J. Cingulum Correlates of Cognitive Functions in Patients with Mild Cognitive Impairment and Early Alzheimer’s Disease: A Diffusion Spectrum Imaging Study. Brain Topogr. 2014, 27, 393–402. [Google Scholar] [CrossRef]
- Neil, J.J. Diffusion imaging concepts for clinicians. J. Magn. Reson. Imaging 2008, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.J.; Westin, C.F. An introduction to diffusion tensor image analysis. Neurosurg. Clin. N. Am. 2011, 22, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Larroza, A.; Moratal, D.; D’ocón Alcañiz, V.; Arana, E. Tractography of the uncinate fasciculus and the posterior cingulate fasciculus in patients with mild cognitive impairment and Alzheimer disease. Neurología 2014, 29, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bathelt, J.; Johnson, A.; Zhang, M.; Astle, D.E. The cingulum as a marker of individual differences in neurocognitive development. Sci. Rep. 2019, 9, 2281. [Google Scholar] [CrossRef]
- Ahn, H.J.; Chin, J.; Park, A.; Lee, B.H.; Suh, M.K.; Seo, S.W.; Na, D.L. Seoul neuropsychological screening battery-dementia version (SNSB-D): A useful tool for assessing and monitoring cognitive impairments in dementia patients. J. Korean Med. Sci. 2010, 25, 1071–1076. [Google Scholar] [CrossRef]
- Lyketsos, C.G.; Lopez, O.; Jones, B.; Fitzpatrick, A.L.; Breitner, J.; DeKosky, S. Prevalence of Neuropsychiatric Symptoms in Dementia and Mild Cognitive Impairment. JAMA 2002, 288, 1475–1483. [Google Scholar] [CrossRef]
- Emrani, S.; Libon, D.J.; Lamar, M.; Price, C.C.; Jefferson, A.L.; Gifford, K.A.; Hohman, T.J.; Nation, D.A.; Delano-Wood, L.; Jak, A.; et al. Assessing working memory in mild cognitive impairment with serial order recall. J. Alzheimer’s Dis. 2018, 61, 917–928. [Google Scholar] [CrossRef]
- Zhang, Y.; Schuff, N.; Jahng, G.H.; Bayne, W.; Mori, S.; Schad, L.; Mueller, S.; Du, A.T.; Kramer, J.H.; Yaffe, K.; et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology 2007, 68, 13–19. [Google Scholar] [CrossRef]
- Selden, N.R.; Gitelman, D.R.; Mesulam, M.M. Trajectories of corticopetal cholinergic pathways within the cerebral hemispheres of the human brain. Neuroimage 1998, 7, 2249–2257. [Google Scholar] [CrossRef]
- Villano, I.; Messina, A.; Valenzano, A.; Moscatelli, F.; Esposito, T.; Monda, V.; Esposito, M.; Precenzano, F.; Carotenuto, M.; Viggiano, A.; et al. Basal forebrain cholinergic system and orexin neurons: Effects on attention. Front. Behav. Neurosci. 2017, 11, 10. [Google Scholar] [CrossRef]
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L.W. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Seo, J.P. Diffusion Tensor Tractography Studies on Injured Anterior Cingulum Recovery Mechanisms: A Mini-Review. Front. Neurol. 2018, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Van Den Heuvel, M.; Mandl, R.; Luigjes, J.; Pol, H.H. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J. Neurosci. 2008, 28, 10844–10851. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Sachdev, P.S.; Trollor, J.N.; Reppermund, S.; Kochan, N.A.; Brodaty, H.; Wen, W. Microstructural White Matter Changes, Not Hippocampal Atrophy, Detect Early Amnestic Mild Cognitive Impairment. PLoS ONE 2013, 8, e58887. [Google Scholar] [CrossRef]
- Metzler-Baddeley, C.; Jones, D.K.; Belaroussi, B.; Aggleton, J.P.; O’Sullivan, M.J. Frontotemporal connections in episodic memory and aging: A diffusion MRI tractography study. J. Neurosci. 2011, 31, 13236–13245. [Google Scholar] [CrossRef]
- Arpawong, T.E.; Pendleton, N.; Mekli, K.; McArdle, J.J.; Gatz, M.; Armoskus, C.; Knowles, J.A.; Prescott, C.A. Genetic variants specific to aging-related verbal memory: Insights from GWASs in a population-based cohort. PLoS ONE 2017, 12, e0182448. [Google Scholar] [CrossRef]
| Control (n = 14, M/F: 5/9) | MCI (n = 16, M/F: 9/7) | p-Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age, year | 66.00 | 4.95 | 71.38 | 8.61 | 0.044 * |
| K-MMSE | 27.84 | 1.28 | 25.73 | 3.23 | 0.037 * |
| Digit span | |||||
| Forward | 7.00 | 1.15 | 6.33 | 1.45 | 0.194 |
| Backward | 4.85 | 1.77 | 3.47 | 1.36 | 0.028 * |
| Language | |||||
| Short-BNT | 12.46 | 1.50 | 9.73 | 2.60 | 0.003 ** |
| Visuospatial function | |||||
| RCFT (copy score) | 33.77 | 2.95 | 26.33 | 10.87 | 0.025 * |
| Memory (SVLT-E) | |||||
| Immediate recall | 19.84 | 3.87 | 14.20 | 4.51 | 0.002 ** |
| Delayed recall | 6.46 | 1.45 | 2.00 | 2.32 | 0.000 ** |
| Frontal/Executive functions | |||||
| Go-No-Go | 19.38 | 0.96 | 16.33 | 5.61 | 0.065 |
| Control | MCI | p-Value | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| FA of Cingulum | Right | 0.54 | 0.03 | 0.52 | 0.02 | 0.106 |
| Left | 0.54 | 0.02 | 0.53 | 0.02 | 0.194 | |
| FA of UF | Right | 0.48 | 0.03 | 0.47 | 0.03 | 0.639 |
| Left | 0.48 | 0.02 | 0.47 | 0.045 | 0.723 | |
| The number of fibers of Cingulum | Right | 1251.00 | 399.19 | 907.87 | 262.37 | 0.011 * |
| Left | 1217.85 | 289.94 | 983.07 | 332.48 | 0.059 | |
| The number of fibers of UF | Right | 912.15 | 228.76 | 587.40 | 234.05 | 0.001 * |
| Left | 590.69 | 270.43 | 434.80 | 236.15 | 0.115 | |
| Age | Right Cingulum | Left Cingulum | Right UF | Left UF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | The Number of Fibers | FA | The Number of Fibers | FA | The Number of Fibers | FA | The Number of Fibers | ||||
| Right Cingulum | FA | r | −0.132 | - | |||||||
| The number of fibers | r | −0.144 | 0.499 ** | - | |||||||
| Left Cingulum | FA | r | −0.285 | 0.671 ** | 0.366 | - | |||||
| The number of fibers | r | −0.126 | 0.301 | 0.415 ** | 0.236 | - | |||||
| Right UF | FA | r | −0.066 | 0.434 * | 0.236 | 0.453 ** | −0.108 | - | |||
| The number of fibers | r | 0.193 | 0.065 | 0.295 | −0.044 | 0.032 | −0.089 | - | |||
| Left UF | FA | r | −0.230 | 0.370 | 0.219 | 0.387 ** | 0.111 | 0.647 ** | −0.162 | - | |
| The number of fibers | r | −0.331 | -0.191 | 0.141 | 0.124 | −0.034 | 0.171 | 0.302 | 0.408 ** | - | |
| Digit span | Forward | r | −0.287 | 0.263 | 0.119 | 0.103 | −0.174 | −0.081 | 0.305 | 0.285 | 0.116 |
| Backward | r | −0.335 | 0.252 | 0.150 | 0.003 | 0.363 | −0.190 | 0.325 | 0.211 | 0.169 | |
| Language | Short BNT | r | −0.359 | 0.194 | 0.384 ** | 0.013 | 0.230 | −0.058 | 0.341 | 0.249 | 0.303 |
| Visuospatial function | RCFT | r | −0.322 | 0.301 | 0.378 ** | 0.200 | 0.223 | −0.014 | 0.249 | 0.269 | 0.096 |
| Memory (SVLT-E) | Immediate recall | r | −0.539 ** | 0.155 | −0.013 | −0.034 | 0.223 | −0.153 | 0.248 | 0.012 | 0.109 |
| Delayed recall | r | −0.145 | 0.144 | 0.161 | 0.031 | 0.207 | −0.156 | 0.424 ** | 0.067 | 0.370 | |
| Frontal/Executive function | Go-no-Go | r | −0.329 | 0.369 | 0.324 | 0.174 | 0.181 | −0.070 | 0.217 | 0.198 | 0.042 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-H.; Kim, S.-H.; Jung, H.-Y. Characteristics of the Uncinate Fasciculus and Cingulum in Patients with Mild Cognitive Impairment: Diffusion Tensor Tractography Study. Brain Sci. 2019, 9, 377. https://doi.org/10.3390/brainsci9120377
Park C-H, Kim S-H, Jung H-Y. Characteristics of the Uncinate Fasciculus and Cingulum in Patients with Mild Cognitive Impairment: Diffusion Tensor Tractography Study. Brain Sciences. 2019; 9(12):377. https://doi.org/10.3390/brainsci9120377
Chicago/Turabian StylePark, Chan-Hyuk, Su-Hong Kim, and Han-Young Jung. 2019. "Characteristics of the Uncinate Fasciculus and Cingulum in Patients with Mild Cognitive Impairment: Diffusion Tensor Tractography Study" Brain Sciences 9, no. 12: 377. https://doi.org/10.3390/brainsci9120377
APA StylePark, C.-H., Kim, S.-H., & Jung, H.-Y. (2019). Characteristics of the Uncinate Fasciculus and Cingulum in Patients with Mild Cognitive Impairment: Diffusion Tensor Tractography Study. Brain Sciences, 9(12), 377. https://doi.org/10.3390/brainsci9120377





