Cortical Thickness Links Impulsive Personality Traits and Risky Behavior
Abstract
1. Introduction
1.1. Neuroanatomical Correlates of Impulsivity
1.2. Impulsive Traits and Risky Behavior
1.3. Current Study
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Data Analysis
3. Results
3.1. Impulsive Traits and Risky Behavior
3.2. Impulsive Trait Associations with Cortical Thickness
3.3. Cortical Thickness Associations with Risky Behavior
3.4. Cortical Thickness Mediates the Association between Sensation Seeking and Recent Risky Behavior
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cross, C.P.; Copping, L.T.; Campbell, A. Sex differences in impulsivity: A meta-analysis. Psychol. Bull. 2011, 137, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Cyders, M.A. Impulsivity and the Sexes: Measurement and Structural Invariance of the UPPS-P Impulsive Behavior Scale. Assessment 2013, 20, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.T.; McCrae, R.R. Personality Disorders and The Five-Factor Model of Personality. J. Personal. Disord. 1990, 4, 362–371. [Google Scholar] [CrossRef]
- Berg, J.M.; Latzman, R.D.; Bliwise, N.G.; Lilienfeld, S.O. Parsing the heterogeneity of impulsivity: A meta-analytic review of the behavioral implications of the UPPS for psychopathology. Psychol. Assess. 2015, 27, 1129–1146. [Google Scholar] [CrossRef]
- Cyders, M.A.; Smith, G.T. Mood-based rash action and its components: Positive and negative urgency. Personal. Individ. Differ. 2007, 43, 839–850. [Google Scholar] [CrossRef]
- Sharma, L.; Kohl, K.; Morgan, T.A.; Clark, L.A. “Impulsivity”: Relations between self-report and behavior. J. Pers. Soc. Psychol. 2013, 104, 559–575. [Google Scholar] [CrossRef]
- Whiteside, S.P.; Lynam, D.R. The Five Factor Model and impulsivity: Using a structural model of personality to understand impulsivity. Personal. Individ. Differ. 2001, 30, 669–689. [Google Scholar] [CrossRef]
- Carlson, S.R.; Pritchard, A.A.; Dominelli, R.M. Externalizing behavior, the UPPS-P Impulsive Behavior scale and Reward and Punishment Sensitivity. Personal. Individ. Differ. 2013, 54, 202–207. [Google Scholar] [CrossRef]
- Netter, P.; Hennig, J.; Roed, I.S. Serotonin and Dopamine as Mediators of Sensation Seeking Behavior. Neuropsychobiology 1996, 34, 155–165. [Google Scholar] [CrossRef]
- Anestis, M.D.; Bagge, C.L.; Tull, M.T.; Joiner, T.E. Clarifying the role of emotion dysregulation in the interpersonal-psychological theory of suicidal behavior in an undergraduate sample. J. Psychiatr. Res. 2011, 45, 603–611. [Google Scholar] [CrossRef]
- Kabbaj, M.; Devine, D.P.; Savage, V.R.; Akil, H. Neurobiological Correlates of Individual Differences in Novelty-Seeking Behavior in the Rat: Differential Expression of Stress-Related Molecules. J. Neurosci. 2000, 20, 6983–6988. [Google Scholar] [CrossRef] [PubMed]
- Piazza, P.V.; Deroche, V.; Deminière, J.M.; Maccari, S.; Moal, M.L.; Simon, H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: Implications for sensation-seeking behaviors. Proc. Natl. Acad. Sci. USA 1993, 90, 11738–11742. [Google Scholar] [CrossRef] [PubMed]
- Philippe, G.; Courvoisier, D.S.; Billieux, J.; Rochat, L.; Schmidt, R.E.; Van der Linden, M. Can the distinction between intentional and unintentional interference control help differentiate varieties of impulsivity? J. Res. Personal. 2010, 44, 46–52. [Google Scholar] [CrossRef]
- Miller, D.J.; Derefinko, K.J.; Lynam, D.R.; Milich, R.; Fillmore, M.T. Impulsivity and Attention Deficit-Hyperactivity Disorder: Subtype Classification Using the UPPS Impulsive Behavior Scale. J. Psychopathol. Behav. Assess. 2010, 32, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zermatten, A.; Van der Linden, M.; d’Acremont, M.; Jermann, F.; Bechara, A. Impulsivity and Decision Making. J. Nerv. Ment. Dis. 2005, 193, 647. [Google Scholar] [CrossRef]
- Besteher, B.; Gaser, C.; Nenadić, I. Brain structure and trait impulsivity: A comparative VBM study contrasting neural correlates of traditional and alternative concepts in healthy subjects. Neuropsychologia 2019, 131, 139–147. [Google Scholar] [CrossRef]
- Schilling, C.; Kühn, S.; Romanowski, A.; Schubert, F.; Kathmann, N.; Gallinat, J. Cortical thickness correlates with impulsiveness in healthy adults. NeuroImage 2012, 59, 824–830. [Google Scholar] [CrossRef]
- Tu, P.C.; Kuan, Y.H.; Li, C.T.; Su, T.P. Structural correlates of trait impulsivity in patients with bipolar disorder and healthy controls: A surface-based morphometry study. Psychol. Med. 2017, 47, 1292–1299. [Google Scholar] [CrossRef]
- Mitchell, M.R.; Potenza, M.N. Recent Insights into the Neurobiology of Impulsivity. Curr. Addict. Rep. 2014, 1, 309–319. [Google Scholar] [CrossRef]
- Muhlert, N.; Lawrence, A.D. Brain structure correlates of emotion-based rash impulsivity. NeuroImage 2015, 115, 138–146. [Google Scholar] [CrossRef]
- Holmes, A.J.; Hollinshead, M.O.; Roffman, J.L.; Smoller, J.W.; Buckner, R.L. Individual Differences in Cognitive Control Circuit Anatomy Link Sensation Seeking, Impulsivity, and Substance Use. J. Neurosci. 2016, 36, 4038–4049. [Google Scholar] [CrossRef] [PubMed]
- Um, M.; Whitt, Z.T.; Revilla, R.; Hunton, T.; Cyders, M.A. Shared Neural Correlates Underlying Addictive Disorders and Negative Urgency. Brain Sci. 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Latzman, R.D.; Chan, W.Y.; Shishido, Y. Impulsivity moderates the association between racial discrimination and alcohol problems. Addict. Behav. 2013, 38, 2898–2904. [Google Scholar] [CrossRef] [PubMed]
- Nock, M.K.; Prinstein, M.J. A Functional Approach to the Assessment of Self-Mutilative Behavior. J. Consult. Clin. Psychol. 2004, 72, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.; Shea, M.T.; Sanislow, C.A.; Skodol, A.E.; Grilo, C.M.; Edelen, M.O.; Stout, R.L.; Morey, L.C.; Zanarini, M.C.; Markowitz, J.C.; et al. Personality traits as prospective predictors of suicide attempts. Acta Psychiatr. Scand. 2009, 120, 222–229. [Google Scholar] [CrossRef]
- Claes, L.; Vandereycken, W.; Vertommen, H. Impulsivity-related traits in eating disorder patients. Personal. Individ. Differ. 2005, 39, 739–749. [Google Scholar] [CrossRef]
- Cyders, M.A.; Smith, G.T. Emotion-based dispositions to rash action: Positive and negative urgency. Psychol. Bull. 2008, 134, 807–828. [Google Scholar] [CrossRef]
- Magid, V.; MacLean, M.G.; Colder, C.R. Differentiating between sensation seeking and impulsivity through their mediated relations with alcohol use and problems. Addict. Behav. 2007, 32, 2046–2061. [Google Scholar] [CrossRef]
- Glenn, C.R.; Klonsky, E.D. A multimethod analysis of impulsivity in nonsuicidal self-injury. Personal. Disord. Theory Res. Treat. 2010, 1, 67–75. [Google Scholar] [CrossRef]
- Witte, T.K.; Gordon, K.H.; Smith, P.N.; Van Orden, K.A. Stoicism and sensation seeking: Male vulnerabilities for the acquired capability for suicide. J. Res. Personal. 2012, 46, 384–392. [Google Scholar] [CrossRef]
- Smith, G.T.; Fischer, S.; Cyders, M.A.; Annus, A.M.; Spillane, N.S.; McCarthy, D.M. On the Validity and Utility of Discriminating Among Impulsivity-Like Traits. Assessment 2007, 14, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Chung, Y.; Jeon, S.-M. Impulsivity and Substance Use in Young Adulthood. Am. J. Addict. 2013, 22, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lynam, D.R.; Miller, J.D.; Miller, D.J.; Bornovalova, M.A.; Lejuez, C.W. Testing the relations between impulsivity-related traits, suicidality, and nonsuicidal self-injury: A test of the incremental validity of the UPPS model. Personal. Disord. Theory Res. Treat. 2011, 2, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, E.; Panayiotou, G.; Konstantinou, N.; Loutsiou-Ladd, A.; Kapardis, A. Risky and aggressive driving in young adults: Personality matters. Accid. Anal. Prev. 2011, 43, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Curry, I.; Luk, J.W.; Trim, R.S.; Hopfer, C.J.; Hewitt, J.K.; Stallings, M.C.; Brown, S.A.; Wall, T.L. Impulsivity Dimensions and Risky Sex Behaviors in an At-Risk Young Adult Sample. Arch. Sex. Behav. 2018, 47, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, N.; Baskin-Sommers, A. Risky, Impulsive, and Self-Destructive Behavior Questionnaire (RISQ): A Validation Study. Assessment 2017, 24, 1080–1094. [Google Scholar] [CrossRef]
- Venables, N.C.; Patrick, C.J. Validity of the Externalizing Spectrum Inventory in a criminal offender sample: Relations with disinhibitory psychopathology, personality, and psychopathic features. Psychol. Assess. 2012, 24, 88–100. [Google Scholar] [CrossRef]
- Wilmington, DE Crime Rates. Available online: https://www.neighborhoodscout.com/de/wilmington/crime (accessed on 2 December 2019).
- Lynam, D.R.; Smith, G.T.; Whiteside, S.P.; Cyders, M.A. The UPPS-P: Assessing Five Personality Pathways to Impulsive Behavior; West Lafayette Purdue University: Seraphite, IN, USA, 2006. [Google Scholar]
- Van der Kouwe, A.J.W.; Benner, T.; Salat, D.H.; Fischl, B. Brain morphometry with multiecho MPRAGE. NeuroImage 2008, 40, 559–569. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Fischl, B.; Salat, D.H.; van der Kouwe, A.J.W.; Makris, N.; Ségonne, F.; Quinn, B.T.; Dale, A.M. Sequence-independent segmentation of magnetic resonance images. NeuroImage 2004, 23, S69–S84. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Liu, A.; Dale, A.M. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 2001, 20, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Schamsnky, N.J.; Rosas, H.D.; Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage 2012, 61, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [PubMed]
- Hagler, D.J.; Saygin, A.P.; Sereno, M.I. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage 2006, 33, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Greve, D.N.; Fischl, B. False positive rates in surface-based anatomical analysis. NeuroImage 2018, 171, 6–14. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide, 8th ed.; Muthén & Muthén: Los Angeles, CA, USA, 2017. [Google Scholar]
- Malach, R.; Reppas, J.B.; Benson, R.R.; Kwong, K.K.; Jiang, H.; Kennedy, W.A.; Ledden, P.J.; Brady, T.J.; Rosen, B.R.; Tootell, R.B. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc. Natl. Acad. Sci. USA 1995, 92, 8135–8139. [Google Scholar] [CrossRef]
- Schwarzkopf, D.S.; Song, C.; Rees, G. The surface area of human V1 predicts the subjective experience of object size. Nat. Neurosci. 2011, 14, 28–30. [Google Scholar] [CrossRef]
- Lawson, A.L.; Liu, X.; Joseph, J.; Vagnini, V.L.; Kelly, T.H.; Jiang, Y. Sensation Seeking Predicts Brain Responses in the Old-New Task: Converging Multimodal Neuroimaging Evidence. Int. J. Psychophysiol. 2012, 84, 260–269. [Google Scholar] [CrossRef]
- Chase, H.W.; Fournier, J.C.; Bertocci, M.A.; Greenberg, T.; Aslam, H.; Stiffler, R.; Lockovich, J.; Graur, S.; Bebko, G.; Forbes, E.E.; et al. A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: Identifying neural markers for new interventions. Transl. Psychiatry 2017, 7, e1096. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Fan, L.; Xu, J.; Li, C.; Liu, Y.; Fox, P.T.; Eickhoff, S.B.; Yu, C.; Jiang, T. Convergent functional architecture of the superior parietal lobule unraveled with multimodal neuroimaging approaches: Parcellation of Superior Parietal Lobule. Hum. Brain Mapp. 2015, 36, 238–257. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, R.; Ferraina, S.; Johnson, P.B. The Sources of Visual Information to the Primate Frontal Lobe: A Novel Role for the Superior Parietal Lobule. Cereb. Cortex 1996, 6, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E. The Precuneus and Consciousness. CNS Spectr. 2007, 12, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Utevsky, A.V.; Smith, D.V.; Huettel, S.A. Precuneus Is a Functional Core of the Default-Mode Network. J. Neurosci. 2014, 34, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Dinner, D.S.; Pillay, P.K.; Lüders, H.; Morris, H.H.; Klem, G.; Wyllie, E.; Awad, I.A. Functional anatomy of the human supplementary sensorimotor area: Results of extraoperative electrical stimulation. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 179–193. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.M.; Kochunov, P.; Blangero, J.; Almasy, L.; Zilles, K.; Fox, P.T.; Glahn, D.C. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 2010, 53, 1135–1146. [Google Scholar] [CrossRef]
- Moreno-López, L.; Catena, A.; Fernández-Serrano, M.J.; Delgado-Rico, E.; Stamatakis, E.A.; Pérez-García, M.; Verdejo-García, A. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012, 125, 208–214. [Google Scholar] [CrossRef]
- Dir, A.L.; Karyadi, K.; Cyders, M.A. The uniqueness of negative urgency as a common risk factor for self-harm behaviors, alcohol consumption, and eating problems. Addict. Behav. 2013, 38, 2158–2162. [Google Scholar] [CrossRef]
- Cyders, M.A.; Coskunpinar, A. Is urgency emotionality? Separating urgent behaviors from effects of emotional experiences. Personal. Individ. Differ. 2010, 48, 839–844. [Google Scholar] [CrossRef]
- Hershberger, A.R.; Um, M.; Cyders, M.A. The relationship between the UPPS-P impulsive personality traits and substance use psychotherapy outcomes: A meta-analysis. Drug Alcohol Depend. 2017, 178, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Littlefield, A.K.; Stevens, A.K.; Ellingson, J.M.; King, K.M.; Jackson, K.M. Changes in negative urgency, positive urgency, and sensation seeking across adolescence. Personal. Individ. Differ. 2016, 90, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Cyders, M.A.; Coskunpinar, A. Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clin. Psychol. Rev. 2011, 31, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Weafer, J.; Baggott, M.J.; de Wit, H. Test-retest reliability of behavioral measures of impulsive choice, impulsive action, and inattention. Exp. Clin. Psychopharmcol. 2013, 21, 475. [Google Scholar] [CrossRef]

| Demographics | |
| Age (M/SD) | 32.1/9.4 |
| Biological Sex (n, %) | |
| Female | 49/ 45.8% |
| Male | 58/ 54.2% |
| BMI (M/SD) | 27.0/5.2 |
| Ethnicity (n, %) | |
| White | 53/49.5% |
| Black/African American | 42/39.3% |
| Hispanic/Latino | 18/16.8% |
| Asian | 7/6.5% |
| Other | 5/4.7% |
| Household Income (M/SD) | $42,629/$38,301 |
| Justice System (n, %) | 58/54.2% |
| Study Variables | |
| Impulsive Urgency (M/SD) | 2.2/0.7 |
| Sensation Seeking (M/SD) | 2.8/0.7 |
| Low Conscientiousness (M/SD) | 1.8/0.4 |
| Past Month Risky Behaviors (M/SD) | 5.5/5.1 |
| Lifetime Risky Behaviors (M/SD) | 30.2/19.4 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|---|
| 1. Sensation Seeking | -- | ||||||
| 2. Impulsive Urgency | 0.25 * | -- | |||||
| 3. Low Conscientiousness | −0.01 | 0.50 ** | -- | ||||
| 4. Risky Behavior (Past Month) | 0.30 ** | 0.46 ** | 0.16 | -- | |||
| 5. Risky Behavior (Lifetime) | 0.21 * | 0.55 ** | 0.32 ** | 0.71 ** | -- | ||
| 6. Age | −0.24 * | −0.04 | −0.10 | 0.04 | 0.23 * | -- | |
| 7. Male Sex | 0.37 ** | 0.13 | 0.22 * | 0.17 | 0.17 | 0.06 | -- |
| 8. BMI | −0.06 | −0.06 | −0.01 | 0.05 | 0.01 | 0.24 * | 0.01 |
| Hemisphere | Annotation | Peak F-Value | Peak MNI (x,y,z) | No. of Vertices | Cluster Size (mm2) |
|---|---|---|---|---|---|
| Sensation Seeking | |||||
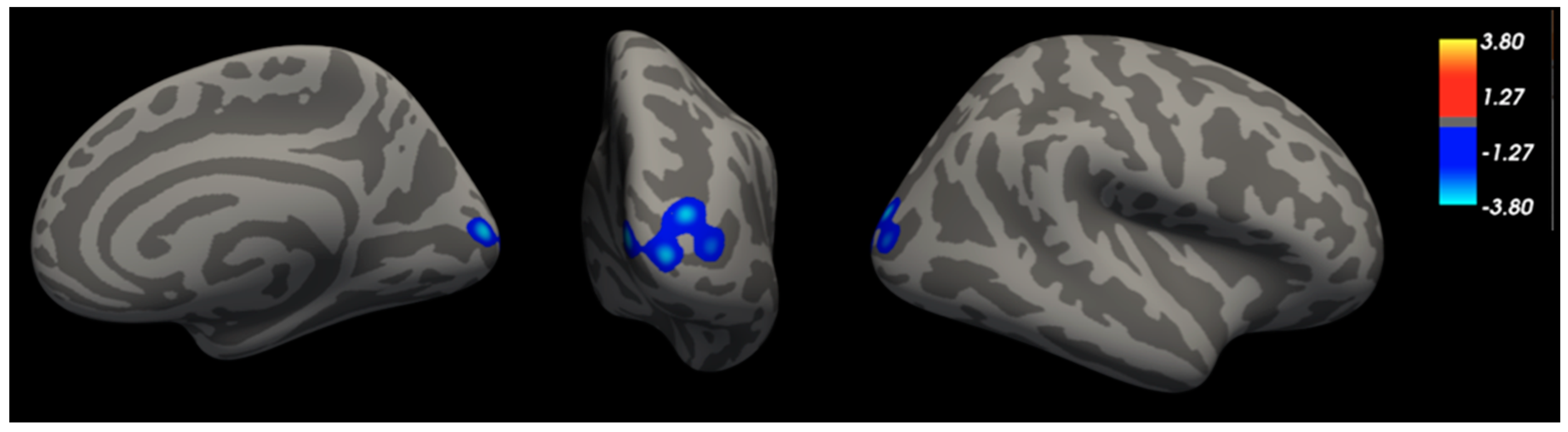
| RH | Pericalcarine CUN OP SOG MOG | −3.50 | 5.9, −87.8, 7.5 | 1707 | 1262.62 |
| Impulsive Urgency | |||||
| LH | PCUN SPG SOG CUN | 2.71 | −19.0, −72.8, 26.2 | 1783 | 969.34 |
| RH | Paracentral PCUN PCG | 3.81 | 17.8, −40.2, 42.4 | 2395 | 833.68 |
| Total Past Month Risky Behavior | Total Lifetime Risky Behavior | |
|---|---|---|
| β (SE) | β (SE) | |
| Step 1 | ||
| Age | 0.03 (0.05) | 0.22 (0.20) |
| Sex | 0.11 (1.00) | 0.11 (3.74) |
| Step 2 | ||
| RH Pericalcarine (Sensation Seeking) | −0.34 (3.59) ** | −0.32 (13.00) ** |
| RH Paracentral (Impulsive Urgency) | 0.20 (2.64) | 0.22 (9.58) + |
| LH Superior Parietal (Impulsive Urgency) | 0.20 (3.78) | 0.34 (13.70) ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miglin, R.; Bounoua, N.; Goodling, S.; Sheehan, A.; Spielberg, J.M.; Sadeh, N. Cortical Thickness Links Impulsive Personality Traits and Risky Behavior. Brain Sci. 2019, 9, 373. https://doi.org/10.3390/brainsci9120373
Miglin R, Bounoua N, Goodling S, Sheehan A, Spielberg JM, Sadeh N. Cortical Thickness Links Impulsive Personality Traits and Risky Behavior. Brain Sciences. 2019; 9(12):373. https://doi.org/10.3390/brainsci9120373
Chicago/Turabian StyleMiglin, Rickie, Nadia Bounoua, Shelly Goodling, Ana Sheehan, Jeffrey M. Spielberg, and Naomi Sadeh. 2019. "Cortical Thickness Links Impulsive Personality Traits and Risky Behavior" Brain Sciences 9, no. 12: 373. https://doi.org/10.3390/brainsci9120373
APA StyleMiglin, R., Bounoua, N., Goodling, S., Sheehan, A., Spielberg, J. M., & Sadeh, N. (2019). Cortical Thickness Links Impulsive Personality Traits and Risky Behavior. Brain Sciences, 9(12), 373. https://doi.org/10.3390/brainsci9120373





