Protective Effects of Scolopendra Water Extract on Trimethyltin-Induced Hippocampal Neurodegeneration and Seizures in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of SWE
2.2. Primary Hippocampal Cell Culture and SWE Treatment
2.3. Cytotoxicity and Cell Viability Examination
2.4. Preparation of SWE
2.5. Drugs, Treatments, and Tissue Sampling
2.6. Seizure Scoring
2.7. Immunohistochemistry: Microglia and Astrocyte Staining
2.8. Fluoro-Jade C (FJC) Staining
2.9. Statistical Analyses
3. Results
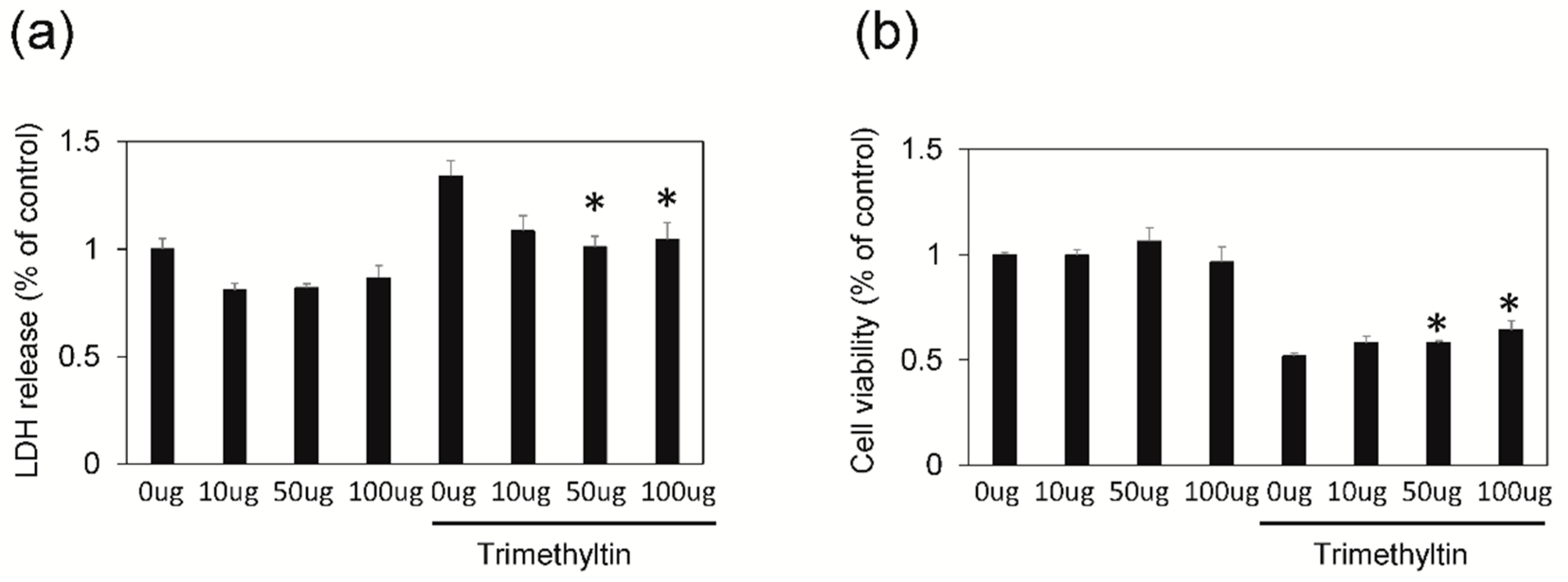
3.1. SWE Protects Hippocampal Cultured Neurons against TMT-Induced Neural Injury
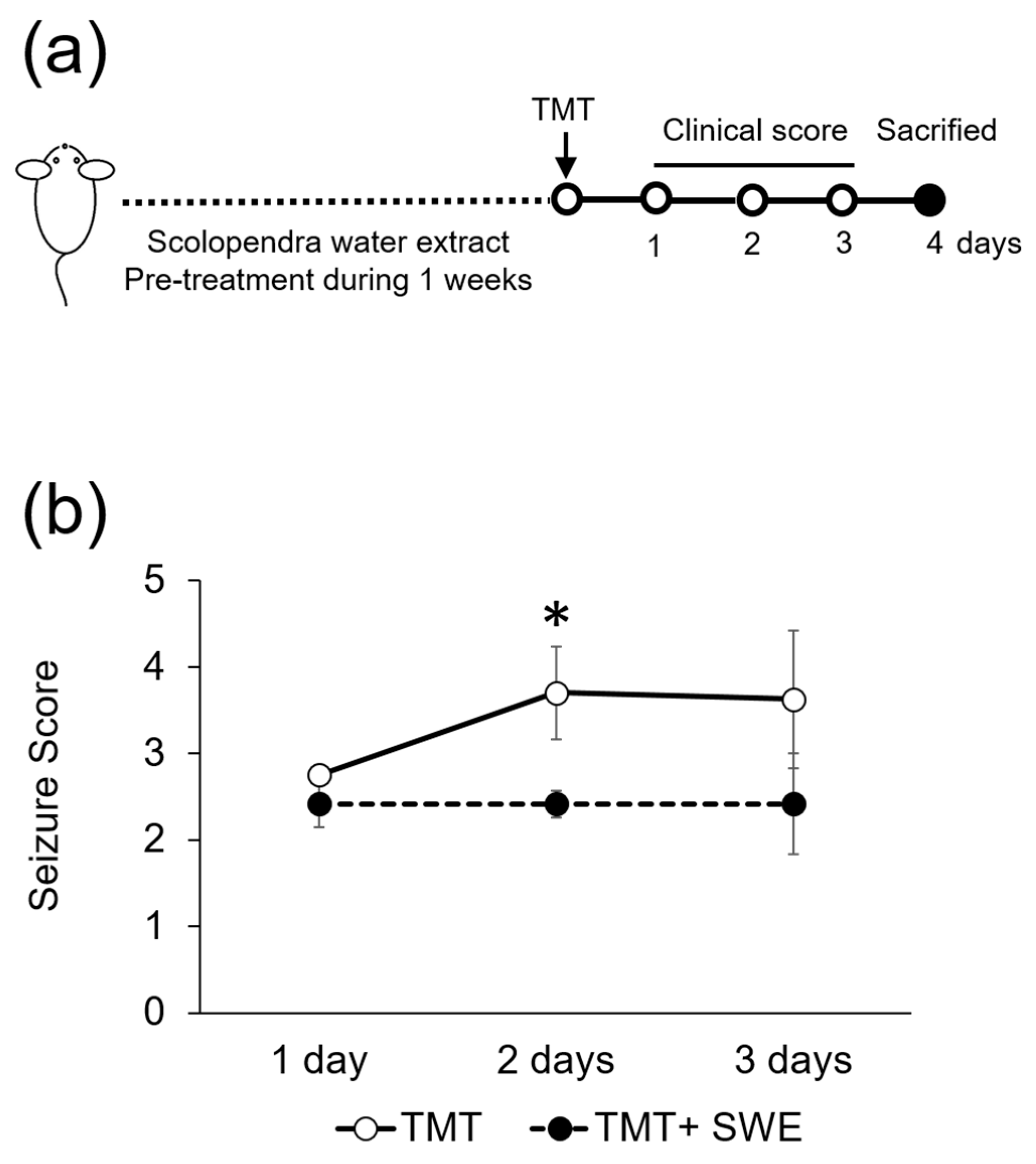
3.2. SWE Protects from TMT-Induced Seizures
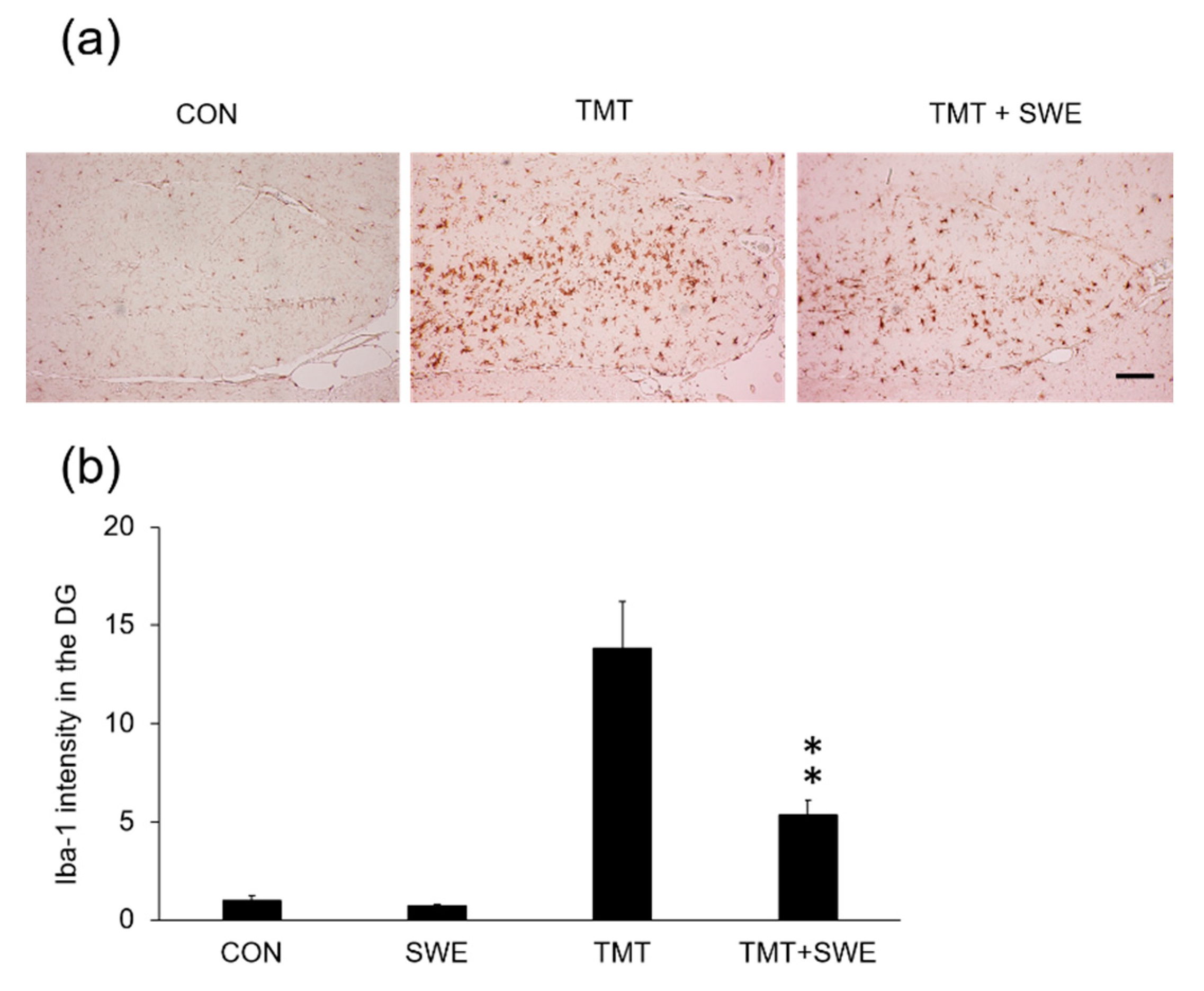
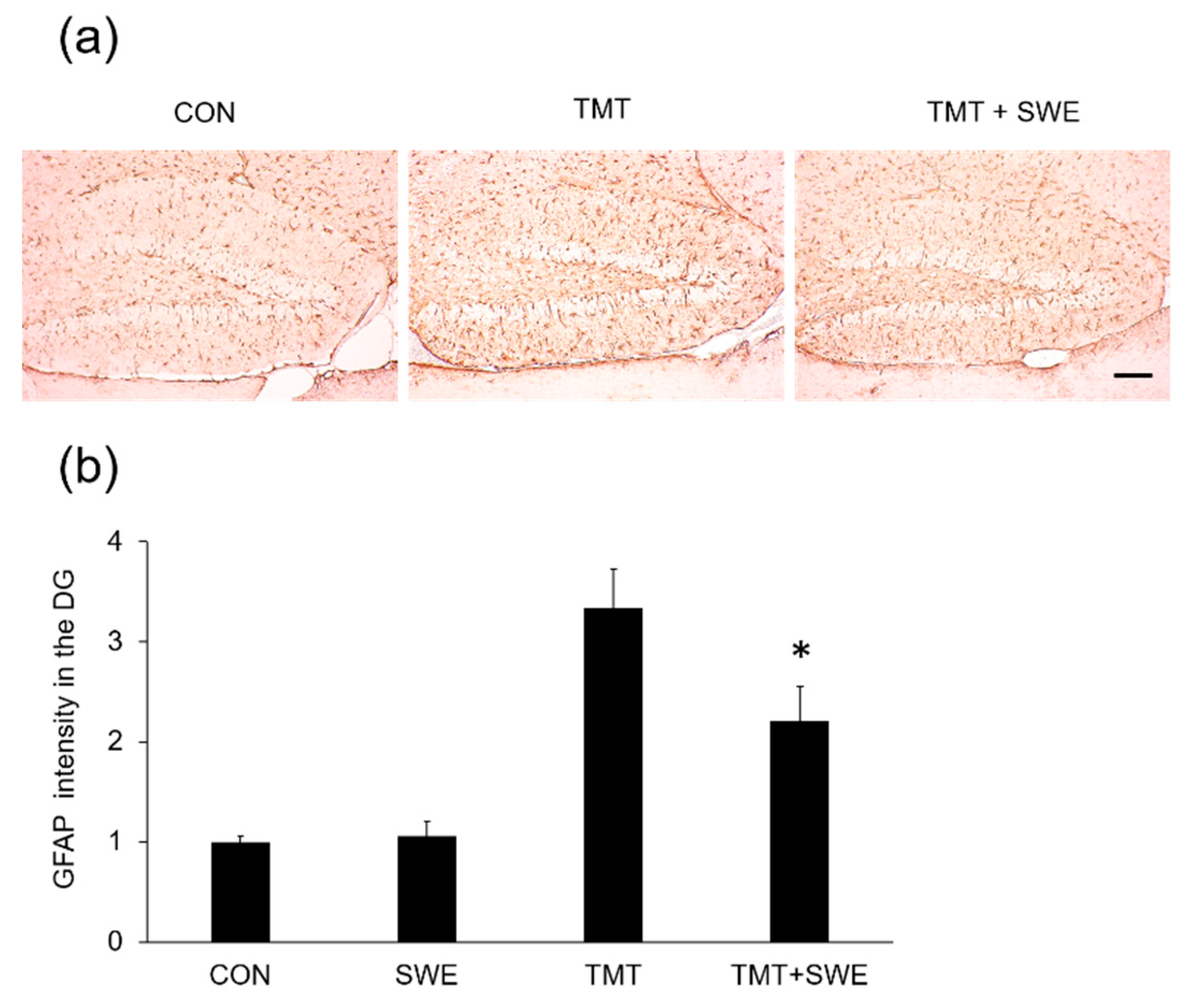
3.3. SWE Treatment Results in Decreased Levels of Iba-1- and GFAP-Positive Cells in the Hippocampi of TMT-Treated Mice
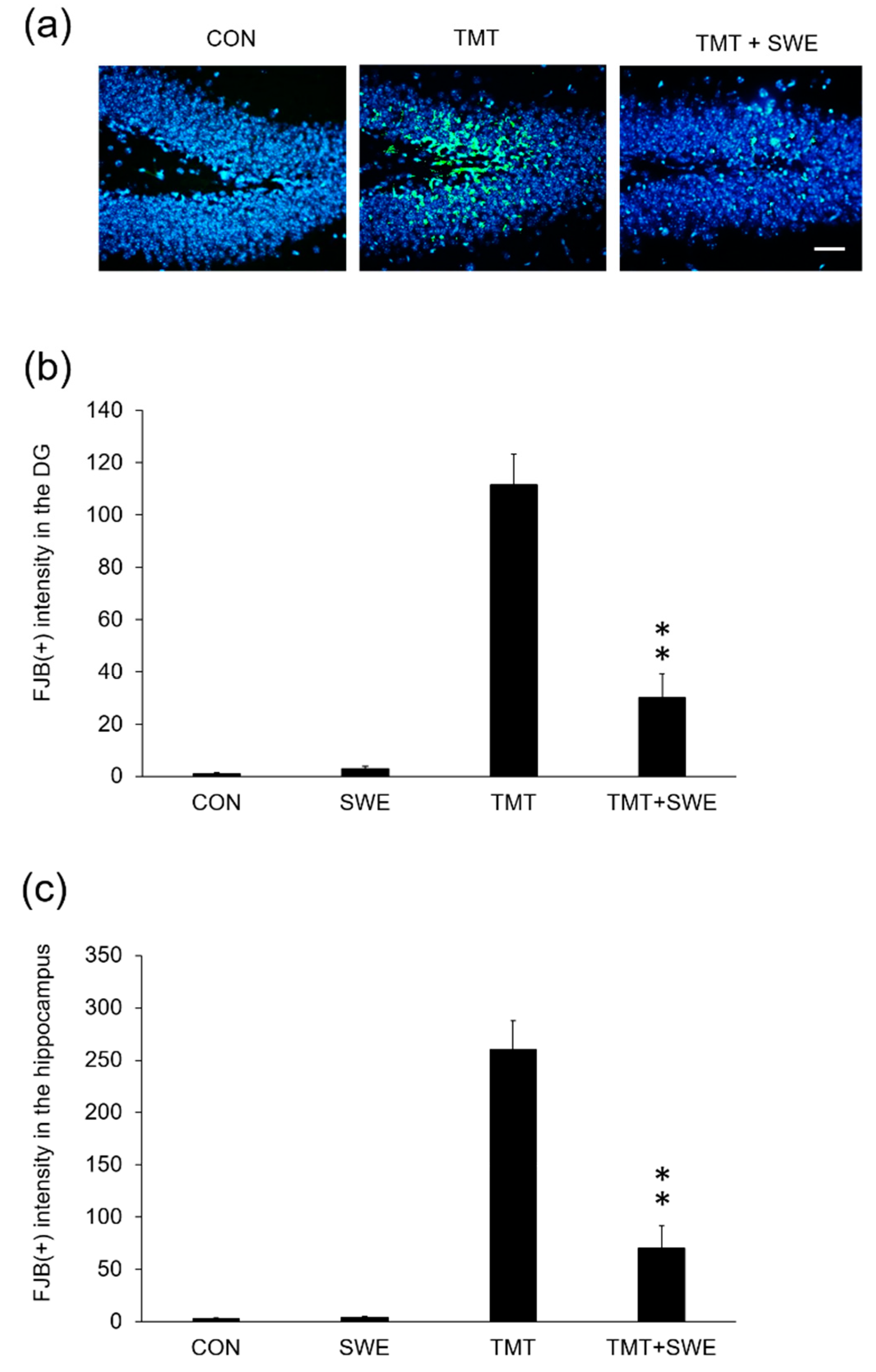
3.4. SWE Treatment Ameliorates Hippocampal Neuron Degeneration in TMT-Treated Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moon, S.-S.; Cho, N.; Shin, J.; Seo, Y.; Lee, C.O.; Choi, S.U. Jineol, a cytotoxic alkaloid from the centipede Scolopendra subspinipes. J. Nat. Prod. 1996, 59, 777–779. [Google Scholar] [CrossRef]
- Undheim, E.A.; King, G.F. On the venom system of centipedes (Chilopoda), a neglected group of venomous animals. Toxicon 2011, 57, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, D.; Zheng, L.; Zhan, Y.; Zhang, Y. Potential roles of Centipede Scolopendra extracts as a strategy against EGFR-dependent cancers. Am. J. Transl. Res. 2015, 7, 39–52. [Google Scholar] [PubMed]
- Lee, Y.-K.; Lim, S.-C.; Jung, T.-Y.; Han, S.-W.; Seo, J.-C. A case of radial nerve palsy treated with additional scolopendrae corpus herbal-acupuncture. J. Pharmacopunct. 2005, 8, 91–95. [Google Scholar]
- Jung, B.; Jang, K.; Song, C.; Ahn, C.B.; Acupunct, A.T. The Effects of Scolopendra subspinipes mutilans L. Koch aquacupuncture Extract Solution on the Analgesia and Anticonvulsion. Acupunct 1997, 14, 219–230. [Google Scholar]
- Kim, S.-C. The analysis of present condition and the method of medical treatment studies on Scolopendrid Herbal Acupuncture. J. Pharmacopunct. 2006, 9, 113–127. [Google Scholar]
- Kim, S.-C.; Seo, G.-Y.; Lee, S.-W.; Park, S.-J.; Kim, J.-H.; Ahn, S.-H.; Hwang, S.-Y. Biological activities of scolopendrid pharmacopuncture. J. Pharmacopunct. 2010, 13, 5–13. [Google Scholar] [CrossRef][Green Version]
- Besser, R.; Kramer, G.; Thumler, R.; Bohl, J.; Gutmann, L.; Hopf, H.C. Acute trimethyltin limbic-cerebellar syndrome. Neurology 1987, 37, 945–950. [Google Scholar] [CrossRef]
- Earley, B.; Burke, M.; Leonard, B.E. Behavioural, biochemical and histological effects of trimethyltin (TMT) induced brain damage in the rat. Neurochem. Int. 1992, 21, 351–366. [Google Scholar] [CrossRef]
- Feldman, R.G.; White, R.F.; Eriator, I.I. Trimethyltin encephalopathy. Arch. Neurol. 1993, 50, 1320–1324. [Google Scholar] [CrossRef]
- Perretta, G.; Righi, F.R.; Gozzo, S. Neuropathological and behavioral toxicology of trimethyltin exposure. Ann. Ist. Super. Sanita 1993, 29, 167–174. [Google Scholar] [PubMed]
- Lee, S.; Yang, M.; Kim, J.; Son, Y.; Kim, J.; Kang, S.; Ahn, W.; Kim, S.H.; Kim, J.C.; Shin, T.; et al. Involvement of BDNF/ERK signaling in spontaneous recovery from trimethyltin-induced hippocampal neurotoxicity in mice. Brain Res. Bull. 2016, 121, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yang, M.; Kim, J.; Kang, S.; Kim, J.; Kim, J.C.; Jung, C.; Shin, T.; Kim, S.H.; Moon, C. Trimethyltin-induced hippocampal neurodegeneration: A mechanism-based review. Brain Res. Bull. 2016, 125, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Geloso, M.C.; Corvino, V.; Michetti, F. Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes. Neurochem. Int. 2011, 58, 729–738. [Google Scholar] [CrossRef]
- Yang, M.; Kim, J.S.; Kim, J.; Kim, S.H.; Kim, J.C.; Kim, J.; Wang, H.; Shin, T.; Moon, C. Neurotoxicity of methotrexate to hippocampal cells in vivo and in vitro. Biochem. Pharmacol. 2011, 82, 72–80. [Google Scholar] [CrossRef]
- Yang, M.; Kim, J.; Kim, T.; Kim, S.H.; Kim, J.C.; Kim, J.; Takayama, C.; Hayashi, A.; Joo, H.G.; Shin, T.; et al. Possible involvement of galectin-3 in microglial activation in the hippocampus with trimethyltin treatment. Neurochem. Int. 2012, 61, 955–962. [Google Scholar] [CrossRef]
- Kim, J.; Yang, M.; Kim, S.H.; Kim, J.C.; Wang, H.; Shin, T.; Moon, C. Possible role of the glycogen synthase kinase-3 signaling pathway in trimethyltin-induced hippocampal neurodegeneration in mice. PLoS ONE 2013, 8, e70356. [Google Scholar] [CrossRef]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]
- Lee, S.; Kang, S.; Kim, J.; Yoon, S.; Kim, S.H.; Moon, C. Enhanced expression of immediate-early genes in mouse hippocampus after trimethyltin treatment. Acta Histochem. 2016, 118, 679–684. [Google Scholar] [CrossRef]
- Kurkowska-Jastrzebska, I.; Joniec, I.; Zaremba, M.; Fiedorowicz, A.; Czlonkowska, A.; Oderfeld-Nowak, B. Anti-myelin basic protein T cells protect hippocampal neurons against trimethyltin-induced damage. Neuroreport 2007, 18, 425–429. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Corvino, V.; Marchese, E.; Michetti, F.; Geloso, M.C. Neuroprotective strategies in hippocampal neurodegeneration induced by the neurotoxicant trimethyltin. Neurochem. Res. 2013, 38, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yang, M.; Kim, J.; Kim, J.; Son, Y.; Kwon, S.; Kim, S.H.; Kim, J.C.; Kang, S.S.; Wang, H.; et al. Nestin expression and glial response in the hippocampus of mice after trimethyltin treatment. Acta Histochem. 2014, 116, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, M.; Son, Y.; Jang, H.; Kim, D.; Kim, J.C.; Kim, S.H.; Kang, M.J.; Im, H.I.; Shin, T.; et al. Glial activation with concurrent up-regulation of inflammatory mediators in trimethyltin-induced neurotoxicity in mice. Acta Histochem. 2014, 116, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Mittelbronn, M.; Brabeck, C.; Mueller, C.A.; Schluesener, H.J. Expression of interleukin-16 by microglial cells in inflammatory, autoimmune, and degenerative lesions of the rat brain. J. Neuroimmunol. 2004, 146, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Teismann, P.; Schulz, J.B. Cellular pathology of Parkinson’s disease: Astrocytes, microglia and inflammation. Cell Tissue Res. 2004, 318, 149–161. [Google Scholar] [CrossRef]
- Liu, W.; Tang, Y.; Feng, J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life Sci. 2011, 89, 141–146. [Google Scholar] [CrossRef]
- Lenkov, D.N.; Volnova, A.B.; Pope, A.R.; Tsytsarev, V. Advantages and limitations of brain imaging methods in the research of absence epilepsy in humans and animal models. J. Neurosci. Methods 2013, 212, 195–202. [Google Scholar] [CrossRef]
- Vitaliti, G.; Pavone, P.; Marino, S.; Saporito, M.A.N.; Corsello, G.; Falsaperla, R. Molecular Mechanism Involved in the Pathogenesis of Early-Onset Epileptic Encephalopathy. Front. Mol. Neurosci. 2019, 12, 118. [Google Scholar] [CrossRef]
- Terrone, G.; Salamone, A.; Vezzani, A. Inflammation and Epilepsy: Preclinical Findings and Potential Clinical Translation. Curr. Pharm. Des. 2017, 23, 5569–5576. [Google Scholar] [CrossRef]
- Fabrizi, C.; Pompili, E.; Somma, F.; De Vito, S.; Ciraci, V.; Artico, M.; Lenzi, P.; Fornai, F.; Fumagalli, L. Lithium limits trimethyltin-induced cytotoxicity and proinflammatory response in microglia without affecting the concurrent autophagy impairment. J. Appl. Toxicol. 2017, 37, 207–213. [Google Scholar] [CrossRef] [PubMed]
- You, W.K.; Sohn, Y.D.; Kim, K.Y.; Park, D.H.; Jang, Y.; Chung, K.H. Purification and molecular cloning of a novel serine protease from the centipede, Scolopendra subspinipes mutilans. Insect Biochem. Mol. Biol. 2004, 34, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Han, K.; Yoon, J. Stability Test and Quantitative and Qualitative Analyses of the Amino Acids in Pharmacopuncture Extracted from Scolopendra subspinipes mutilans. J. Pharmacopunct. 2015, 18, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Datta, A.; Sarangi, B.; Kar, P.K.; Lahiri, S.C. Isolation, purification & pharmacodynamics of a toxin from the venom of the centipede Scolopendra subspinipes dehaani Brandt. Indian J. Exp. Biol. 1983, 21, 203–207. [Google Scholar]
- Li, X.; Chen, H.; Gan, M. Anti-inflammatory and analgesic effects of scolopendra from different habitats. Zhongguo Zhong Yao Za Zhi. 1996, 21, 498–499. [Google Scholar]
- Ren, Y.; Houghton, P.; Hider, R.C. Relevant activities of extracts and constituents of animals used in traditional Chinese medicine for central nervous system effects associated with Alzheimer’s disease. J. Pharm. Pharmacol. 2006, 58, 989–996. [Google Scholar] [CrossRef]
- Cai, M.; Choi, S.M.; Song, B.K.; Son, I.; Kim, S.; Yang, E.J. Scolopendra subspinipes mutilans attenuates neuroinflammation in symptomatic hSOD1(G93A) mice. J. Neuroinflamm. 2013, 10, 131. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.-S.; Ang, M.J.; Moon, B.C.; Kim, H.S.; Choi, G.; Lim, H.-S.; Kang, S.; Jeon, M.; Kim, S.-H.; Moon, C.; et al. Protective Effects of Scolopendra Water Extract on Trimethyltin-Induced Hippocampal Neurodegeneration and Seizures in Mice. Brain Sci. 2019, 9, 369. https://doi.org/10.3390/brainsci9120369
Seo Y-S, Ang MJ, Moon BC, Kim HS, Choi G, Lim H-S, Kang S, Jeon M, Kim S-H, Moon C, et al. Protective Effects of Scolopendra Water Extract on Trimethyltin-Induced Hippocampal Neurodegeneration and Seizures in Mice. Brain Sciences. 2019; 9(12):369. https://doi.org/10.3390/brainsci9120369
Chicago/Turabian StyleSeo, Yun-Soo, Mary Jasmin Ang, Byeong Cheol Moon, Hyo Seon Kim, Goya Choi, Hye-Sun Lim, Sohi Kang, Mijin Jeon, Sung-Ho Kim, Changjong Moon, and et al. 2019. "Protective Effects of Scolopendra Water Extract on Trimethyltin-Induced Hippocampal Neurodegeneration and Seizures in Mice" Brain Sciences 9, no. 12: 369. https://doi.org/10.3390/brainsci9120369
APA StyleSeo, Y.-S., Ang, M. J., Moon, B. C., Kim, H. S., Choi, G., Lim, H.-S., Kang, S., Jeon, M., Kim, S.-H., Moon, C., & Kim, J. S. (2019). Protective Effects of Scolopendra Water Extract on Trimethyltin-Induced Hippocampal Neurodegeneration and Seizures in Mice. Brain Sciences, 9(12), 369. https://doi.org/10.3390/brainsci9120369





