Brain Connectivity Analysis Under Semantic Vigilance and Enhanced Mental States
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
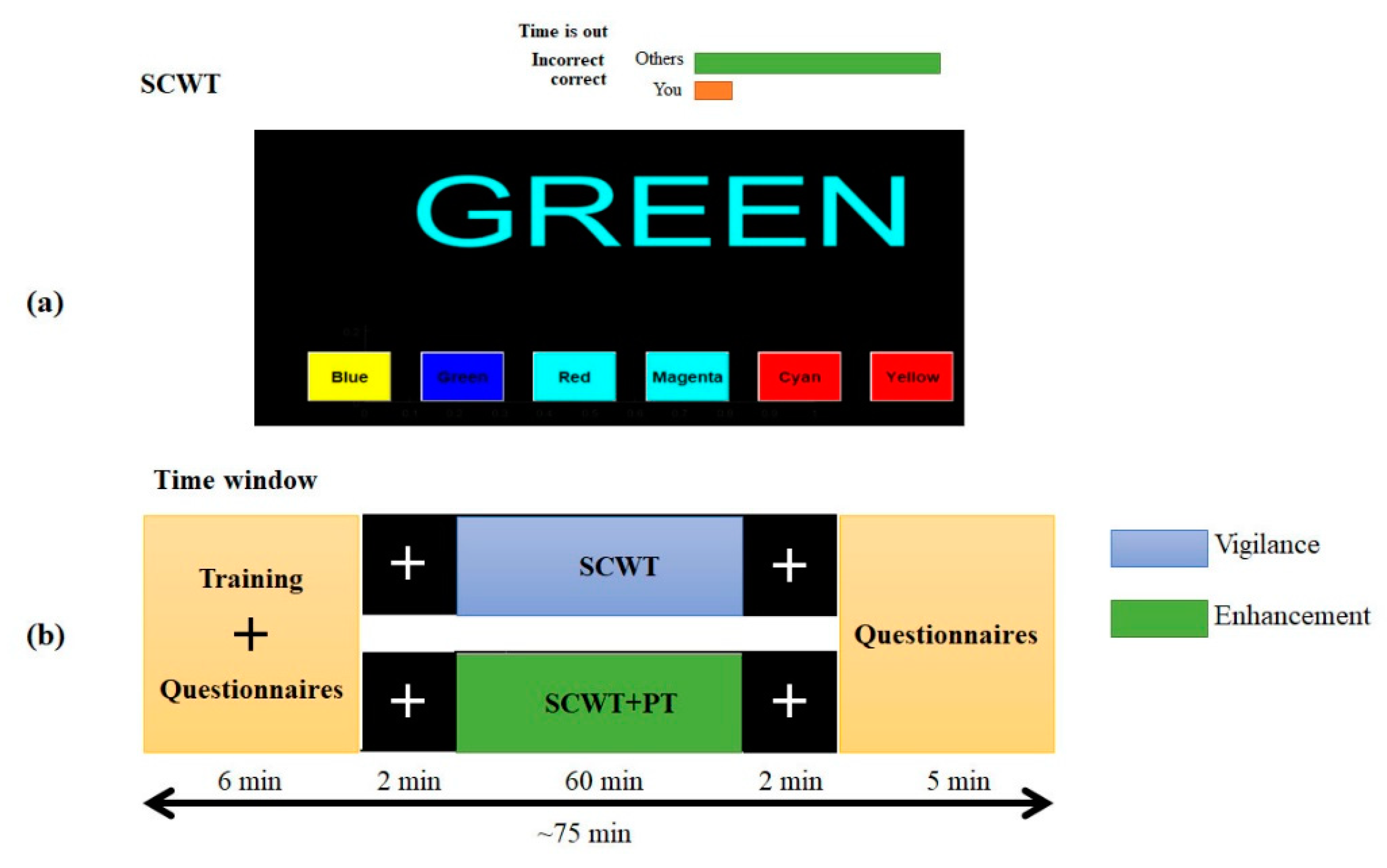
2.2. Experiment Design
2.2.1. Semantic Vigilance Task
2.2.2. Audio Stimulation
2.3. Subjective Evaluation
2.4. EEG Data Acquistion and Pre-Processing
2.5. Functional Connectivity
2.6. Graph Theory Analysis
2.6.1. Nodal Degree ()
2.6.2. Nodal Strength ()
2.6.3. Clustering Coefficient (CC)
2.6.4. Characteristics Path Length (PL)
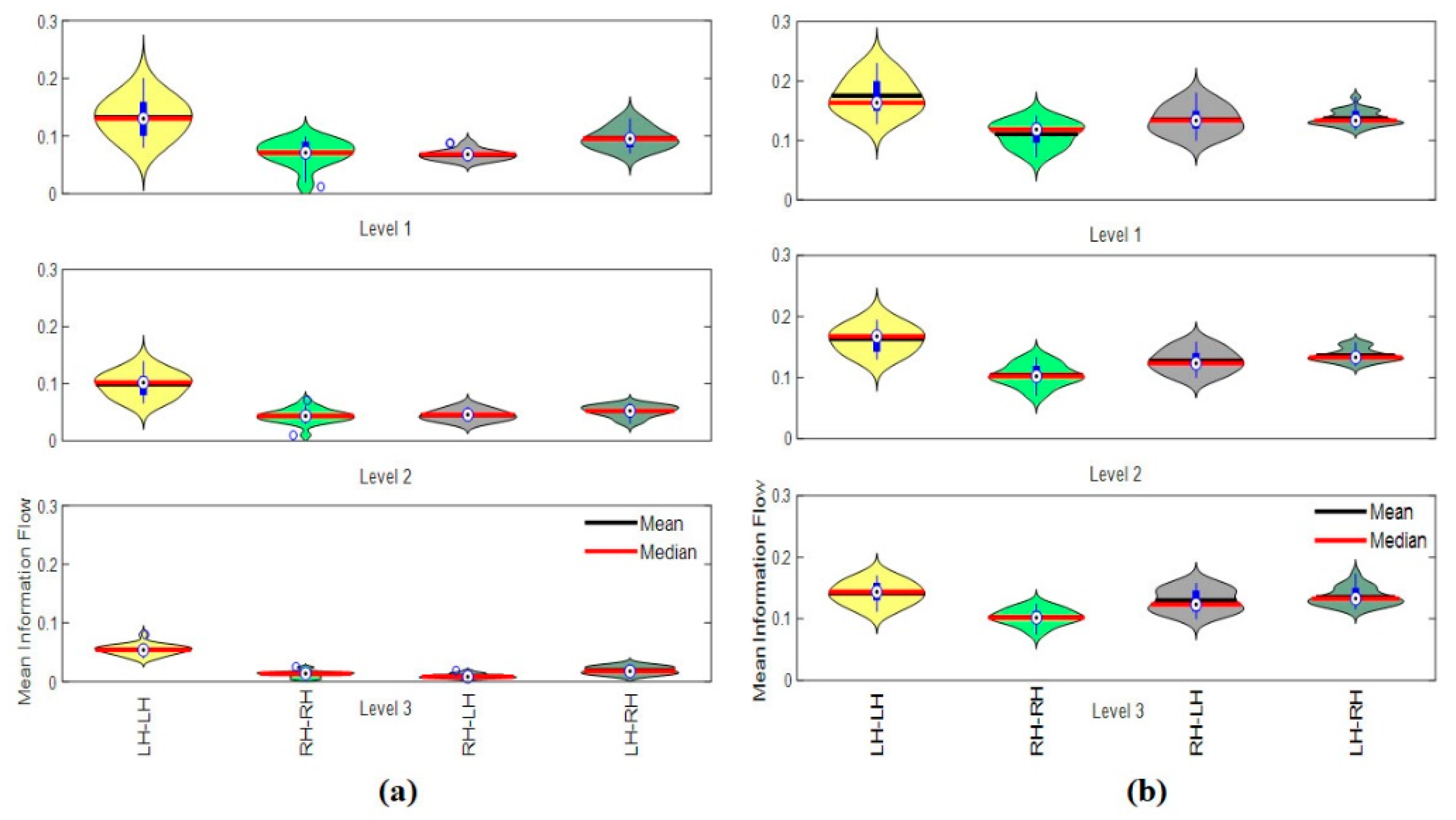
2.6.5. Hemispheric Information Flow (HIF)
2.7. Statistical Analysis
3. Results
3.1. Behavioral Data
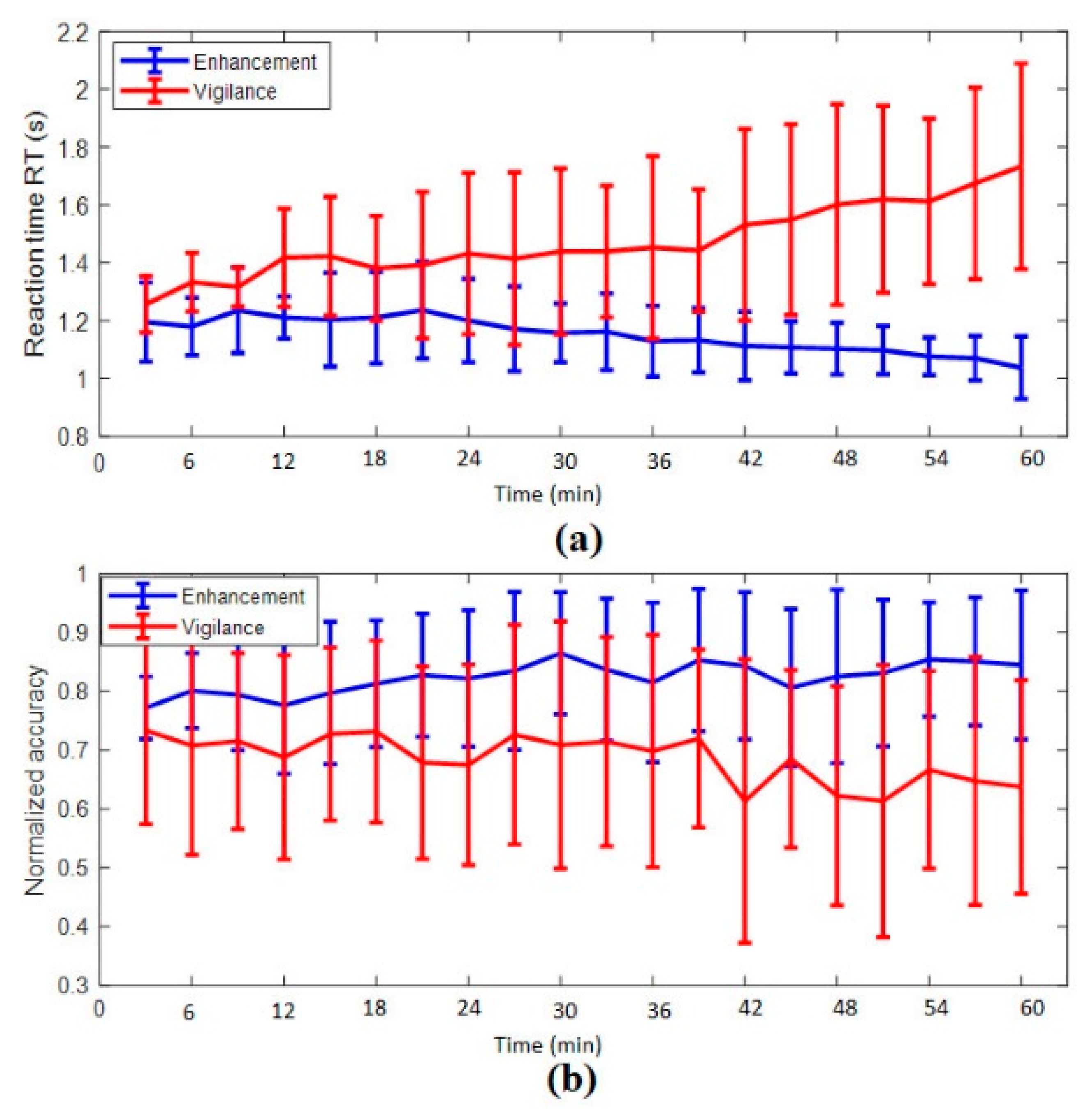
3.1.1. Reaction Time
3.1.2. Accuracy
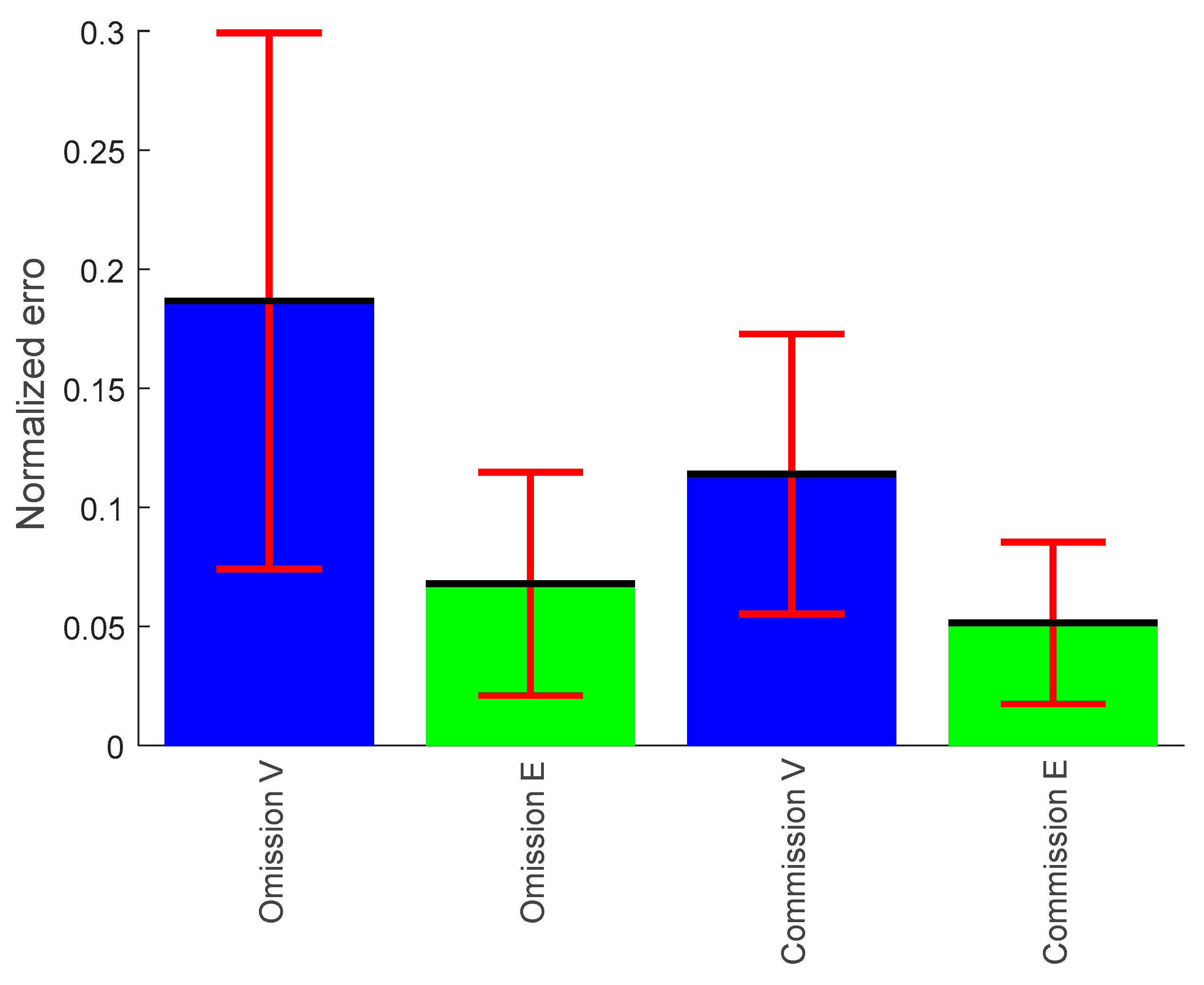
3.1.3. Omission Error
3.1.4. Commission Error
3.2. Subjective Data
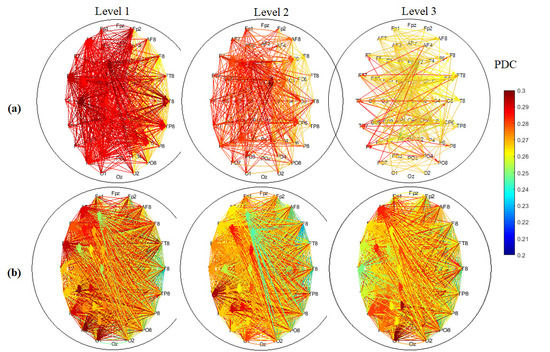
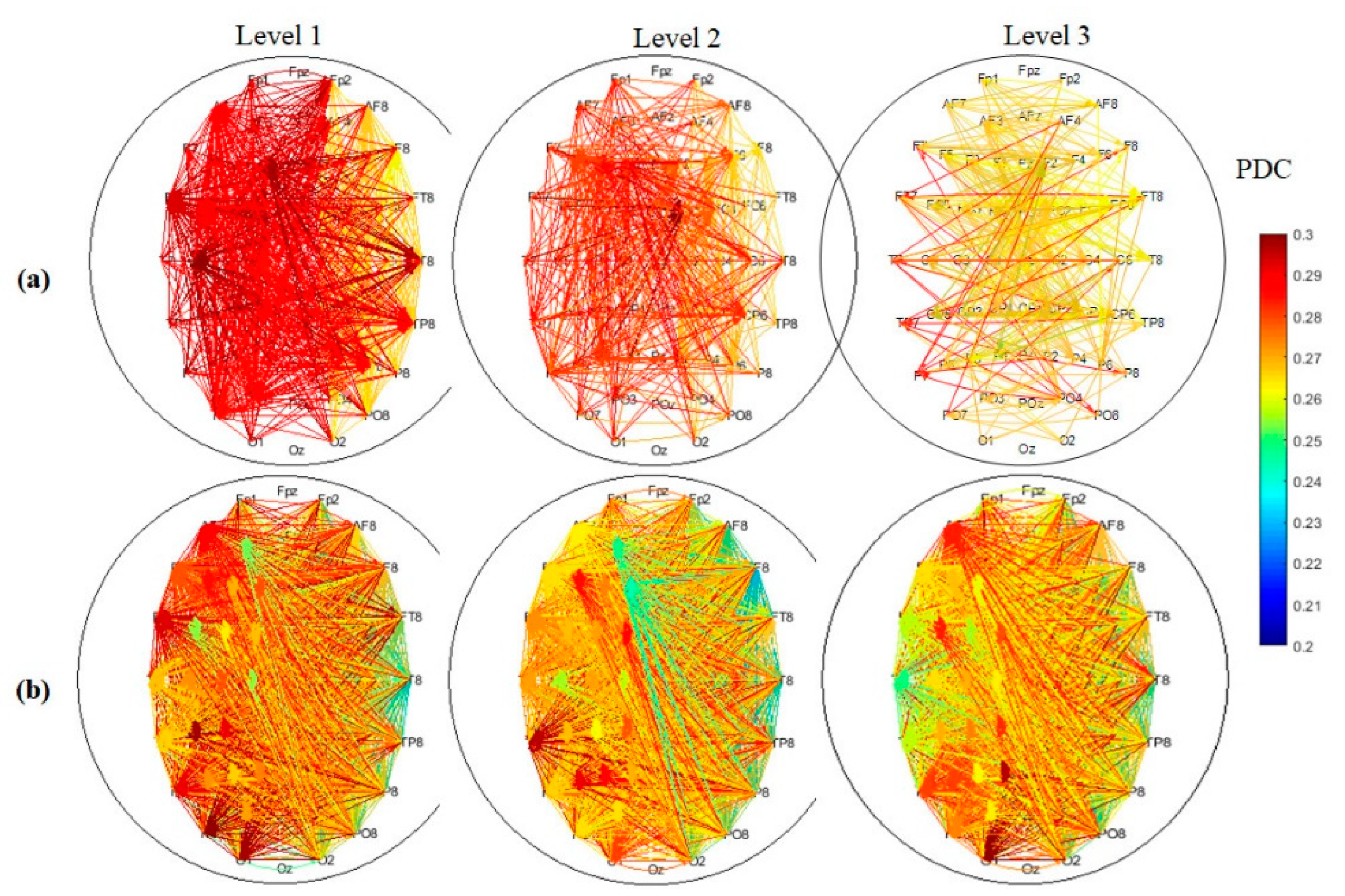
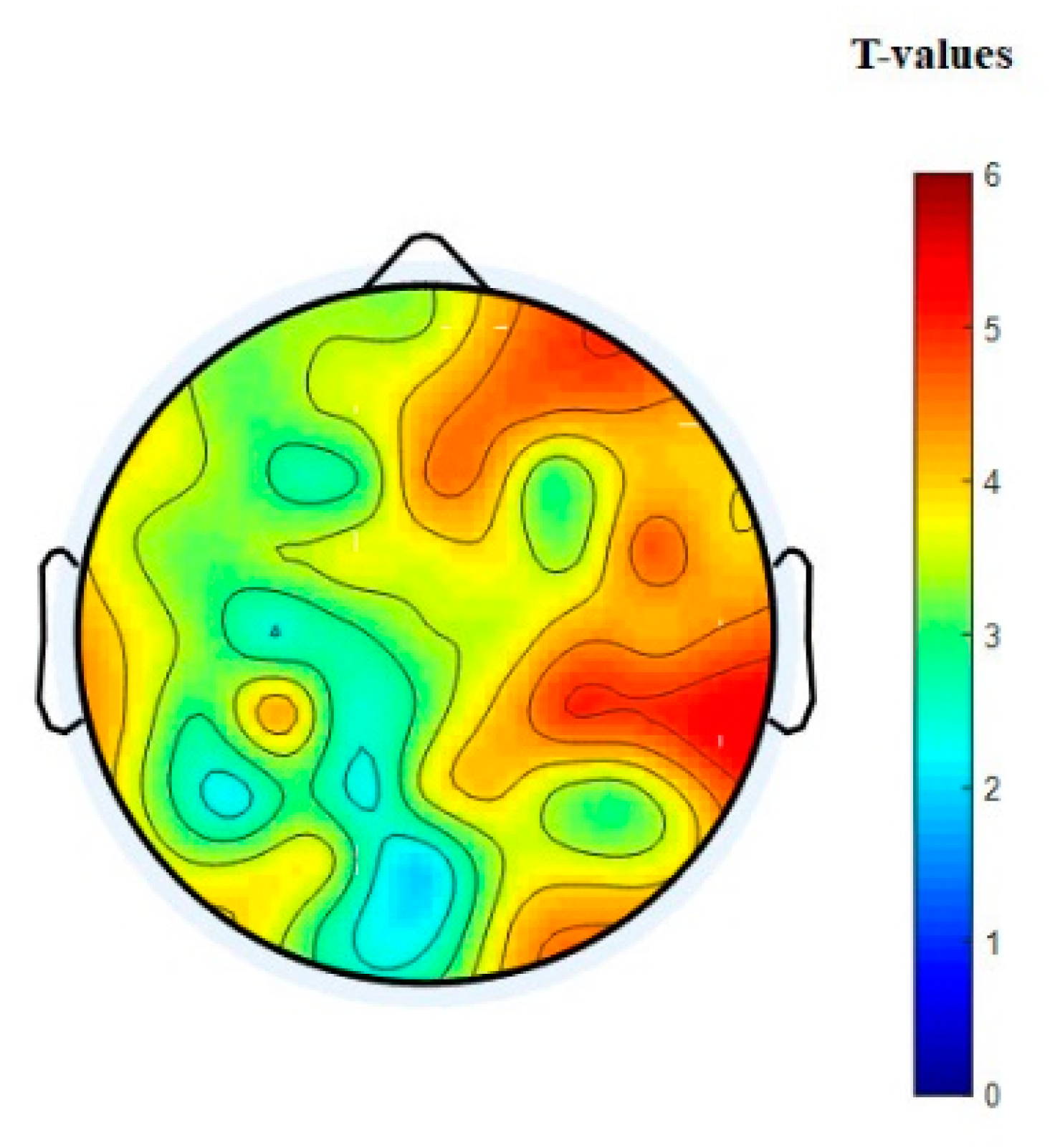
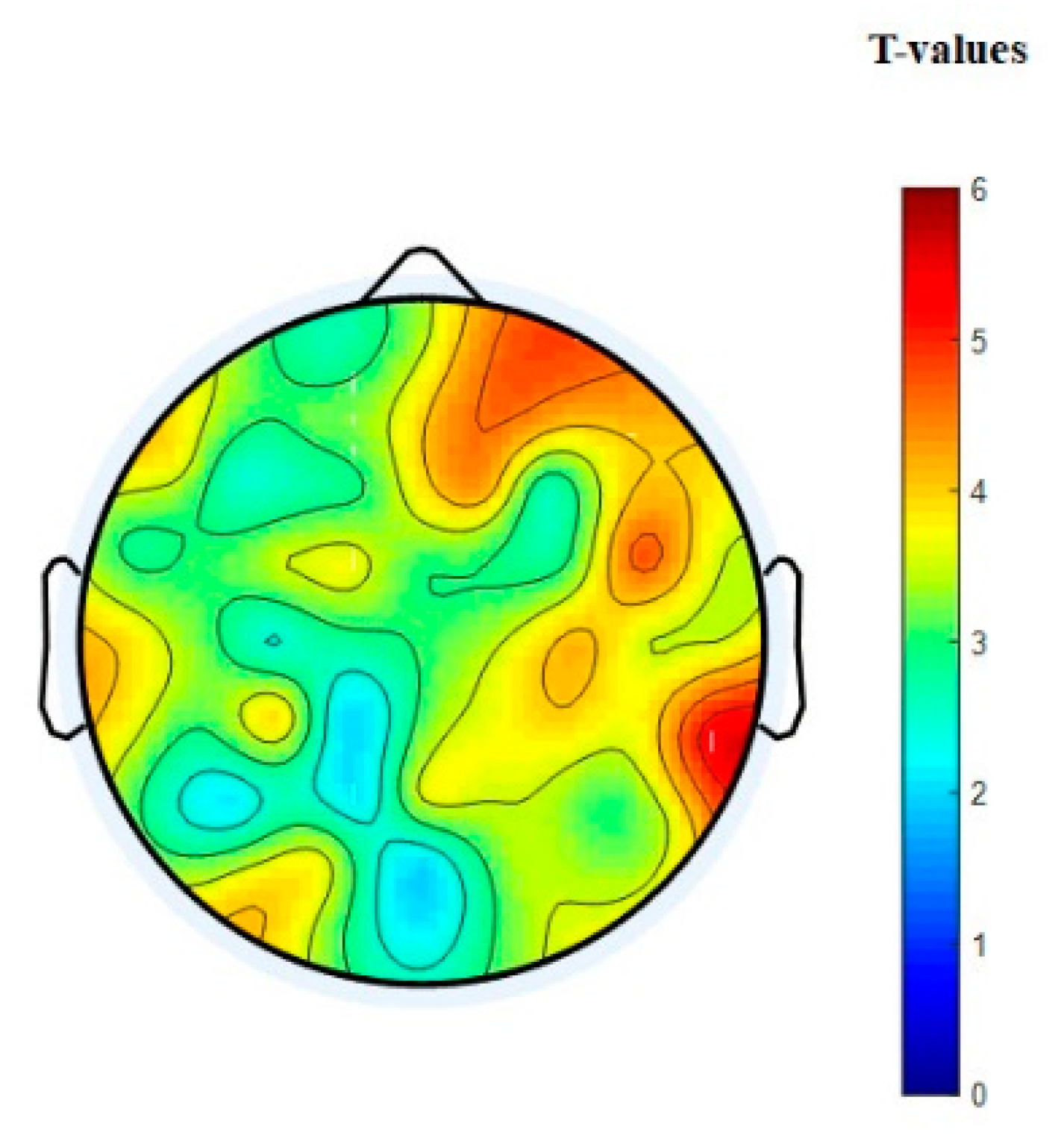
3.3. EEG Connectivity
3.4. Graph Theory Analysis of Brain Network
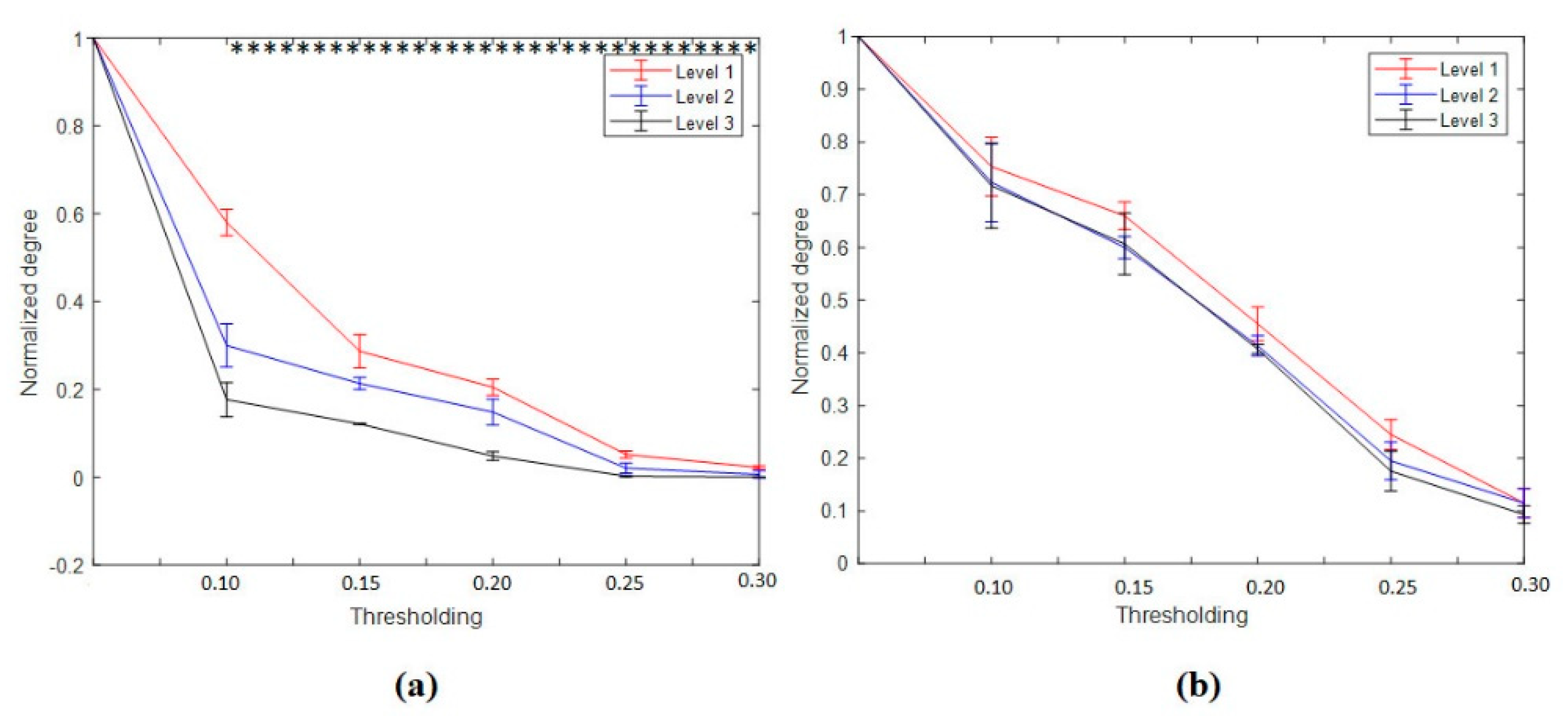
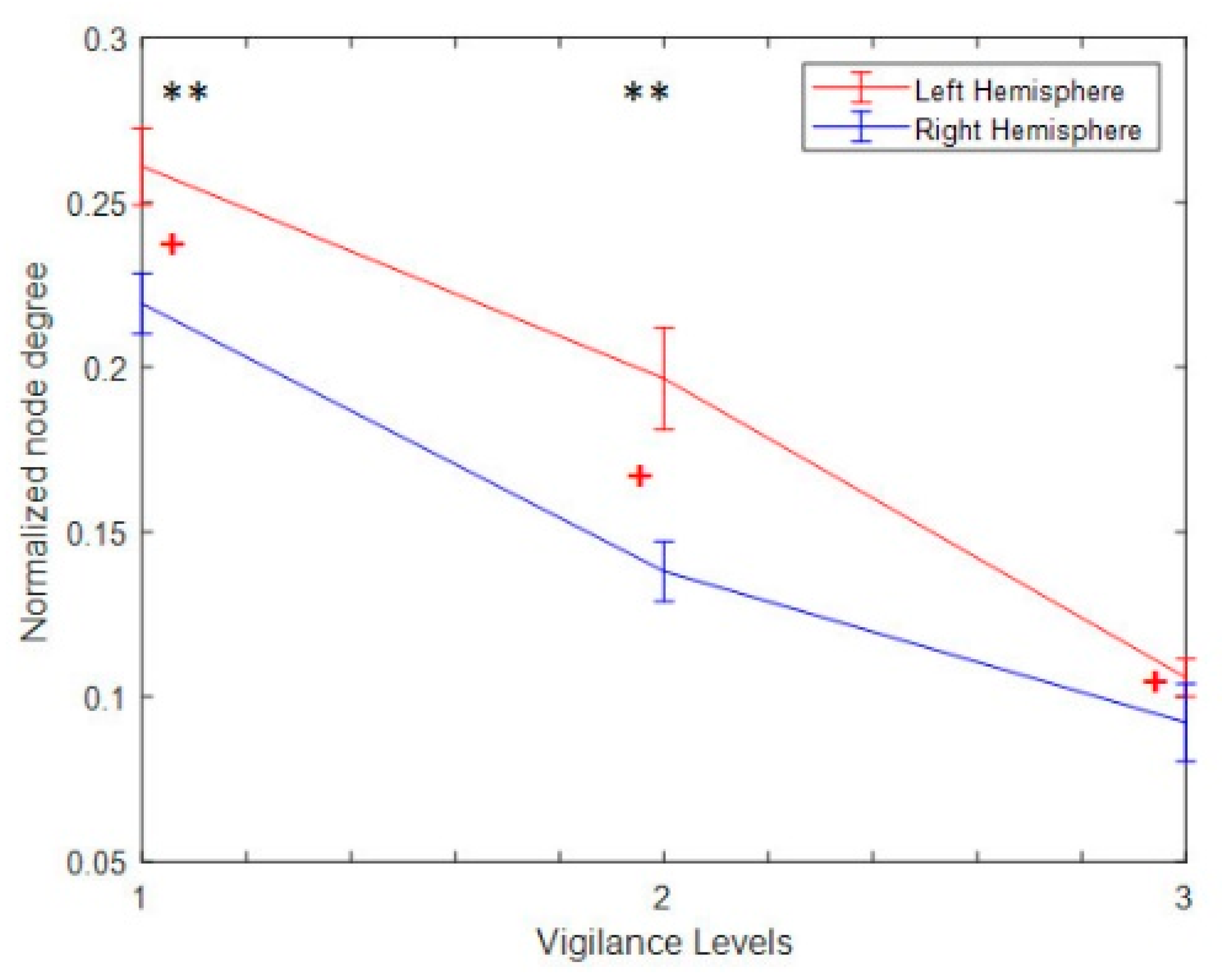
3.4.1. Nodal Degree Index
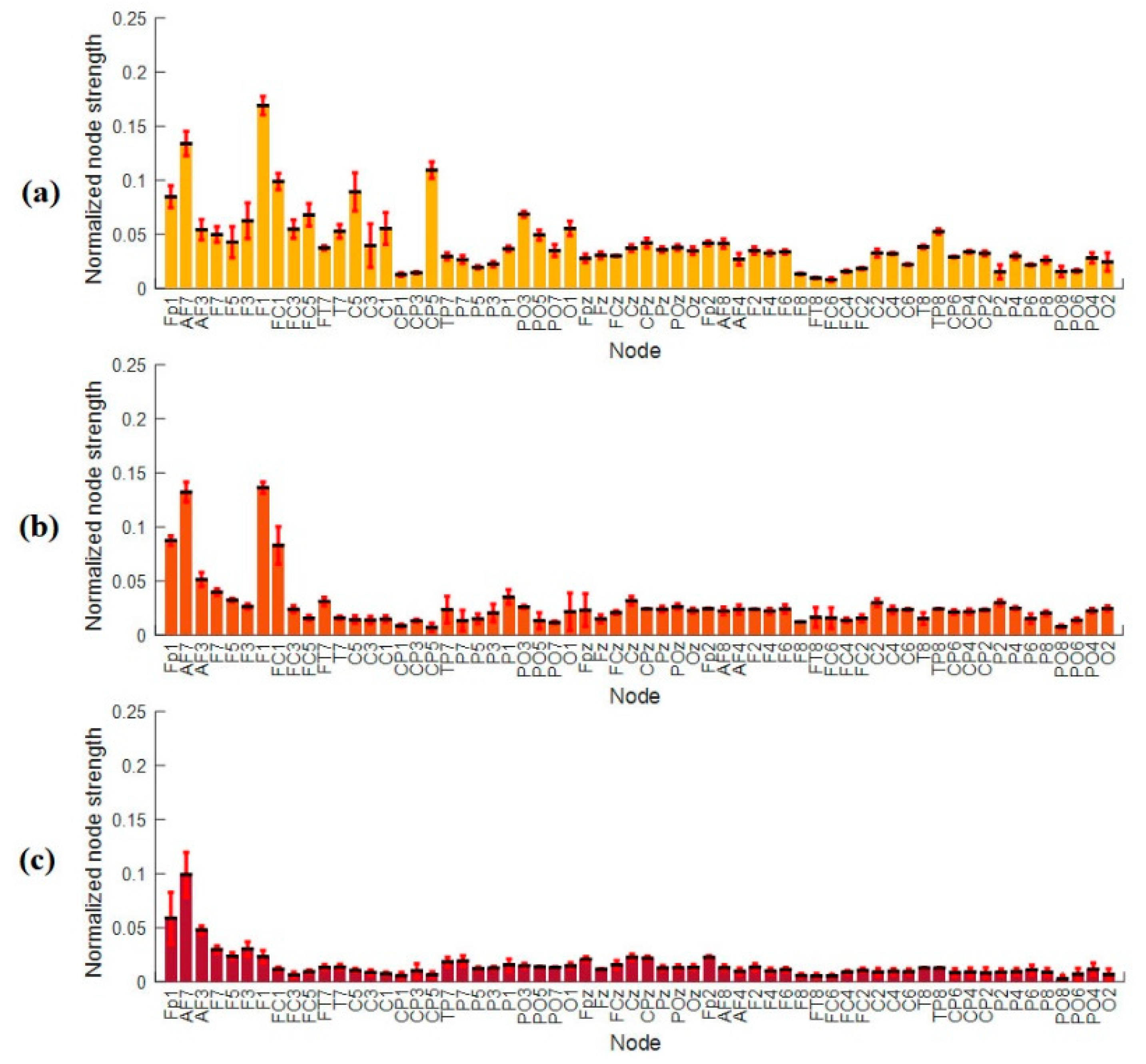
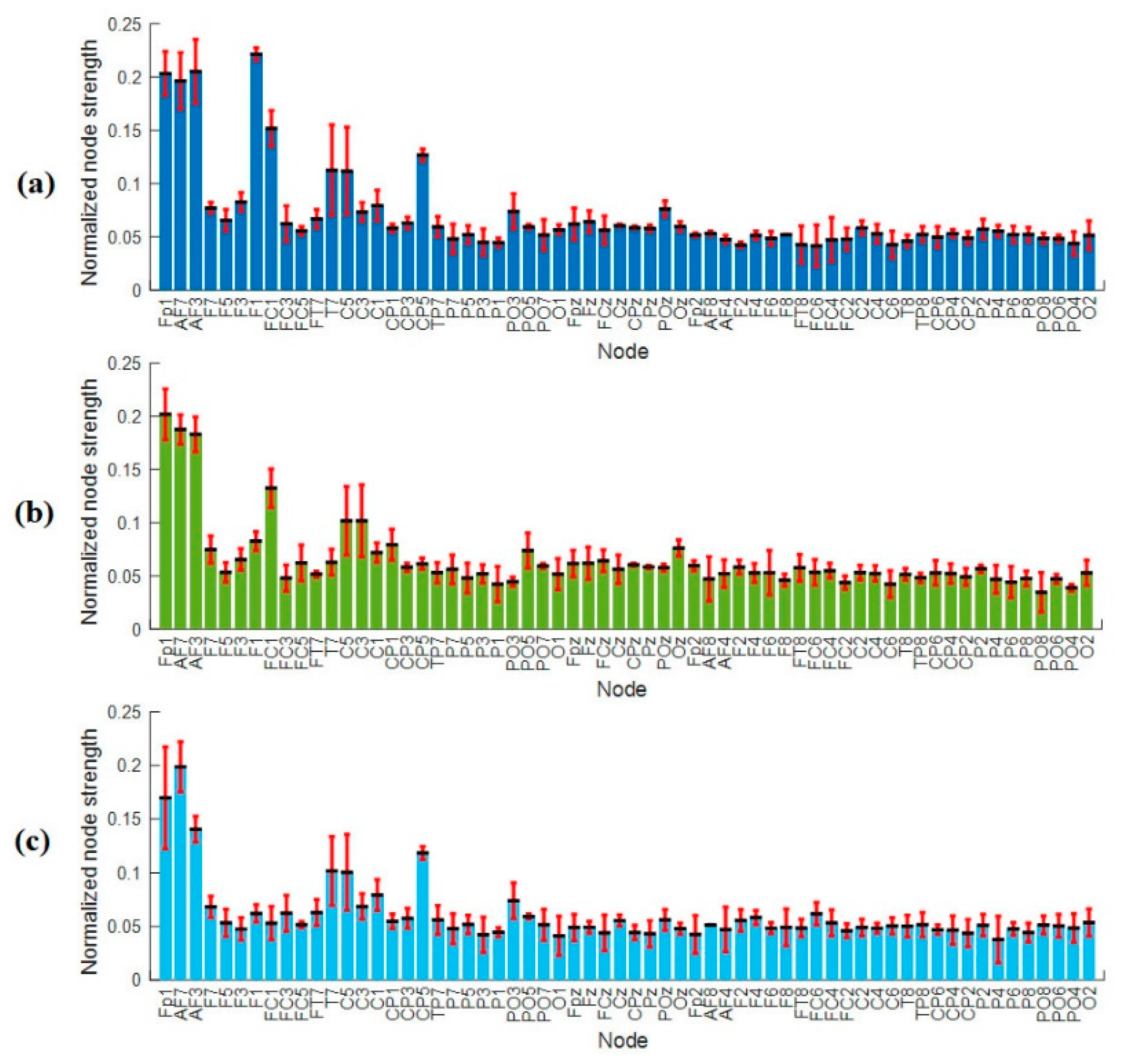
3.4.2. Nodal Strength
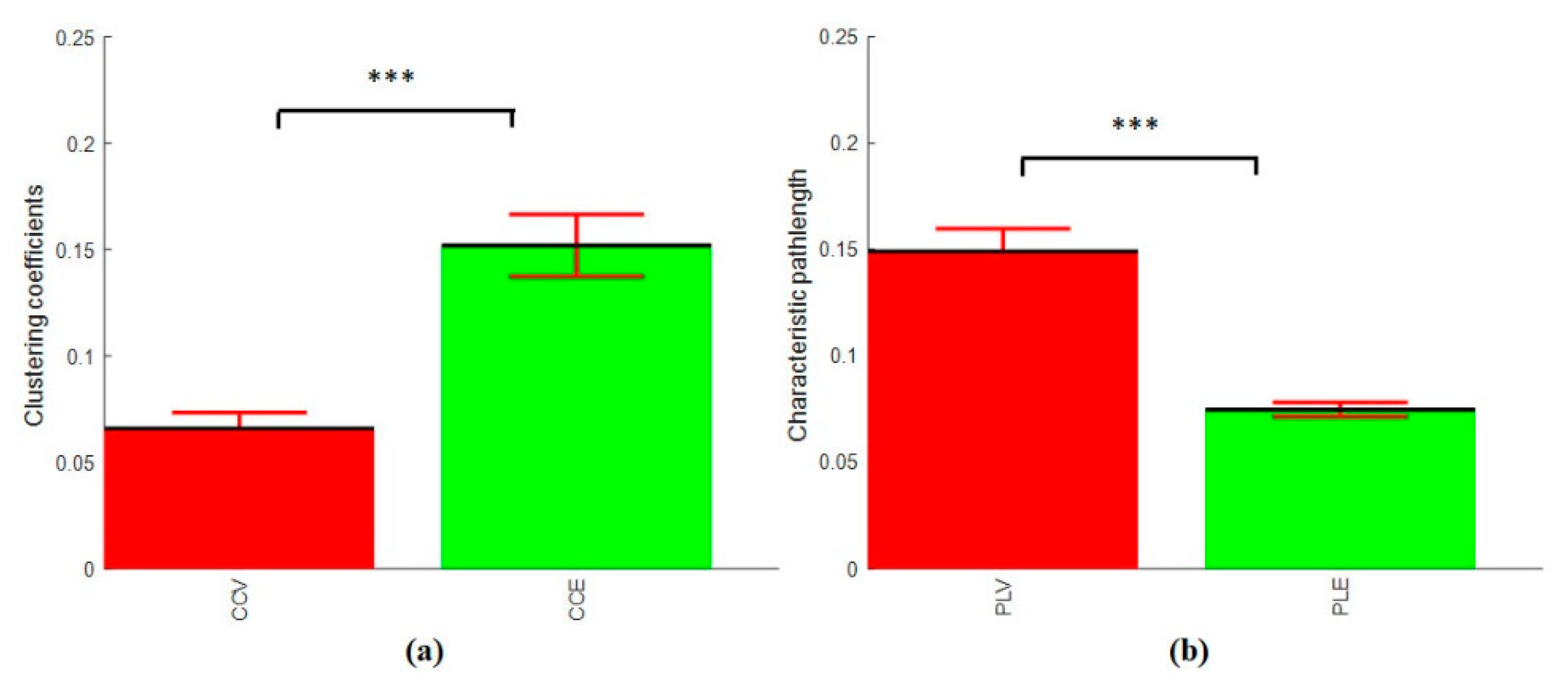
3.4.3. Clustering Coefficients and Characteristics Path Length
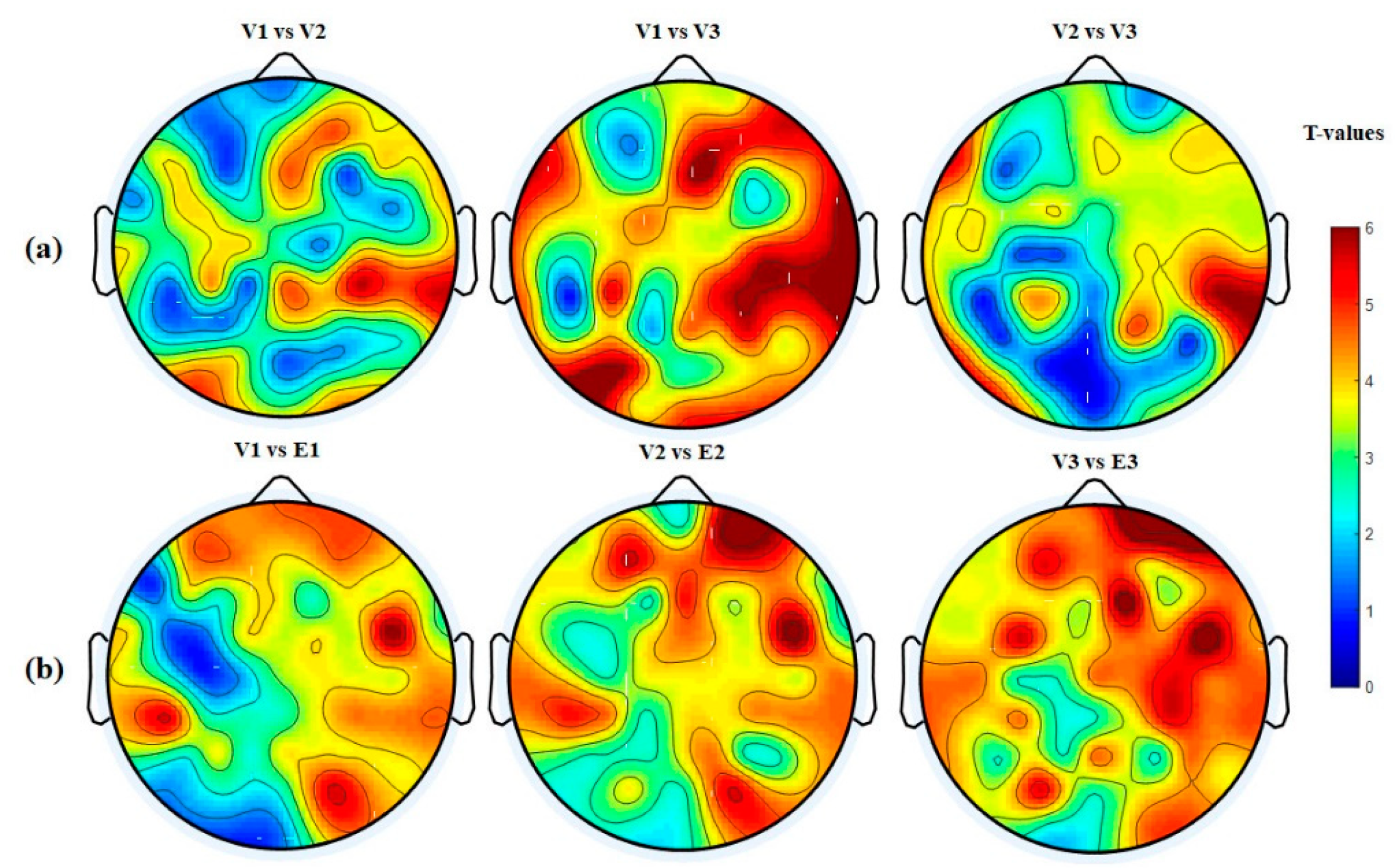
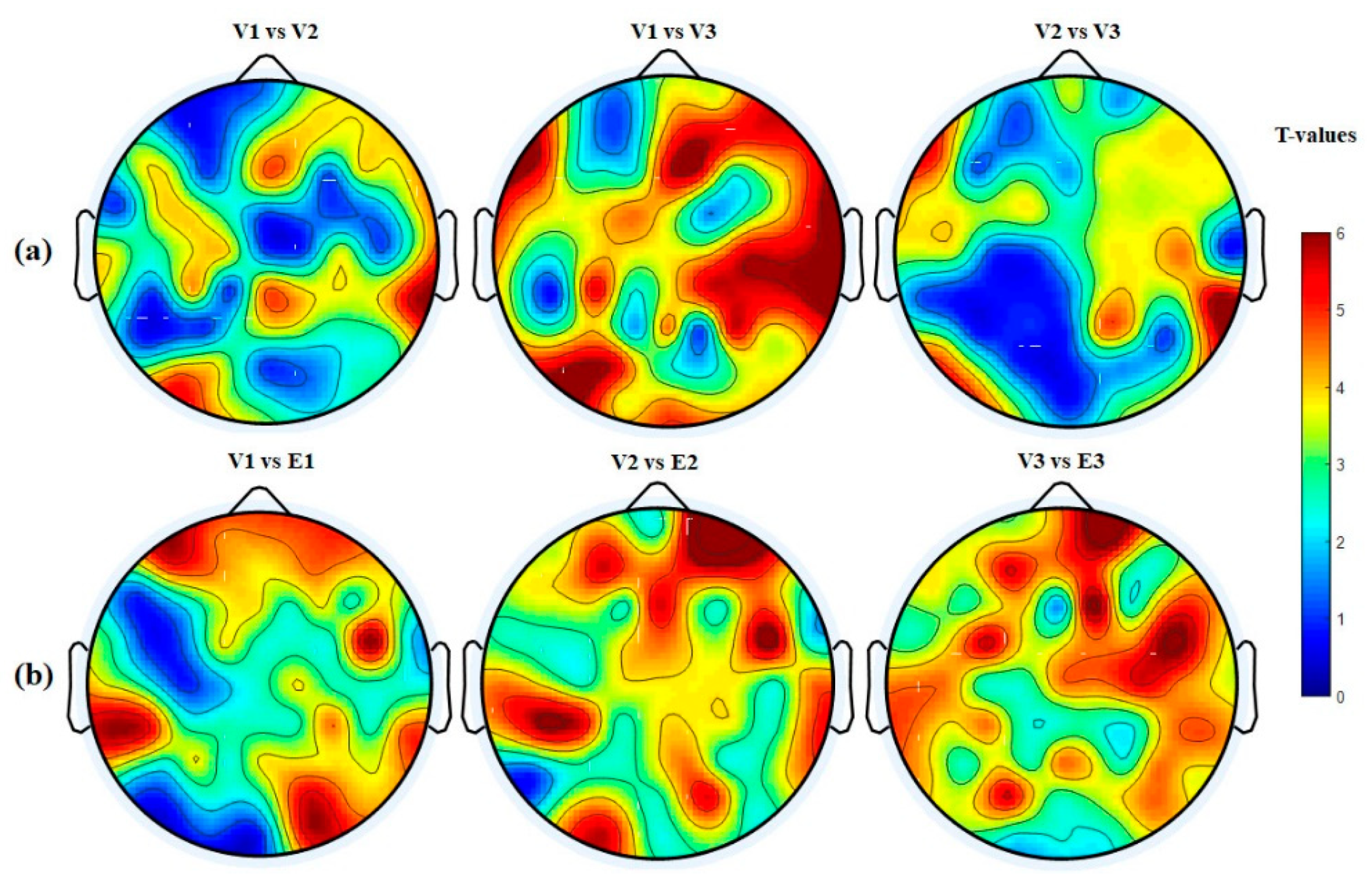
3.4.4. Hemispheric Analysis
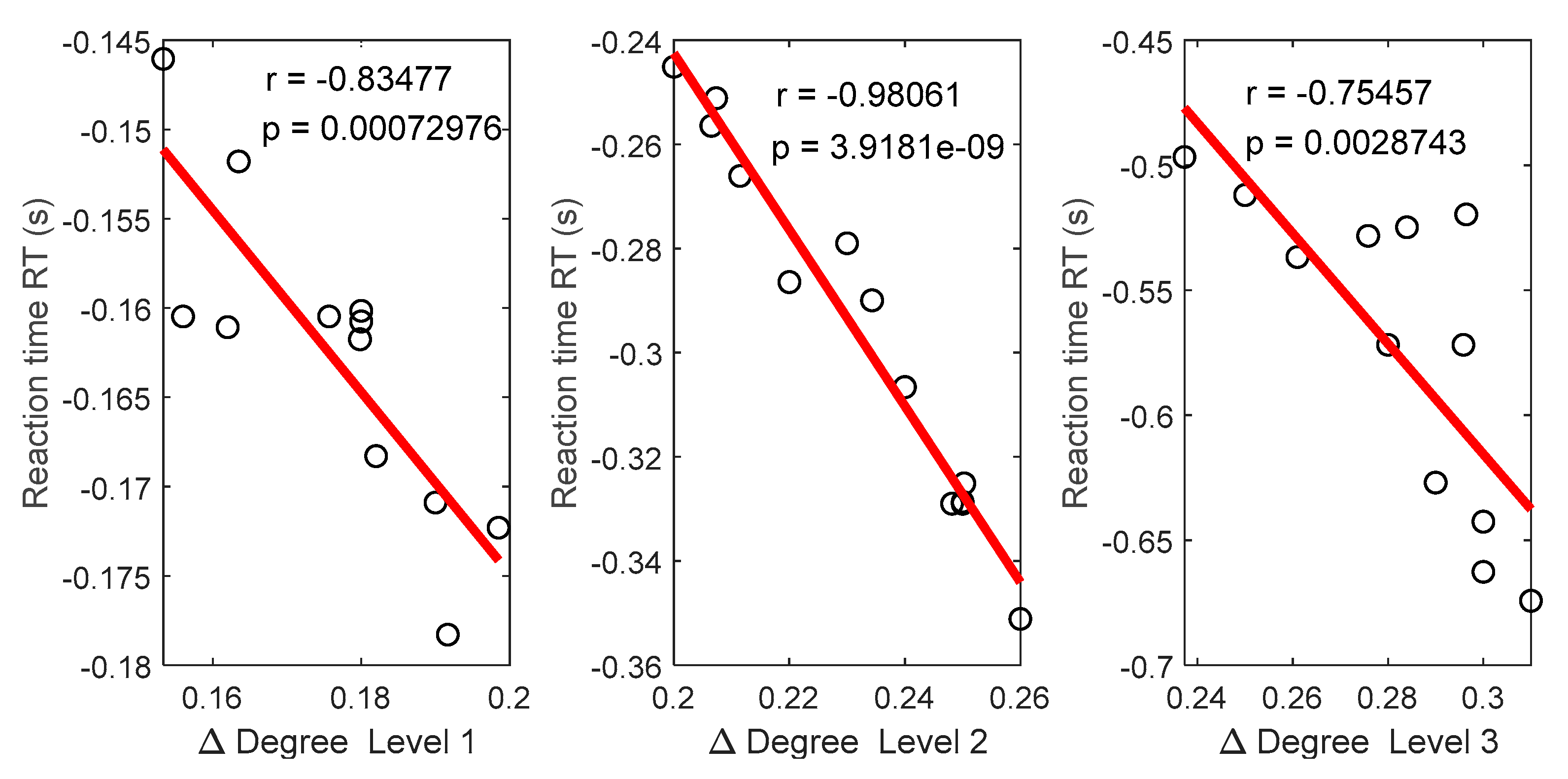
3.5. Relationships between Functional Connectivity Estimators and Behavioral Responses
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meuter, R.F.; Lacherez, P.F. When and why threats go undetected: Impacts of event rate and shift length on threat detection accuracy during airport baggage screening. Hum. Factors 2016, 58, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Körber, M.; Cingel, A.; Zimmermann, M.; Bengler, K. Vigilance decrement and passive fatigue caused by monotony in automated driving. Procedia Manuf. 2015, 3, 2403–2409. [Google Scholar] [CrossRef]
- Näsholm, E.; Rohlfing, S.; Sauer, J.D. Pirate stealth or inattentional blindness? The effects of target relevance and sustained attention on security monitoring for experienced and naïve operators. PLoS ONE 2014, 9, e86157. [Google Scholar]
- Sawyer, B.D.; Finomore, V.S.; Funke, G.J.; Mancuso, V.F.; Funke, M.E.; Matthews, G.; Warm, J.S. Cyber vigilance: Effects of signal probability and event rate. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2014, 58, 1771–1775. [Google Scholar] [CrossRef]
- Small, A.J.; Wiggins, M.W.; Loveday, T. Cue-based processing capacity, cognitive load and the completion of simulated short-duration vigilance tasks in power transmission control. Appl. Cogn. Psychol. 2014, 28, 481–487. [Google Scholar] [CrossRef]
- Ko, L.-W.; Komarov, O.; Hairston, W.D.; Jung, T.-P.; Lin, C.-T. Sustained attention in real classroom settings: An eeg study. Front. Hum. Neurosci. 2017, 11, 388. [Google Scholar] [CrossRef]
- Helton, W.S.; Warm, J.S. Signal salience and the mindlessness theory of vigilance. Acta Psychol. 2008, 129, 18–25. [Google Scholar] [CrossRef]
- Thomson, D.R.; Besner, D.; Smilek, D. A resource-control account of sustained attention: Evidence from mind-wandering and vigilance paradigms. Perspect. Psychol. Sci. 2015, 10, 82–96. [Google Scholar] [CrossRef]
- Kurzban, R.; Duckworth, A.; Kable, J.W.; Myers, J. An opportunity cost model of subjective effort and task performance. Behav. Brain Sci. 2013, 36, 661–679. [Google Scholar] [CrossRef]
- Gartenberg, D.; Veksler, B.Z.; Gunzelmann, G.; Trafton, J.G. An ACT-R process model of the signal duration phenomenon of vigilance. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2014, 58, 909–913. [Google Scholar] [CrossRef]
- Gartenberg, D.; Gunzelmann, G.; Hassanzadeh-Behbahani, S.; Trafton, J.G. Examining the role of task requirements in the magnitude of the vigilance decrement. Front. Psychol. 2018, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Borragán, G.; Slama, H.; Bartolomei, M.; Peigneux, P. Cognitive fatigue: A time-based resource-sharing account. Cortex 2017, 89, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Teichner, W.H. The detection of a simple visual signal as a function of time of watch. Hum. Factors 1974, 16, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Al-Shargie, F.; Tariq, U.; Mir, H.; Alawar, H.; Babiloni, F.; Al-Nashash, H. Vigilance decrement and enhancement techniques: A review. Brain Sci. 2019, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Warm, J.S.; Parasuraman, R.; Matthews, G. Vigilance requires hard mental work and is stressful. Hum. Factors 2008, 50, 433–441. [Google Scholar] [CrossRef] [PubMed]
- See, J.E.; Howe, S.R.; Warm, J.S.; Dember, W.N. Meta-analysis of the sensitivity decrement in vigilance. Psychol. Bull. 1995, 117, 230. [Google Scholar] [CrossRef]
- Lewin, J.S.; Friedman, L.; Wu, D.; Miller, D.A.; Thompson, L.A.; Klein, S.K.; Wise, A.L.; Hedera, P.; Buckley, P.; Meltzer, H.J. Cortical localization of human sustained attention: Detection with functional MR using a visual vigilance paradigm. J. Comput. Assist. Tomogr. 1996, 20, 695–701. [Google Scholar] [CrossRef]
- Pardo, J.V.; Fox, P.T.; Raichle, M.E. Localization of a human system for sustained attention by positron emission tomography. Nature 1991, 349, 61. [Google Scholar] [CrossRef]
- Shaw, T.H.; Funke, M.E.; Dillard, M.; Funke, G.J.; Warm, J.S.; Parasuraman, R. Event-related cerebral hemodynamics reveal target-specific resource allocation for both “go” and “no-go” response-based vigilance tasks. Brain Cogn. 2013, 82, 265–273. [Google Scholar] [CrossRef]
- Stroobant, N.; Vingerhoets, G. Transcranial Doppler ultrasonography monitoring of cerebral hemodynamics during performance of cognitive tasks: A review. Neuropsychol. Rev. 2000, 10, 213–231. [Google Scholar] [CrossRef]
- Warm, J.S.; Matthews, G.; Parasuraman, R. Cerebral hemodynamics and vigilance performance. Mil. Psychol. 2009, 21, S75–S100. [Google Scholar] [CrossRef]
- Borghini, G.; Astolfi, L.; Vecchiato, G.; Mattia, D.; Babiloni, F.B. Measuring neurophysiological signals in aircraft pilots and car drivers for the assessment of mental workload, fatigue and drowsiness. Neurosci. Biobehav. Rev. 2014, 44, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; McCandliss, B.D.; Fossella, J.; Flombaum, J.I.; Posner, M.I. The activation of attentional networks. Neuroimage 2005, 26, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Wu, W.-C.; Wang, J.; Detre, J.A.; Dinges, D.F.; Rao, H. Imaging brain fatigue from sustained mental workload: An ASL perfusion study of the time-on-task effect. Neuroimage 2010, 49, 3426–3435. [Google Scholar] [CrossRef] [PubMed]
- Warm, J.S.; Finomore, V.S.; Vidulich, M.A.; Funke, M.E. Vigilance: A perceptual challenge. In Cambridge Handbooks in Psychology: The Cambridge Handbook of Applied Perception Research; Hoffman, R.R., Hancock, P.A., Scerbo, M.W., Parasuraman, R., Szalma, J.L., Eds.; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Boksem, M.A.; Meijman, T.F.; Lorist, M.M. Effects of mental fatigue on attention: An ERP study. Cogn. Brain Res. 2005, 25, 107–116. [Google Scholar] [CrossRef]
- Haubert, A.; Walsh, M.; Boyd, R.; Morris, M.; Wiedbusch, M.; Krusmark, M.; Gunzelmann, G. Relationship of event-related potentials to the vigilance decrement. Front. Psychol. 2018, 9, 237. [Google Scholar] [CrossRef]
- Möckel, T.; Beste, C.; Wascher, E. The effects of time on task in response selection-an ERP study of mental fatigue. Sci. Rep. 2015, 5, 10113. [Google Scholar] [CrossRef]
- Hopstaken, J.F.; Van Der Linden, D.; Bakker, A.B.; Kompier, M.A. A multifaceted investigation of the link between mental fatigue and task disengagement. Psychophysiology 2015, 52, 305–315. [Google Scholar] [CrossRef]
- Yu, K.; Prasad, I.; Mir, H.; Thakor, N.; Al-Nashash, H. Cognitive workload modulation through degraded visual stimuli: A single-trial EEG study. J. Neural Eng. 2015, 12, 046020. [Google Scholar] [CrossRef]
- Bodala, I.P.; Li, J.; Thakor, N.V.; Al-Nashash, H. EEG and eye tracking demonstrate vigilance enhancement with challenge integration. Front. Hum. Neurosci. 2016, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Bergasa, L.M.; Nuevo, J.; Sotelo, M.A.; Barea, R.; Lopez, M.E. Real-time system for monitoring driver vigilance. IEEE Trans. Intell. Transp. Syst. 2006, 7, 63–77. [Google Scholar] [CrossRef]
- Corsi-Cabrera, M.; Arce, C.; Ramos, J.; Lorenzo, I.; Guevara, M. Time course of reaction time and EEG while performing a vigilance task during total sleep deprivation. Sleep 1996, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, D.; Rasim, Y.; Li, Y.; Meng, G.; Xu, J.; Wang, C. A vehicle active safety model: Vehicle speed control based on driver vigilance detection using wearable EEG and sparse representation. Sensors 2016, 16, 242. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Liu, Y.; Zhang, Z.; Wang, Z.; Luo, D.; Zhou, X.; Zhu, M.; Salman, W.; Hu, G. Design of a fatigue detection system for high-speed trains based on driver vigilance using a wireless wearable EEG. Sensors 2017, 17, 486. [Google Scholar] [CrossRef]
- Martel, A.; Dähne, S.; Blankertz, B.J. EEG predictors of covert vigilant attention. J. Neural Eng. 2014, 11, 035009. [Google Scholar] [CrossRef]
- Wascher, E.; Rasch, B.; Sänger, J.; Hoffmann, S.; Schneider, D.; Rinkenauer, G.; Heuer, H.; Gutberlet, I. Frontal theta activity reflects distinct aspects of mental fatigue. Biol. Psychol. 2014, 96, 57–65. [Google Scholar] [CrossRef]
- Kamzanova, A.T.; Kustubayeva, A.M.; Matthews, G. Use of EEG workload indices for diagnostic monitoring of vigilance decrement. Hum. Factors 2014, 56, 1136–1149. [Google Scholar] [CrossRef]
- Makeig, S.; Jung, T.-P. Tonic, phasic, and transient EEG correlates of auditory awareness in drowsiness. Cogn. Brain Res. 1996, 4, 15–25. [Google Scholar] [CrossRef]
- Claypoole, V.L.; Dever, D.A.; Denues, K.L.; Szalma, J.L. The effects of event rate on a cognitive vigilance task. Hum. Factors 2019, 61, 440–450. [Google Scholar] [CrossRef]
- Han, C.; Sun, X.; Yang, Y.; Che, Y.; Qin, Y. Brain Complex Network Characteristic Analysis of Fatigue during Simulated Driving Based on Electroencephalogram Signals. Entropy 2019, 21, 353. [Google Scholar] [CrossRef]
- Qi, P.; Ru, H.; Gao, L.; Zhang, X.; Zhou, T.; Tian, Y.; Thakor, N.; Bezerianos, A.; Li, J.; Sun, Y. Neural mechanisms of mental fatigue revisited: New insights from the brain connectome. Engineering. 2019, 5, 276–286. [Google Scholar] [CrossRef]
- Liu, J.-P.; Zhang, C.; Zheng, C.-X. Estimation of the cortical functional connectivity by directed transfer function during mental fatigue. Appl. Ergon. 2010, 42, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Wang, H. Functional brain network analysis of the fatigue state for coach bus driver. In Proceedings of the 2015 IEEE 7th International Conference on Awareness Science and Technology (iCAST), Qinhuangdao, China, 22–24 September 2015; pp. 43–48. [Google Scholar]
- Claypoole, V.L.; Neigel, A.R.; Fraulini, N.W.; Hancock, G.M.; Szalma, J.L. Can vigilance tasks be administered online? A replication and discussion. J. Exp. Psychol. Hum. Percept. Perform. 2018, 44, 1348. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.R.; Besner, D.; Smilek, D. A critical examination of the evidence for sensitivity loss in modern vigilance tasks. Psychol. Rev. 2016, 123, 70. [Google Scholar] [CrossRef] [PubMed]
- Valdez, P.; Ramírez, C.; García, A.; Talamantes, J.; Armijo, P.; Borrani, J. Circadian rhythms in components of attention. Biol. Rhythm Res. 2005, 36, 57–65. [Google Scholar] [CrossRef]
- Brandt, R.; Herrero, D.; Massetti, T.; Crocetta, T.B.; Guarnieri, R.; de Mello Monteiro, C.B.; da Silveira Viana, M.; Bevilacqua, G.G.; de Abreu, L.C.; Andrade, A. The Brunel Mood Scale Rating in mental health for physically active and apparently healthy populations. Health 2016, 8, 125. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Al-Shargie, F.; Tang, T.B.; Kiguchi, M. Stress assessment based on decision fusion of EEG and fNIRS signals. IEEE Access 2017, 5, 19889–19896. [Google Scholar] [CrossRef]
- Al-Shargie, F.; Kiguchi, M.; Badruddin, N.; Dass, S.C.; Hani, A.F.M.; Tang, T.B. Mental stress assessment using simultaneous measurement of EEG and fNIRS. Biomed. Opt. Express 2016, 7, 3882–3898. [Google Scholar] [CrossRef]
- Cui, J.; Xu, L.; Bressler, S.L.; Ding, M.; Liang, H. BSMART: A Matlab/C toolbox for analysis of multichannel neural time series. Neural Netw. 2008, 21, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Fagiolo, G. Clustering in complex directed networks. Psychophysiology 2007, 76, 026107. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Schack, B.; Vath, N.; Petsche, H.; Geissler, H.-G.; Möller, E. Phase-coupling of theta–gamma EEG rhythms during short-term memory processing. Psychophysiology 2002, 44, 143–163. [Google Scholar] [CrossRef]
- Davies, D.R.; Parasuraman, R. The Psychology of Vigilance; Academic Press: Waltham, MA, USA, 1982. [Google Scholar]
- Hester, R.; Foxe, J.J.; Molholm, S.; Shpaner, M.; Garavan, H. Neural mechanisms involved in error processing: A comparison of errors made with and without awareness. Neuroimage 2005, 27, 602–608. [Google Scholar] [CrossRef]
- Helton, W.S.; Russell, P.N.J.P.R. Feature absence–presence and two theories of lapses of sustained attention. Psychol. Res. 2011, 75, 384–392. [Google Scholar] [CrossRef]
- Mackworth, N.H. The breakdown of vigilance during prolonged visual search. Q. J. Exp. Psychol. 1948, 1, 6–21. [Google Scholar] [CrossRef]
- Babiloni, C.; Ferri, R.; Binetti, G.; Cassarino, A.; Dal Forno, G.; Ercolani, M.; Ferreri, F.; Frisoni, G.B.; Lanuzza, B.; Miniussi, C. Fronto-parietal coupling of brain rhythms in mild cognitive impairment: A multicentric EEG study. Brain Res. Bull. 2006, 69, 63–73. [Google Scholar] [CrossRef]
- Sun, Y.; Lim, J.; Kwok, K.; Bezerianos, A. Functional cortical connectivity analysis of mental fatigue unmasks hemispheric asymmetry and changes in small-world networks. Brain Cogn. 2014, 85, 220–230. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Wang, Q.; Hua, C. Exploring the fatigue affecting electroencephalography based functional brain networks during real driving in young males. Neuropsychologia 2019, 129, 200–211. [Google Scholar] [CrossRef]
- MacDonald, A.W.; Cohen, J.D.; Stenger, V.A.; Carter, C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000, 288, 1835–1838. [Google Scholar] [CrossRef] [PubMed]
- Heekeren, H.R.; Marrett, S.; Ruff, D.A.; Bandettini, P.; Ungerleider, L.G. Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc. Natl. Acad. Sci. USA 2006, 103, 10023–10028. [Google Scholar] [CrossRef]
- Demeter, E.; Hernandez-Garcia, L.; Sarter, M.; Lustig, C. Challenges to attention: A continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. Neuroimage 2011, 54, 1518–1529. [Google Scholar] [CrossRef]
- Helton, W.S.; Warm, J.S.; Tripp, L.D.; Matthews, G.; Parasuraman, R.; Hancock, P.A. Cerebral lateralization of vigilance: A function of task difficulty. Neuropsychologia 2010, 48, 1683–1688. [Google Scholar] [CrossRef]
- Dimond, S.J.; Beaumont, J.G. Difference in the vigilance performance of the right and left hemispheres. Cortex 1973, 9, 259–265. [Google Scholar] [CrossRef]
- Hitchcock, E.M.; Warm, J.S.; Matthews, G.; Dember, W.N.; Shear, P.K.; Tripp, L.D.; Mayleben, D.W.; Parasuraman, R. Automation cueing modulates cerebral blood flow and vigilance in a simulated air traffic control task. Theor. Issues Ergon. Sci. 2003, 4, 89–112. [Google Scholar] [CrossRef]
- Shaw, T.H.; Warm, J.S.; Finomore, V.; Tripp, L.; Matthews, G.; Weiler, E.; Parasuraman, R. Effects of sensory modality on cerebral blood flow velocity during vigilance. Neurosci. Lett. 2009, 461, 207–211. [Google Scholar] [CrossRef]
- Grier, R.A. How high is high? A meta-analysis of NASA-TLX global workload scores. Hum. Factors Ergon. Soc. Annu. Meet. 2015, 59, 1727–1731. [Google Scholar] [CrossRef]
- Al-Shargie, F.; Tang, T.B.; Badruddin, N.; Kiguchi, M. Towards multilevel mental stress assessment using SVM with ECOC: An EEG approach. Med. Biol. Eng. Comput. 2018, 56, 125–136. [Google Scholar] [CrossRef]
- Al-Shargie, F.; Tang, T.B.; Kiguchi, M. Assessment of mental stress effects on prefrontal cortical activities using canonical correlation analysis: An fNIRS-EEG study. Biomed. Opt. Express 2017, 8, 2583–2598. [Google Scholar] [CrossRef]
- Al-shargie, F.; Tang, T.B.; Kiguchi, M. Mental stress grading based on fNIRS signals. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 17–20 August 2016; pp. 5140–5143. [Google Scholar]
- Chokron, S.; Brickman, A.M.; Wei, T.; Buchsbaum, M.S. Hemispheric asymmetry for selective attention. Cogn. Brain Res. 2000, 9, 85–90. [Google Scholar] [CrossRef]
- Cate, A.D.; Herron, T.J.; Yund, E.W.; Stecker, G.C.; Rinne, T.; Kang, X.; Petkov, C.I.; Disbrow, E.A.; Woods, D.L. Auditory attention activates peripheral visual cortex. PLoS ONE 2009, 4, e4645. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, C.H.; Gutschalk, A. Transient human auditory cortex activation during volitional attention shifting. PLoS ONE 2017, 12, e0172907. [Google Scholar] [CrossRef][Green Version]
- Grady, C.L.; Van Meter, J.W.; Maisog, J.M.; Pietrini, P.; Krasuski, J.; Rauschecker, J.P. Attention-related modulation of activity in primary and secondary auditory cortex. Neuroreport 1997, 8, 2511–2516. [Google Scholar] [CrossRef]
- Wang, C.; Trongnetrpunya, A.; Samuel, I.B.H.; Ding, M.; Kluger, B.M. Compensatory neural activity in response to cognitive fatigue. J. Neurosci. 2016, 36, 3919–3924. [Google Scholar] [CrossRef]
- Matsubara, T.; Ogata, K.; Hironaga, N.; Kikuchi, Y.; Uehara, T.; Chatani, H.; Mitsudo, T.; Shigeto, H.; Tobimatsu, S.J.E. Altered neural synchronization to pure tone stimulation in patients with mesial temporal lobe epilepsy: An MEG study. Epilepsy Behav. 2018, 88, 96–105. [Google Scholar] [CrossRef]
- Helton, W.S.; Matthews, G.; Warm, J.S. Stress state mediation between environmental variables and performance: The case of noise and vigilance. Acta Psychol. 2009, 130, 204–213. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Liu, C.; Liu, D.; Ding, X.; Zhou, C. Graph theoretical analysis of EEG functional connectivity during music perception. Brain Res. 2012, 1483, 71–81. [Google Scholar] [CrossRef]
- Rosen, A.C.; Prull, M.W.; O’Hara, R.; Race, E.A.; Desmond, J.E.; Glover, G.H.; Yesavage, J.A.; Gabrieli, J.D. Variable effects of aging on frontal lobe contributions to memory. Neuroreport 2002, 13, 2425–2428. [Google Scholar] [CrossRef] [PubMed]
- Sikström, S.; Söderlund, G. Stimulus-dependent dopamine release in attention-deficit/hyperactivity disorder. Psychol. Rev. 2007, 114, 1047. [Google Scholar] [CrossRef] [PubMed]
- Rausch, V.H.; Bauch, E.M.; Bunzeck, N. White noise improves learning by modulating activity in dopaminergic midbrain regions and right superior temporal sulcus. J. Cogn. Neurosci. 2014, 26, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Reches, A.; Netser, S.; Gutfreund, Y. Interactions between stimulus-specific adaptation and visual auditory integration in the forebrain of the barn owl. J. Neurosci. 2010, 30, 6991–6998. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Factors | Items | Vigilance Mean ± SD | Enhancement Mean ± SD | Significance |
|---|---|---|---|---|
| Anger | Annoyed | 1.50 ± 1.51 | 0.90 ± 1.22 | ↓↓ |
| Bitter | 0.83 ± 1.16 | 0.27 ± 0.46 | ↓↓ | |
| Angry | 0.50 ± 1.22 | 0.27 ± 0.90 | ↓ | |
| Bad tempered | 0.66 ± 1.21 | 0.54 ± 0.93 | ||
| Tension | Panicky | 0.66 ± 1.21 | 1.10 ± 1.59 | ↑↑ |
| Anxious | 0.66 ± 1.21 | 1.09 ± 1.51 | ||
| Worried | 1.16 ± 1.16 | 1.0 ± 1.54 | ||
| Nervous | 0.16 ± 0.40 | 0.63 ± 0.28 | ||
| Depression | Depressed | 0.50 ± 0.54 | 0.27 ± 0.46 | ↓ |
| Downhearted | 0.66 ± 1.21 | 0.36 ± 0.67 | ↓↓ | |
| Unhappy | 0.83 ± 1.16 | 0.27 ± 0.46 | ↓↓ | |
| Miserable | 1.16 ± 1.47 | 0.36 ± 0.92 | ↓↓ | |
| Vigor | Lively | 2.00 ± 1.41 | 2.27 ± 1.42 | |
| Energetic | 1.66 ± 1.36 | 2.0 ± 1.48 | ↑↑ | |
| Active | 2.16 ± 1.83 | 2.27 ± 1.81 | ||
| Alert | 2.16 ± 1.83 | 2.27 ± 1.71 | ||
| Fatigue | Worn out | 2.16 ± 1.32 | 1.18 ± 1.32 | ↓↓ |
| Exhausted | 2.33 ± 1.50 | 1.36 ± 1.50 | ↓↓ | |
| Sleepy | 2.33 ± 1.36 | 1.36 ± 1.36 | ↓↓ | |
| Tired | 2.83 ± 1.16 | 1.72 ± 1.48 | ↓↓ | |
| Confusion | Confused | 0.66 ± 0.40 | 0.30 ± 0.30 | ↓ |
| Muddled | 0.66 ± 1.03 | 0.18 ± 1.40 | ↓ | |
| Mixed up | 0.66 ± 0.81 | 0.60 ± 1.30 | ||
| Uncertain | 1.00 ± 0.89 | 0.54 ± 0.93 | ↓ | |
| Happy | Cheerful | 2.00 ± 1.26 | 2.63 ± 1.40 | ↑↑ |
| Content | 1.66 ± 1.21 | 2.27 ± 1.27 | ↑↑ | |
| Happy | 2.33 ± 1.21 | 2.27 ± 1.19 | ||
| Satisfied | 1.66 ± 1.12 | 2.45 ± 1.36 | ↑↑ | |
| Calmness | Calm | 2.16 ± 1.47 | 2.45 ± 1.03 | ↑↑ |
| Composed | 2.00 ± 0.63 | 2.45 ± 0.93 | ↑↑ | |
| Relaxed | 1.83 ± 1.16 | 2.45 ± 1.21 | ↑↑ | |
| Restful | 1.36 ± 1.36 | 2.00 ± 1.34 | ↑↑ |
| Hemisphere | V1 vs. V2 p-Value (<) | V1 vs. V3 p-Value (<) | V2 vs. V3 p-Value (<) | V1 vs. E1 p-Value (<) | V2 vs. E2 p-Value (<) | V3 vs. E3 p-Value (<) |
|---|---|---|---|---|---|---|
| LH → LH | 0.0411 | 0.0002 | 0.0401 | 0.0201 | 0.0041 | 0.0001 |
| RH → RH | 0.0001 | 0.0001 | 0.0051 | 0.0021 | 0.0001 | 0.0001 |
| RH → LH | 0.0401 | 0.0011 | 0.0031 | 0.0001 | 0.0001 | 0.0001 |
| LH → RH | 0.0311 | 0.0001 | 0.0031 | 0.005 | 0.0051 | 0.0001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Shargie, F.; Tariq, U.; Hassanin, O.; Mir, H.; Babiloni, F.; Al-Nashash, H. Brain Connectivity Analysis Under Semantic Vigilance and Enhanced Mental States. Brain Sci. 2019, 9, 363. https://doi.org/10.3390/brainsci9120363
Al-Shargie F, Tariq U, Hassanin O, Mir H, Babiloni F, Al-Nashash H. Brain Connectivity Analysis Under Semantic Vigilance and Enhanced Mental States. Brain Sciences. 2019; 9(12):363. https://doi.org/10.3390/brainsci9120363
Chicago/Turabian StyleAl-Shargie, Fares, Usman Tariq, Omnia Hassanin, Hasan Mir, Fabio Babiloni, and Hasan Al-Nashash. 2019. "Brain Connectivity Analysis Under Semantic Vigilance and Enhanced Mental States" Brain Sciences 9, no. 12: 363. https://doi.org/10.3390/brainsci9120363
APA StyleAl-Shargie, F., Tariq, U., Hassanin, O., Mir, H., Babiloni, F., & Al-Nashash, H. (2019). Brain Connectivity Analysis Under Semantic Vigilance and Enhanced Mental States. Brain Sciences, 9(12), 363. https://doi.org/10.3390/brainsci9120363