The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease
Abstract
1. Introduction
2. Synthesis, Metabolism and Action Mechanism of Melatonin
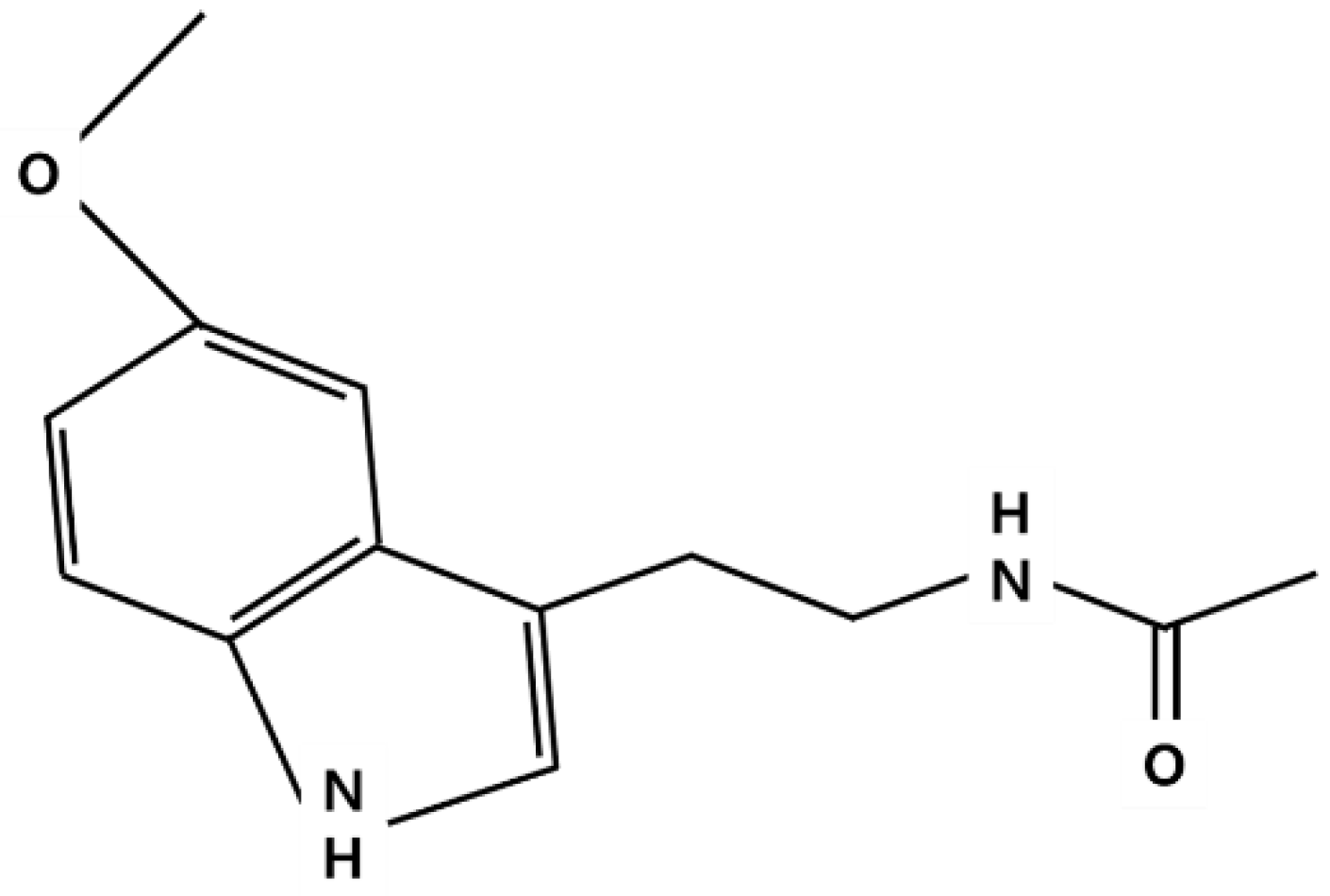
2.1. Synthesis and Metabolism of Melatonin
2.2. Action Mechanism of Melatonin
3. The Role of Melatonin in the CNS
3.1. Source of Melatonin in the Human Body
3.2. Melatonin in the CNS
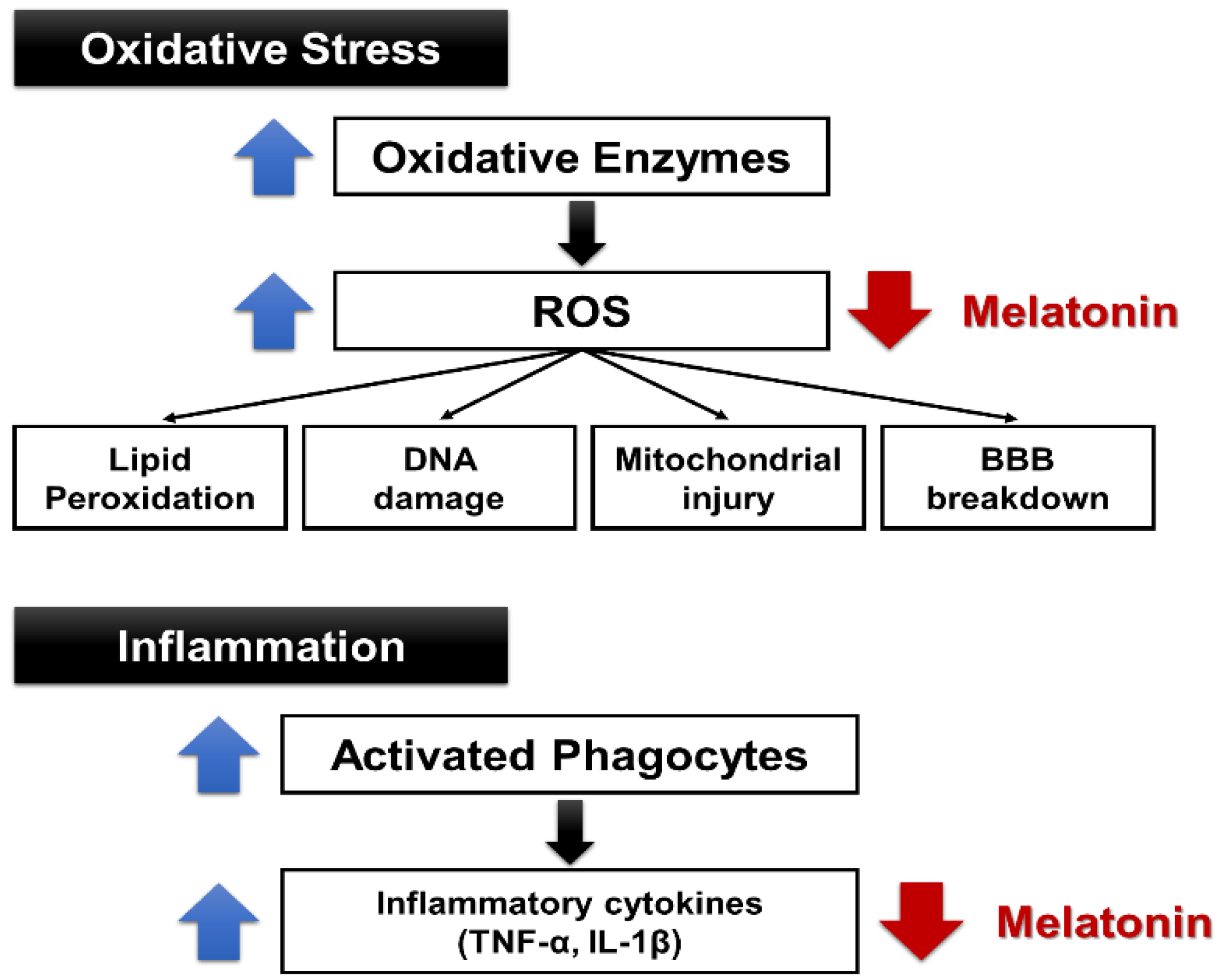
4. Anti-Ischemic and Antioxidant Effects of Melatonin
5. Antioxidant Effects of Melatonin in Alzheimer’s Disease (AD)
6. Antidepressant-Like Actions of Melatonin
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| 5-HT | 5-hydroxytryptamine |
| AANAT | arylalkylamine N-acetyltransferase |
| Aβ | amyloid-β |
| AD | Alzheimer’s disease |
| ADAS-Cog | Alzheimer’s disease assessment scale-cognition |
| Akt | Protein kinase B (PKB) |
| AFMK | N-acetyl-N2-inyl-5-methoxykynuramine |
| AMK | N-acetyl-5-methosykynuramine |
| APP+PS1 | amyloid precursor protein and presenilin-1 |
| AQP4 | aquaporin 4 |
| ATP | adenosine triphosphate |
| BAD | BCL2 (B-cell lymphoma 2) associated agonist of cell death |
| βAPP | β-amyloid precursor protein |
| BAX | Bcl-2-associated X protein |
| CA | Cornu Ammonis |
| Ca2+ | calcium ion |
| CaMKII | Ca 2+/calmodulin-dependent protein kinase II |
| cAMP | cyclic adenosine monophosphate |
| cGMP | cyclic guanosine monophosphate |
| CMS | chronic mild stress |
| CNS | central nervous system |
| CREB | cAMP response element-binding protein |
| CRMP-2 | collapsin response mediator protein 2 |
| CYP1A2 | cytochrome P450 1A2 |
| EEG | electroencephalography |
| FSL | flinders sensitive line |
| FST | forced swim test |
| GABAA | gamma-Aminobutyric acid A |
| GSK-3β | glycogen synthase kinase 3 beta |
| HIE | hypoxic-ischemic encephalopathy |
| HIOMT | hydroxyindole-O-methyltransferase |
| IL-1β | interleukin 1 beta |
| K+ | potassium ion |
| MCA | middle cerebral artery |
| MDA | malondialdehyde |
| MK-801 | dizocilpine |
| mRNA | messenger ribonucleic acid (RNA) |
| MT1 | melatonin receptor type 1A |
| MT2 | melatonin receptor 1B |
| NMDA | N-methyl-d-aspartic acid |
| MRI | magnetic resonance imaging |
| Na+ | sodium ion |
| NO | nitric oxide |
| Nox | nicotinamide adenine dinucleotide phosphate (NADPH) oxidase |
| PLC | phospholipase C |
| PKA | protein kinase A |
| PKC | protein kinase C |
| PSD-95 | postsynaptic density protein 95 |
| PVN | paraventricular nucleus |
| ROS | reactive oxygen species |
| SCN | suprachiasmatic nucleus |
| SOD | superoxide dismutase |
| SON | supraoptic nucleus |
| TNF-α | tumor necrosis factor alpha |
| TST | tail suspension test |
References
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.; Escames, G.; López, L.C.; Venegas, C.; Dayoub, J.C.; García, L.; Acuña-Castroviejo, D. Long-term melatonin administration protects brain mitochondria from aging. J. Pineal Res. 2009, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin metabolism in the central nervous system. Curr. Neuropharmacol. 2010, 8, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.; Cardinali, D.P.; Poeggeler, B.; Hardeland, R. Melatonin: Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.P., 3rd; McLin, D.E., 3rd; Dressman, M.A.; Neubauer, D.N. Comparative Review of Approved Melatonin Agonists for the Treatment of Circadian Rhythm Sleep-Wake Disorders. Pharmacotherapy 2016, 36, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Samejima, M.; Okabe, A.; Fukuda, A. Neuroprotective effects of melatonin against anoxia/aglycemia stress, as assessed by synaptic potentials and superoxide production in rat hippocampal slices. J. Pineal Res. 2004, 37, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.K.; Gupta, M.; Kohli, K. Neuroprotective role of melatonin in oxidative stress vulnerable brain. Indian J. Physiol. Pharmacol. 2003, 47, 373–386. [Google Scholar]
- Rogério, F.; de Souza Queiroz, L.; Teixeira, S.A.; Oliveira, A.L.; de Nucci, G.; Langone, F. Neuroprotective action of melatonin on neonatal rat motoneurons after sciatic nerve transection. Brain Res. 2002, 926, 33–41. [Google Scholar] [CrossRef]
- Persengiev, S.P. The neuroprotective and antiapoptotic effects of melatonin in cerebellar neurons involve glucocorticoid receptor and p130 signal pathways. J. Steroid Biochem. Mol. Biol. 2001, 77, 151–158. [Google Scholar] [CrossRef]
- Ali, S.F.; Martin, J.L.; Black, M.D.; Itzhak, Y. Neuroprotective role of melatonin in methamphetamine- and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity. Ann. N. Y. Acad. Sci. 1999, 890, 119. [Google Scholar] [CrossRef]
- Smale, L.; Cassone, V.M.; Moore, R.Y.; Morin, L.P. Paraventricular nucleus projections mediating pineal melatonin and gonadal responses to photoperiod in the hamster. Brain Res. Bull. 1989, 22, 263–269. [Google Scholar] [CrossRef]
- Bittman, E.L.; Crandell, R.G.; Lehman, M.N. Influences of the paraventricular and suprachiasmatic nuclei and olfactory bulbs on melatonin responses in the golden hamster. Biol. Reprod. 1989, 40, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Physiological crosstalk between the AC/PKA and PLC/PKC pathways modulates melatonin-mediated, monochromatic-light-induced proliferation of T-lymphocytes in chickens. Cell Tissue Res. 2017, 69, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Garratt, P.J.; Tsotinis, A. Synthesis of compounds as melatonin agonists and antagonists. Mini Rev. Med. Chem. 2007, 7, 1075–1088. [Google Scholar] [CrossRef]
- Ackermann, K.; Stehle, J.H. Melatonin synthesis in the human pineal gland: Advantages, implications, and difficulties. Chronobiol. Int. 2006, 23, 369–379. [Google Scholar] [CrossRef]
- Liu, T.; Borjigin, J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J. Pineal Res. 2005, 39, 91–96. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Melatonin and synthetic melatonergic agonists: Actions and metabolism in the central nervous system. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 189–216. [Google Scholar] [CrossRef]
- Hardeland, R. Taxon- and Site-Specific Melatonin Catabolism. Molecules 2017, 21, 2015. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Skaper, S.D.; Floreani, M.; Ceccon, M.; Facci, L.; Giusti, P. Excitotoxicity, oxidative stress, and the neuroprotective potential of melatonin. Ann. N. Y. Acad. Sci. 1999, 890, 107–118. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Benleulmi-Chaachoua, A.; Journé, A.S.; Kamal, M.; Guillaume, J.L.; Dussaud, S.; Gbahou, F.; Yettou, K.; Liu, C.; Contreras-Alcantara, S.; et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci. Signal. 2013, 6, ra89. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Hazlerigg, D.G.; Gonzalez-Brito, A.; Lawson, W.; Hastings, M.H.; Morgan, P.J. Prolonged exposure to melatonin leads to time-dependent sensitization of adenylate cyclase and down-regulates melatonin receptors in pars tuberalis cells from ovine pituitary. Endocrinology 1993, 132, 285–292. [Google Scholar] [CrossRef]
- Tan, D.X.; Zanghi, B.M.; Manchester, L.C.; Reiter, R.J. Melatonin identified in meats and other food stuffs: Potentially nutritional impact. J. Pineal Res. 2014, 57, 213–218. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Sanchez-Barcelo, E.; Mediavilla, M.D.; Reiter, R.J. Significance of high levels of endogenous melatonin in Mammalian cerebrospinal fluid and in the central nervous system. Curr. Neuropharmacol. 2010, 8, 162–167. [Google Scholar] [CrossRef]
- Stefulj, J.; Hörtner, M.; Ghosh, M.; Schauenstein, K.; Rinner, I.; Wölfler, A.; Semmler, J.; Liebmann, P.M. Gene expression of the key enzymes of melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 2001, 30, 243–247. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhuang, J.; Zhu, H.Y.; Shen, Y.X.; Tan, Z.L.; Zhou, J.N. Cultured rat cortical astrocytes synthesize melatonin: Absence of a diurnal rhythm. J. Pineal Res. 2007, 43, 232–238. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef]
- Wu, Y.H.; Zhou, J.N.; Balesar, R.; Unmehopa, U.; Bao, A.; Jockers, R.; Van Heerikhuize, J.; Swaab, D.F. Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: Colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J. Comp. Neurol. 2006, 499, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Lacoste, B.; Angeloni, D.; Dominguez-Lopez, S.; Calderoni, S.; Mauro, A.; Fraschini, F.; Descarries, L.; Gobbi, G. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal Res. 2015, 58, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.Y.; Leong, M.K.; Liang, H.; Paxinos, G. Melatonin receptors: Distribution in mammalian brain and their respective putative functions. Brain Struct. Funct. 2017, 222, 2921–2939. [Google Scholar] [CrossRef] [PubMed]
- Jilg, A.; Moek, J.; Weaver, D.R.; Korf, H.W.; Stehle, J.H.; von Gall, C. Rhythms in clock proteins in the mouse pars tuberalis depend on MT1 melatonin receptor signalling. Eur. J. Neurosci. 2005, 22, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.Z.; Liang, Y.L.; Wang, H.; Chen, X.; Huang, Y.Y.; Zhang, Z.H.; Chen, Y.H.; Zhang, C.; Zhao, M.; Xu, D.X.; et al. Melatonin modulates TLR4-mediated inflammatory genes through MyD88- and TRIF-dependent signaling pathways in lipopolysaccharide-stimulated RAW264.7 cells. J. Pineal Res. 2012, 53, 325–334. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Karaaslan, C.; Suzen, S. Antioxidant properties of melatonin and its potential action in diseases. Curr. Top. Med. Chem. 2015, 15, 894–903. [Google Scholar] [CrossRef]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; Antoli, I.; Herrera, F.; Martin, V.; Rodriguez, C. Melatonin regulation of antioxidant enzyme gene expression. Cell. Mol. Life Sci. 2002, 59, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.W.; MacWalter, R.S.; Doney, A.S. The cost of cerebral ischaemia. Neuropharmacology 2008, 55, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Stys, P.K. General mechanisms of axonal damage and its prevention. J. Neurol. Sci. 2005, 233, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Stys, P.K. Anoxic and ischemic injury of myelinated axons in CNS white matter: From mechanistic concepts to therapeutics. J. Cereb. Blood Flow Metab. 1998, 18, 2–25. [Google Scholar] [CrossRef]
- Hutton, L.C.; Abbass, M.; Dickinson, H.; Ireland, Z.; Walker, D.W. Neuroprotective properties of melatonin in a model of birth asphyxia in the spiny mouse (Acomys cahirinus). Dev. Neurosci. 2009, 31, 437–451. [Google Scholar] [CrossRef]
- Kilic, E.; Ozdemir, Y.G.; Bolay, H.; Keleştimur, H.; Dalkara, T. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J. Cereb. Blood Flow Metab. 1999, 19, 511–516. [Google Scholar] [CrossRef]
- Sinha, K.; Degaonkar, M.N.; Jagannathan, N.R.; Gupta, Y.K. Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur. J. Pharmacol. 2001, 428, 185–192. [Google Scholar] [CrossRef]
- Pei, Z.; Pang, S.F.; Cheung, R.T. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke 2003, 34, 770–775. [Google Scholar] [CrossRef]
- Chen, T.Y.; Lee, M.Y.; Chen, H.Y.; Kuo, Y.L.; Lin, S.C.; Wu, T.S.; Lee, E.J. Melatonin attenuates the postischemic increase in blood-brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J. Pineal Res. 2006, 40, 242–250. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kuan, Y.H.; Chen, H.Y.; Chen, T.Y.; Chen, S.T.; Huang, C.C.; Yang, I.P.; Hsu, Y.S.; Wu, T.S.; Lee, E.J. Intravenous administration of melatonin reduces the intracerebral cellular inflammatory response following transient focal cerebral ischemia in rats. J. Pineal Res. 2007, 42, 297–309. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, M.Y.; Chen, H.Y.; Hsu, Y.S.; Wu, T.S.; Chen, S.T.; Chang, G.L. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J. Pineal Res. 2005, 38, 42–52. [Google Scholar] [CrossRef]
- Letechipía-Vallejo, G.; González-Burgos, I.; Cervantes, M. Neuroprotective effect of melatonin on brain damage induced by acute global cerebral ischemia in cats. Arch. Med. Res. 2001, 32, 186–192. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Sumaya, I.; Moss, D.; Kumazaki, M.; Sakurai, T.; Hida, H.; Nishino, H. Melatonin-secreting pineal gland: A novel tissue source for neural transplantation therapy in stroke. Cell Transplant. 2003, 12, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, C.; Niu, H.J.; Wang, K.; Mo, L.J.; Shao, A.W.; Dixon, B.J.; Zhang, J.M.; Yang, S.X.; Wang, Y.R. Neuroprotective Mechanisms of Melatonin in Hemorrhagic Stroke. Cell. Mol. Neurobiol. 2017, 37, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Pandey, A.K.; Paul, S.; Patnaik, R. Melatonin renders neuroprotection by protein kinase C mediated aquaporin-4 inhibition in animal model of focal cerebral ischemia. Life Sci. 2014, 100, 97–109. [Google Scholar] [CrossRef]
- Koh, P.O. Melatonin regulates the calcium-buffering proteins, parvalbumin and hippocalcin, in ischemic brain injury. J. Pineal Res. 2012, 53, 358–365. [Google Scholar] [CrossRef]
- Chern, C.M.; Liao, J.F.; Wang, Y.H.; Shen, Y.C. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the MT2 melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med. 2012, 52, 1634–1647. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Feng, D.; Liu, Y.; Xu, M.; Gao, A.; Tian, F.; Zhang, L.; Cui, Y.; Wang, Z.; et al. Alterations in the time course of expression of the Nox family in the brain in a rat experimental cerebral ischemia and reperfusion model: Effects of melatonin. J. Pineal Res. 2014, 57, 110–119. [Google Scholar] [CrossRef]
- Aly, H.; Elmahdy, H.; El-Dib, M.; Rowisha, M.; Awny, M.; El-Gohary, T.; Elbatch, M.; Hamisa, M.; El-Mashad, A.R. Melatonin use for neuroprotection in perinatal asphyxia: A randomized controlled pilot study. J. Perinatol. 2015, 35, 186–191. [Google Scholar] [CrossRef]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: Reduction by melatonin. J. Pineal Res. 2001, 31, 343–349. [Google Scholar] [CrossRef]
- Paredes, S.D.; Rancan, L.; Kireev, R.; González, A.; Louzao, P.; González, P.; Rodríguez-Bobada, C.; García, C.; Vara, E.; Tresguerres, J.A. Melatonin Counteracts at a Transcriptional Level the Inflammatory and Apoptotic Response Secondary to Ischemic Brain Injury Induced by Middle Cerebral Artery Blockade in Aging Rats. Biores. Open Access 2015, 4, 407–416. [Google Scholar] [CrossRef]
- Alghamdi, B.S. The neuroprotective role of melatonin in neurological disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N. Oxidative stress and pro-inflammatory cytokines may act as one of the signals for regulating microRNAs expression in Alzheimer’s disease. Mech. Ageing Dev. 2017, 162, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Nesi, G.; Sestito, S.; Digiacomo, M.; Rapposelli, S. Oxidative Stress, Mitochondrial Abnormalities and Proteins Deposition: Multitarget Approaches in Alzheimer’s Disease. Curr. Top. Med. Chem. 2017, 17, 3062–3079. [Google Scholar] [PubMed]
- Zheng, L.; Roberg, K.; Jerhammar, F.; Marcusson, J.; Terman, A. Oxidative stress induces intralysosomal accumulation of Alzheimer amyloid beta-protein in cultured neuroblastoma cells. Ann. N. Y. Acad. Sci. 2006, 1067, 248–251. [Google Scholar] [CrossRef]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef]
- Olcese, J.M.; Cao, C.; Mori, T.; Mamcarz, M.B.; Maxwell, A.; Runfeldt, M.J.; Wang, L.; Zhang, C.; Lin, X.; Zhang, G.; et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J. Pineal Res. 2009, 47, 82–96. [Google Scholar] [CrossRef]
- Fan, R.; Schrott, L.M.; Arnold, T.; Snelling, S.; Rao, M.; Graham, D.; Cornelius, A.; Korneeva, N.L. Chronic oxycodone induces axonal degeneration in rat brain. BMC Neurosci. 2018, 19, 15. [Google Scholar] [CrossRef]
- Rudnitskaya, E.A.; Muraleva, N.A.; Maksimova, K.Y.; Kiseleva, E.; Kolosova, N.G.; Stefanova, N.A. Melatonin Attenuates Memory Impairment, Amyloid-β Accumulation, and Neurodegeneration in a Rat Model of Sporadic Alzheimer’s Disease. J. Alzheimers Dis. 2015, 47, 103–116. [Google Scholar] [CrossRef]
- Stefanova, N.A.; Maksimova, K.Y.; Kiseleva, E.; Rudnitskaya, E.A.; Muraleva, N.A.; Kolosova, N.G. Melatonin attenuates impairments of structural hippocampal neuroplasticity in OXYS rats during active progression of Alzheimer’s disease-like pathology. J. Pineal Res. 2015, 59, 163–177. [Google Scholar] [CrossRef]
- Ozcankaya, R.; Delibas, N. Malondialdehyde, superoxide dismutase, melatonin, iron, copper, and zinc blood concentrations in patients with Alzheimer disease: Cross-sectional study. Croat. Med. J. 2002, 43, 28–32. [Google Scholar]
- Wang, Y.Y.; Zheng, W.; Ng, C.H.; Ungvari, G.S.; Wei, W.; Xiang, Y.T. Meta-analysis of randomized, double-blind, placebo-controlled trials of melatonin in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2017, 32, 50–57. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.L.; Dammer, E.B.; Li, C.B.; Xu, G.; Chen, S.D.; Wang, G. Melatonin for sleep disorders and cognition in dementia: A meta-analysis of randomized controlled trials. Am. J. Alzheimers Dis. Other Demen. 2015, 30, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.G.; Farmer, M.; Harari, G.; Fund, N.; Laudon, M.; Nir, T.; Frydman-Marom, A.; Zisapel, N. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: A 6-month, randomized, placebo-controlled, multicenter trial. Clin. Interv. Aging 2014, 9, 947–961. [Google Scholar]
- Cardinali, D.P.; Srinivasan, V.; Brzezinski, A.; Brown, G.M. Melatonin and its analogs in insomnia and depression. J. Pineal Res. 2012, 52, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Quera Salva, M.A.; Hartley, S.; Barbot, F.; Alvarez, J.C.; Lofaso, F.; Guilleminault, C. Circadian rhythms, melatonin and depression. Curr. Pharm. Des. 2011, 17, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Mechanism of action of agomelatine: A novel antidepressant exploiting synergy between monoaminergic and melatonergic properties. CNS Spectr. 2014, 19, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Akpınar, E.; Cerit, C.; Talas, A.; Tural, Ü. Agomelatine versus Sertraline: An Observational, Open-labeled and 12 Weeks Follow-up Study on Efficacy and Tolerability. Clin. Psychopharmacol. Neurosci. 2016, 14, 351–356. [Google Scholar] [CrossRef][Green Version]
- Overstreet, D.H.; Pucilowski, O.; Retton, M.C.; Delagrange, P.; Guardiola-Lemaitre, B. Effects of melatonin receptor ligands on swim test immobility. Neuroreport 1998, 9, 249–253. [Google Scholar] [CrossRef]
- Overstreet, D.H. The Flinders sensitive line rats: A genetic animal model of depression. Neurosci. Biobehav. Rev. 1993, 17, 51–68. [Google Scholar] [CrossRef]
- Dagyte, G.; Trentani, A.; Postema, F.; Luiten, P.G.; Den Boer, J.A.; Gabriel, C.; Mocaër, E.; Meerlo, P.; Van der Zee, E.A. The novel antidepressant agomelatine normalizes hippocampal neuronal activity and promotes neurogenesis in chronically stressed rats. CNS Neurosci. Ther. 2010, 16, 195–207. [Google Scholar] [CrossRef]
- Chenu, F.; El Mansari, M.; Blier, P. Electrophysiological effects of repeated administration of agomelatine on the dopamine, norepinephrine, and serotonin systems in the rat brain. Neuropsychopharmacology 2013, 38, 275–284. [Google Scholar] [CrossRef]
- Mantovani, M.; Pértile, R.; Calixto, J.B.; Santos, A.R.; Rodrigues, A.L. Melatonin exerts an antidepressant-like effect in the tail suspension test in mice: Evidence for involvement of N-methyl-d-aspartate receptors and the l-arginine-nitric oxide pathway. Neurosci. Lett. 2003, 343, 1–4. [Google Scholar] [CrossRef]
- Detanico, B.C.; Piato, A.L.; Freitas, J.J.; Lhullier, F.L.; Hidalgo, M.P.; Caumo, W.; Elisabetsky, E. Antidepressant-like effects of melatonin in the mouse chronic mild stress model. Eur. J. Pharmacol. 2009, 607, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Arezzi, A.; Rampello, L.; Drago, F. Melatonin affects the immobility time of rats in the forced swim test: The role of serotonin neurotransmission. Eur. Neuropsychopharmacol. 2006, 16, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Alonso, A.; Valdés-Tovar, M.; Solís-Chagoyán, H.; Benítez-King, G. Melatonin stimulates dendrite formation and complexity in the hilar zone of the rat hippocampus: Participation of the Ca++/Calmodulin complex. Int. J. Mol. Sci. 2015, 16, 1907–1927. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wei, N.; Man, H.Y.; Lu, Y.; Zhu, L.Q.; Wang, J.Z. The MT2 receptor stimulates axonogenesis and enhances synaptic transmission by activating Akt signaling. Cell Death Differ. 2015, 22, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Tovar, M.; Estrada-Reyes, R.; Solís-Chagoyán, H.; Argueta, J.; Dorantes-Barrón, A.M.; Quero-Chávez, D.; Cruz-Garduño, R.; Cercós, M.G.; Trueta, C.; Oikawa-Sala, J.; et al. Circadian modulation of neuroplasticity by melatonin: A target in the treatment of depression. Br. J. Pharmacol. 2018, 175, 3200–3208. [Google Scholar] [CrossRef] [PubMed]
- Igwe, S.C.; Brigo, F. Does Melatonin and Melatonin Agonists Improve the Metabolic Side Effects of Atypical Antipsychotics?: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Psychopharmacol. Neurosci. 2018, 16, 235–245. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.G.; Woo, Y.S.; Park, S.W.; Seog, D.-H.; Seo, M.K.; Bahk, W.-M. The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease. Brain Sci. 2019, 9, 285. https://doi.org/10.3390/brainsci9100285
Lee JG, Woo YS, Park SW, Seog D-H, Seo MK, Bahk W-M. The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease. Brain Sciences. 2019; 9(10):285. https://doi.org/10.3390/brainsci9100285
Chicago/Turabian StyleLee, Jung Goo, Young Sup Woo, Sung Woo Park, Dae-Hyun Seog, Mi Kyoung Seo, and Won-Myong Bahk. 2019. "The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease" Brain Sciences 9, no. 10: 285. https://doi.org/10.3390/brainsci9100285
APA StyleLee, J. G., Woo, Y. S., Park, S. W., Seog, D.-H., Seo, M. K., & Bahk, W.-M. (2019). The Neuroprotective Effects of Melatonin: Possible Role in the Pathophysiology of Neuropsychiatric Disease. Brain Sciences, 9(10), 285. https://doi.org/10.3390/brainsci9100285




