High Serum Caspase-Cleaved Cytokeratin-18 Levels and Mortality of Traumatic Brain Injury Patients
Abstract
:1. Introduction
2. Methods
2.1. Design and Subjects
2.2. Serum CCCK-18 Analysis
2.3. Statistical Methods
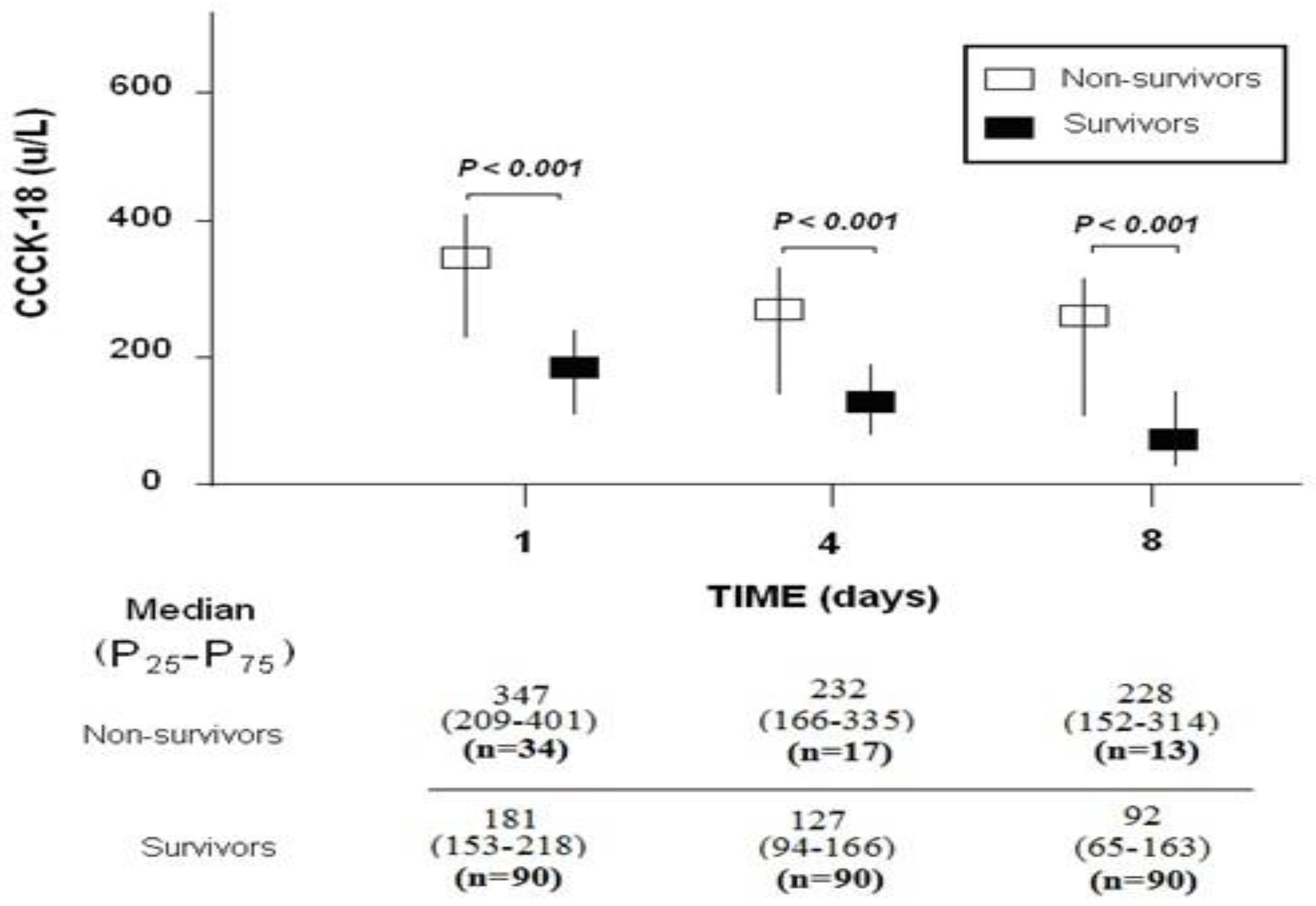
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons. Guidelines for the management of severe traumatic brain injury. J. Neurotrauma 2007, 24 Suppl 1, S1–S106. [Google Scholar] [CrossRef]
- Cavallucci, V.; D’Amelio, M. Matter of life and death: The pharmacological approaches targeting apoptosis in brain diseases. Curr. Pharm. Des. 2011, 17, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, B.; Ma, J. Research progress in traumatic brain penumbra. Chin. Med. J. (Engl) 2014, 127, 1964–1968. [Google Scholar] [PubMed]
- Rovegno, M.; Soto, P.A.; Sáez, J.C.; von Bernhardi, R. Biological mechanisms involved in the spread of traumatic brain damage. Med. Intensiva. 2012, 36, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.; Dirnagl, U.; Mergenthaler, P. Acute pathophysiological processes after ischaemic and traumatic brain injury. Best Pract. Res. Clin. Anaesthesiol. 2010, 24, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Raghupathi, R.; Conti, A.C.; Graham, D.I.; Krajewski, S.; Reed, J.C.; Grady, M.S.; Trojanowski, J.Q.; McIntosh, T.K. Mild traumatic brain injury induces apoptotic cell death in the cortex that is preceded by decreases in cellular Bcl-2 immunoreactivity. Neuroscience 2002, 110, 605–616. [Google Scholar] [CrossRef]
- Villapol, S.; Byrnes, K.R.; Symes, A.J. Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front. Neurol. 2014, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, J.; Jiang, B.; Wan, X.; Liu, H.; Liu, H.; Yang, X.; Wu, X.; Zou, Q.; Yang, W. Study of cell apoptosis in the hippocampus and thalamencephalon in a ventricular fluid impact model. Exp. Ther. Med. 2013, 6, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.S.; Kochanek, P.M.; Chen, M.; Watkins, S.C.; Marion, D.W.; Chen, J.; Hamilton, R.L.; Loeffert, J.E.; Graham, S.H. Increases in Bcl-2 and cleavage of caspase-1 and caspase-3 in human brain after head injury. FASEB J. 1999, 13, 813–821. [Google Scholar] [CrossRef]
- Miñambres, E.; Ballesteros, M.A.; Mayorga, M.; Marin, M.J.; Muñoz, P.; Figols, J.; López-Hoyos, M. Cerebral apoptosis in severe traumatic brain injury patients: An in vitro, in vivo, and postmortem study. J. Neurotrauma 2008, 25, 581–591. [Google Scholar] [CrossRef]
- Chu, P.G.; Weiss, L.M. Keratin expression in human tissues and neoplasms. Histopathology 2002, 40, 403–439. [Google Scholar] [CrossRef]
- Caulín, C.; Salvesen, G.S.; Oshima, R.G. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J. Cell Biol. 1997, 138, 1379–1394. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Martín, M.M.; González-Rivero, A.F.; Ferreres, J.; Solé-Violán, J.; Labarta, L.; Díaz, C.; Jiménez, A.; Borreguero-León, J.M. Serum levels of caspase-cleaved cytokeratin-18 and mortality are associated in severe septic patients: Pilot study. PLoS One 2014, 9, e109618. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Rodriguez, S.T.; Sanz, P.; Pérez-Cejas, A.; Padilla, J.; Díaz, D.; González, A.; Martín, M.M.; Jiménez, A.; Barrera, M.A. Prognostic value of serum caspase-cleaved cytokeratin-18 levels before liver transplantation for one-year survival of patients with hepatocellular carcinoma. Int. J. Mol. Sci. 2016, 17, 1524. [Google Scholar] [CrossRef]
- Luciani, P.; Gelmini, S.; Ferrante, E.; Lania, A.; Benvenuti, S.; Baglioni, S.; Mantovani, G.; Cellai, I.; Ammannati, F.; Spada, A.; et al. Expression of the antiapoptotic gene seladin-1 and octreotide-induced apoptosis in growth hormone-secreting and nonfunctioning pituitary adenomas. J. Clin. Endocrinol. Metab. 2005, 90, 6156–6161. [Google Scholar] [CrossRef] [PubMed]
- Ari, F.; Aztopal, N.; Oran, S.; Bozdemir, S.; Celikler, S.; Ozturk, S.; Ulukaya, E. Parmelia sulcata Taylor and Usnea filipendula Stirt induce apoptosis-like cell death and DNA damage in cancer cells. Cell Prolif. 2014, 47, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.G.; Wang, J.L.; Jin, G.L.; Yu, X.B.; Li, J.Q.; Qiu, T.L.; Dai, R.X. Serum caspase-cleaved cytokeratin-18 levels and outcomes after aneurysmal subarachnoid hemorrhage. J. Neurol. Sci. 2015, 359, 298–304. [Google Scholar] [CrossRef]
- Gu, S.J.; Lu, M.; Xuan, H.F.; Chen, X.Z.; Dong, W.F.; Yan, X.F.; Si, Y.; Gao, G.L.; Hu, D.X.; Miao, J.Q. Predictive value of serum caspase-cleaved cytokeratin-18 concentrations after acute intracerebral hemorrhage. Clin. Chim. Acta 2016, 452, 124–128. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. Association between serum levels of caspase-cleaved cytokeratin-18 and early mortality in patients with severe spontaneous intracerebral hemorrhage. BMC Neurosci. 2018, 19, 23. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Pérez-Cejas, A.; Ramos, L.; Argueso, M.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. High serum levels of caspase-cleaved cytokeratin-18 are associated with malignant middle cerebral artery infarction patient mortality. BMC Neurol. 2018, 18, 32. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; González-Rivero, A.F.; Argueso, M.; Ramos, L.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; Borreguero-León, J.M. Serum levels of caspase-cleaved cytokeratin-18 in patients with severe traumatic brain injury are associated with mortality: A pilot study. PLoS One 2015, 10, e0121739. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessement of coma and impaired conciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Baker, S.P.; O’Neill, B.; Haddon, W.; Jr Long, W.B. The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. J. Trauma 1974, 14, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care. Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.F.; Marshall, S.B.; Klauber, M.R.; Van Berkum Clark, M.; Eisenberg, H.; Jane, J.A.; Luerssen, T.G.; Marmarou, A.; Foulkes, M.A. The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma 1992, 9 Suppl 1, S287–S292. [Google Scholar]
- Lu, J.; Goh, S.J.; Tng, P.Y.; Deng, Y.Y.; Ling, E.A.; Moochhala, S. Systemic inflammatory response following acute traumatic brain injury. Front. Biosci. (Landmark Ed.) 2009, 14, 3795–3813. [Google Scholar] [CrossRef] [PubMed]
- Wesche-Soldato, D.E.; Swan, R.Z.; Chung, C.S.; Ayala, A. The apoptotic pathway as a therapeutic target in sepsis. Curr. Drug Targets 2007, 8, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Saykally, J.N.; Rachmany, L.; Hatic, H.; Shaer, A.; Rubovitch, V.; Pick, C.G.; Citron, B.A. The nuclear factor erythroid 2-like 2 activator, tert-butylhydroquinone, improves cognitive performance in mice after mild traumatic brain injury. Neuroscience 2012, 223, 305–314. [Google Scholar] [CrossRef]
- Abrahamson, E.E.; Ikonomovic, M.D.; Ciallella, J.R.; Hope, C.E.; Paljug, W.R.; Isanski, B.A.; Flood, D.G.; Clark, R.S.; DeKosky, S.T. Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: Implications for clinical outcome. Exp. Neurol. 2006, 197, 437–450. [Google Scholar] [CrossRef]
- Soustiel, J.F.; Palzur, E.; Nevo, O.; Thaler, I.; Vlodavsky, E. Neuroprotective anti-apoptosis effect of estrogens in traumatic brain injury. J. Neurotrauma 2005, 22, 345–352. [Google Scholar] [CrossRef]
- Clausen, F.; Lundqvist, H.; Ekmark, S.; Lewén, A.; Ebendal, T.; Hillered, L. Oxygen free radical-dependent activation of extracellular signal-regulated kinase mediates apoptosis-like cell death after traumatic brain injury. J. Neurotrauma 2004, 21, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.S.; Kochanek, P.M.; Watkins, S.C.; Chen, M.; Dixon, C.E.; Seidberg, N.A.; Melick, J.; Loeffert, J.E.; Nathaniel, P.D.; Jin, K.L.; et al. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J. Neurochem. 2000, 74, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Mejia, R.O.; Ona, V.O.; Li, M.; Friedlander, R.M. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery 2001, 48, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Song, Z.; Wan, Y.; Wang, K.; Mo, L.; Wang, Y. Calcium-sensing receptor antagonist NPS2390 attenuates neuronal apoptosis though intrinsic pathway following traumatic brain injury in rats. Biochem. Biophys. Res. Commun. 2017, 486, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gu, Z.T.; Li, L.; Maegele, M.; Zhou, B.Y.; Li, F.; Zhao, M.; Zhao, K.S. SIRT1 plays a neuroprotective role in traumatic brain injury in rats via inhibiting the p38 MAPK pathway. Acta Pharmacol. Sin. 2017, 38, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Hartings, J.A.; Bullock, M.R.; Okonkwo, D.O.; Murray, L.S.; Murray, G.D.; Fabricius, M.; Maas, A.I.; Woitzik, J.; Sakowitz, O.; Mathern, B.; et al. Spreading depolarisations and outcome after traumatic brain injury: A prospective observational study. Lancet Neurol. 2011, 10, 1058–1064. [Google Scholar] [CrossRef]
- Fabricius, M.; Fuhr, S.; Bhatia, R.; Boutelle, M.; Hashemi, P.; Strong, A.J.; Lauritzen, M. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain 2006, 129, 778–790. [Google Scholar] [CrossRef]
- Jahanbazi Jahan-Abad, A.; Alizadeh, L.; Sahab Negah, S.; Barati, P.; Khaleghi Ghadiri, M.; Meuth, S.G.; Kovac, S.; Gorji, A. Apoptosis Following Cortical Spreading Depression in Juvenile Rats. Mol. Neurobiol. 2018, 55, 4225–4239. [Google Scholar] [CrossRef]
| Survivors (n = 90) | Non-Survivors (n = 34) | p-Value | |
|---|---|---|---|
| Brain CT injury—n (%) | 0.01 | ||
| Diffuse injury I | 0 | 0 | |
| Diffuse injury II | 25 (27.8) | 5 (14.7) | |
| Diffuse injury III | 15 (16.7) | 6 (17.6) | |
| Diffuse injury IV | 13 (14.4) | 9 (26.5) | |
| Evacuated mass lesion V | 32 (35.6) | 6 (17.6) | |
| Non-evacuated mass lesion VI | 5 (5.6) | 8 (23.5) | |
| Brain CT with high death risk (III, IV, VI)—n (%) | 33 (36.7) | 23 (67.6) | 0.002 |
| Gender female—n (%) | 15 (16.7) | 13 (38.2) | 0.02 |
| Decompressive craniectomy—n (%) | 13 (14.4) | 5 (14.7) | 0.99 |
| Age (years)—m (p 25–75) | 46 (28–62) | 65 (55–75) | <0.001 |
| Sodium (mEq/L)—m (p 25–75) | 140 (138–143) | 141 (136–147) | 0.41 |
| Glycemia (g/dL)—m (p 25–75) | 139 (121–167) | 160 (125–191) | 0.11 |
| Lactic acid (mmol/L)—m (p 25–75) | 1.75 (1.10–2.50) | 2.30 (1.25–4.58) | 0.08 |
| Bilirubin (mg/dL)—m (p 25–75) | 0.60 (0.40–0.80) | 0.70 (0.53–1.05) | 0.06 |
| Creatinine (mg/dL)—m (p 25–75) | 0.80 (0.70–1.00) | 0.80 (0.70–1.10) | 0.50 |
| INR—m (p 25–75) | 1.11 (1.00–1.24) | 1.12 (1.03–1.48) | 0.19 |
| Platelets—m × 103/mm3 (p 25–75) | 182 (135–238) | 172 (125–232) | 0.49 |
| aPTT (seconds)—m (p 25–75) | 28 (25–31) | 29 (25–37) | 0.25 |
| PaO2/FI02 ratio—m (p 25–75) | 336 (246–400) | 294 (167–395) | 0.11 |
| PaO2 (mmHg)—m (p 25–75) | 148 (110–242) | 142 (97–195) | 0.45 |
| Leukocytes—m × 103/mm3 (p 25–75) | 13.9 (10.1–19.0) | 14.9 (9.7–21.6) | 0.47 |
| Fibrinogen (mg/dL)—m (p 25–75) | 371 (286–471) | 348 (300–475) | 0.70 |
| Hemoglobin (g/dL)—m (p 25–75) | 11.2 (10.0–13.0) | 11.9 (10.0–13.7) | 0.73 |
| Glasgow Coma Scale—m (p 25–75) | 7 (5–8) | 4 (3–7) | <0.001 |
| ICP peak (mmHg)—m (p 25–75) | 15 (14–20) | 25 (11–30) | 0.08 |
| CPP small (mmHg)—m (p 25–75) | 68 (57–70) | 61 (52–70) | 0.20 |
| APACHE-II—m (p 25–75) | 18 (14–22) | 25 (23–28) | <0.001 |
| ISS—m (p 25–75) | 25 (25–29) | 25 (25–26) | 0.59 |
| CCCK-18 (µ/L)—m (p 25–75) | 181 (153–218) | 347 (209–401) | <0.001 |
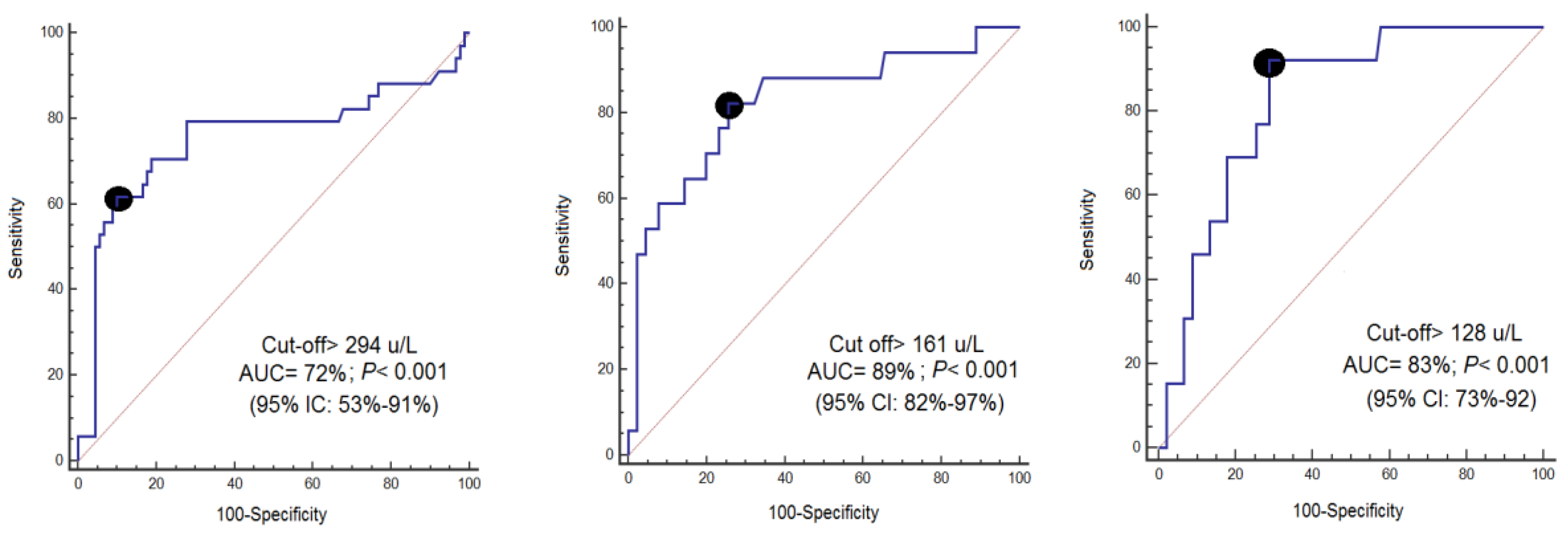
| Day 1 | Day 4 | Day 8 | |
|---|---|---|---|
| Cut-off of CCCK-18 (µ/L) | >294 | >161 | >128 |
| Specificity (95% CI) | 90% (82–95%) | 74% (64–83%) | 71% (61–80%) |
| Sensitivity (95% CI) | 62% (44–78%) | 82% (57–96%) | 92% (64–99%) |
| Negative likelihood ratio (95% CI) | 0.4 (0.3–0.7) | 0.2 (0.1–0.7) | 0.1 (0.02–0.70) |
| Positive likelihood ratio (95% CI) | 6.2 (3.1–12.1) | 3.2 (2.1–4.9) | 3.3 (2.2–4.6) |
| Negative predicted value (95% CI) | 86% (80–91%) | 96% (89–98%) | 98% (91–99%) |
| Positive predicted value (95% CI) | 70% (54–82%) | 38% (29–48%) | 32% (24–40%) |
| Variable | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Serum CCCK-18 (µ/L) | 1.02 | 1.01–1.03 | <0.001 |
| Sex (female vs. male) | 5.77 | 1.17–28.43 | 0.03 |
| CT classification (high vs. low risk of death) | 3.61 | 0.99–13.22 | 0.052 |
| APACHE-II score (points) | 1.38 | 1.17–1.63 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorente, L.; Martín, M.M.; González-Rivero, A.F.; Pérez-Cejas, A.; Argueso, M.; Ramos, L.; Solé-Violán, J.; Cáceres, J.J.; Jiménez, A.; García-Marín, V. High Serum Caspase-Cleaved Cytokeratin-18 Levels and Mortality of Traumatic Brain Injury Patients. Brain Sci. 2019, 9, 269. https://doi.org/10.3390/brainsci9100269
Lorente L, Martín MM, González-Rivero AF, Pérez-Cejas A, Argueso M, Ramos L, Solé-Violán J, Cáceres JJ, Jiménez A, García-Marín V. High Serum Caspase-Cleaved Cytokeratin-18 Levels and Mortality of Traumatic Brain Injury Patients. Brain Sciences. 2019; 9(10):269. https://doi.org/10.3390/brainsci9100269
Chicago/Turabian StyleLorente, Leonardo, María M. Martín, Agustín F. González-Rivero, Antonia Pérez-Cejas, Mónica Argueso, Luis Ramos, Jordi Solé-Violán, Juan J. Cáceres, Alejandro Jiménez, and Victor García-Marín. 2019. "High Serum Caspase-Cleaved Cytokeratin-18 Levels and Mortality of Traumatic Brain Injury Patients" Brain Sciences 9, no. 10: 269. https://doi.org/10.3390/brainsci9100269
APA StyleLorente, L., Martín, M. M., González-Rivero, A. F., Pérez-Cejas, A., Argueso, M., Ramos, L., Solé-Violán, J., Cáceres, J. J., Jiménez, A., & García-Marín, V. (2019). High Serum Caspase-Cleaved Cytokeratin-18 Levels and Mortality of Traumatic Brain Injury Patients. Brain Sciences, 9(10), 269. https://doi.org/10.3390/brainsci9100269





