Comparative Behavioral Phenotypes of Fmr1 KO, Fxr2 Het, and Fmr1 KO/Fxr2 Het Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Behavior Testing
2.3. Open Field
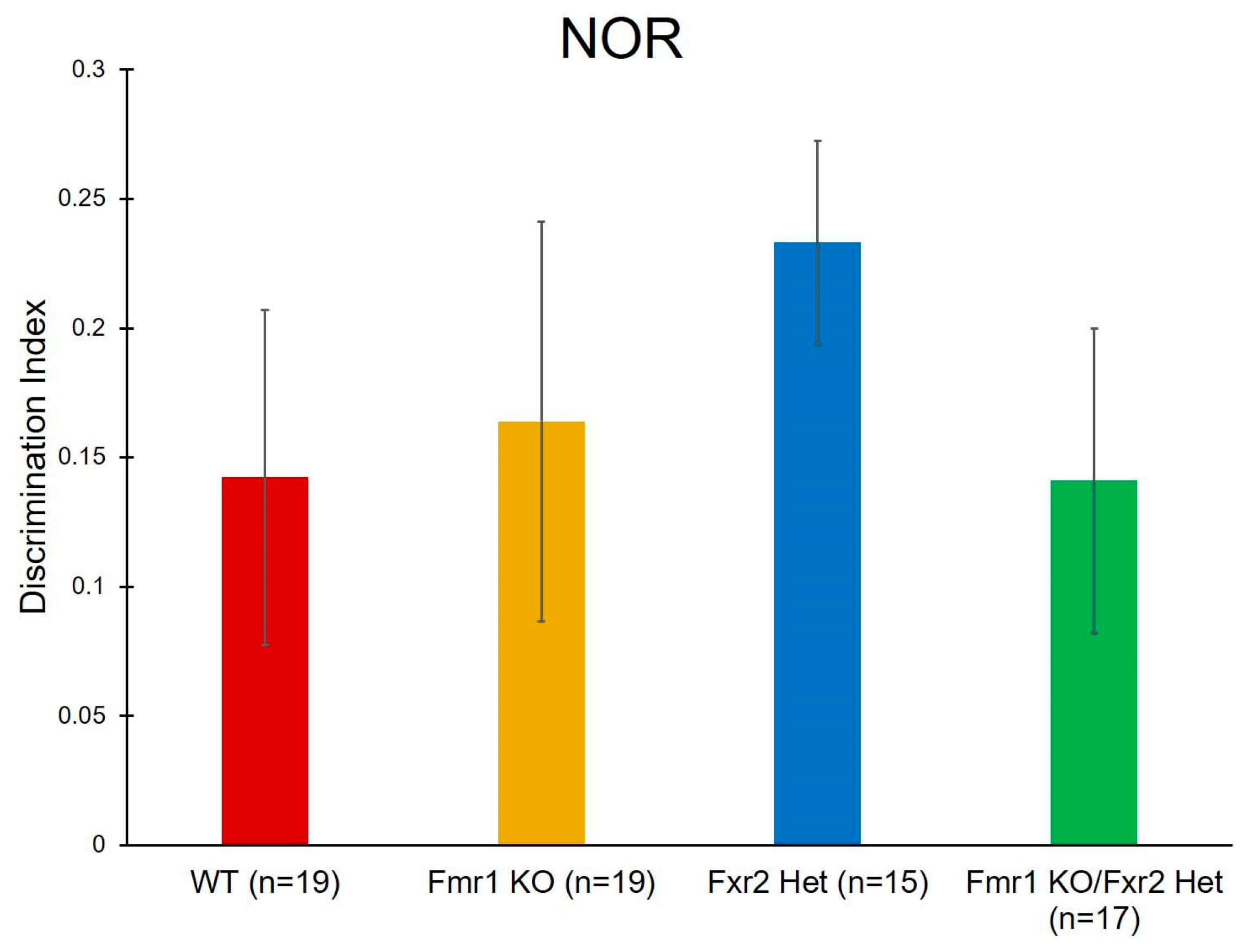
2.4. Novel Object Recognition (NOR)
2.5. Zero Maze
2.6. Marble Burying
2.7. Social Behavior
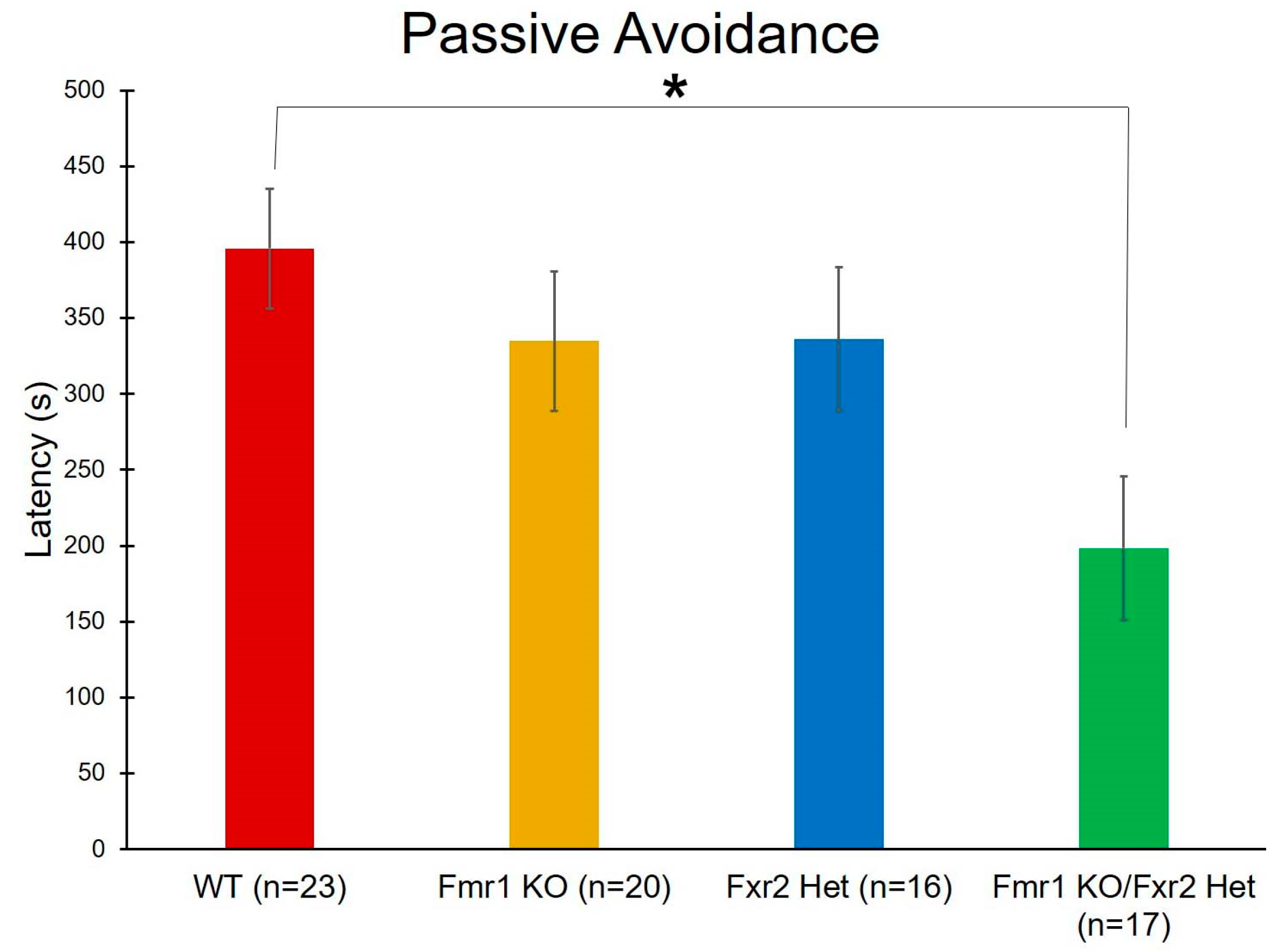
2.8. Passive Avoidance
2.9. Protein Expression
2.10. Statistical Analysis
3. Results
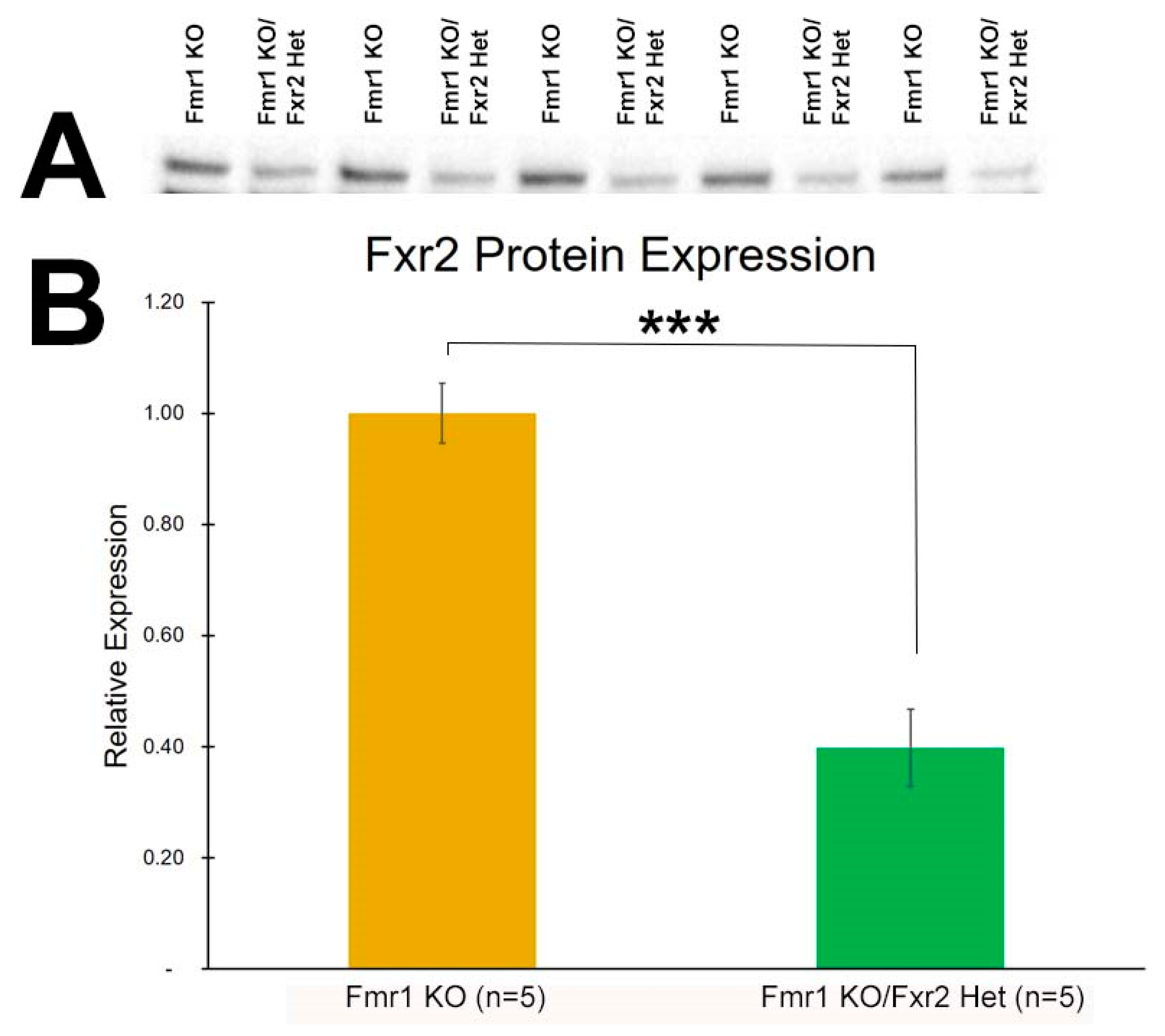
3.1. Expression of FXR2
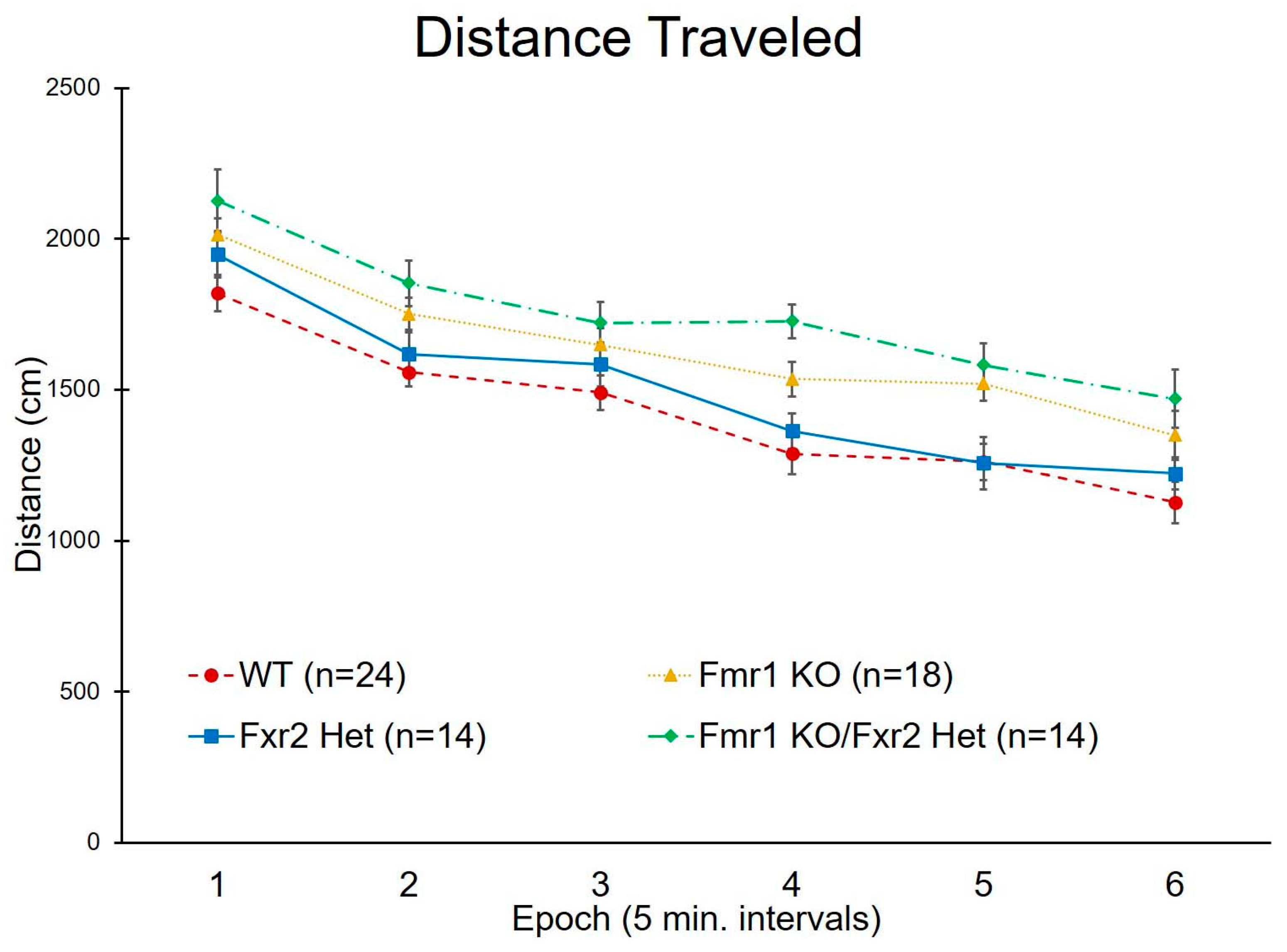
3.2. Activity in the Open Field
3.3. Anxiety-Like Behavior
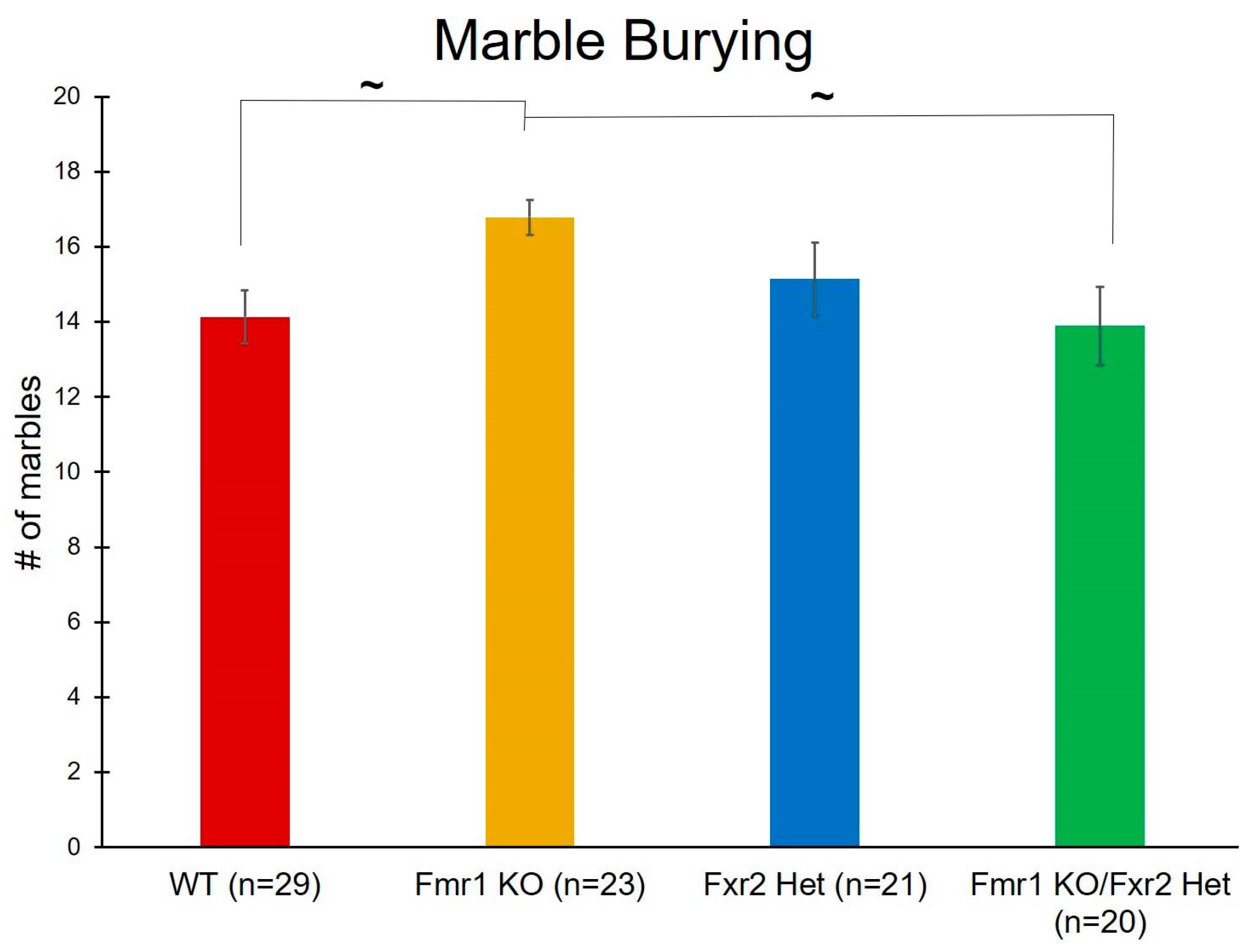
3.4. Repetitive Behavior
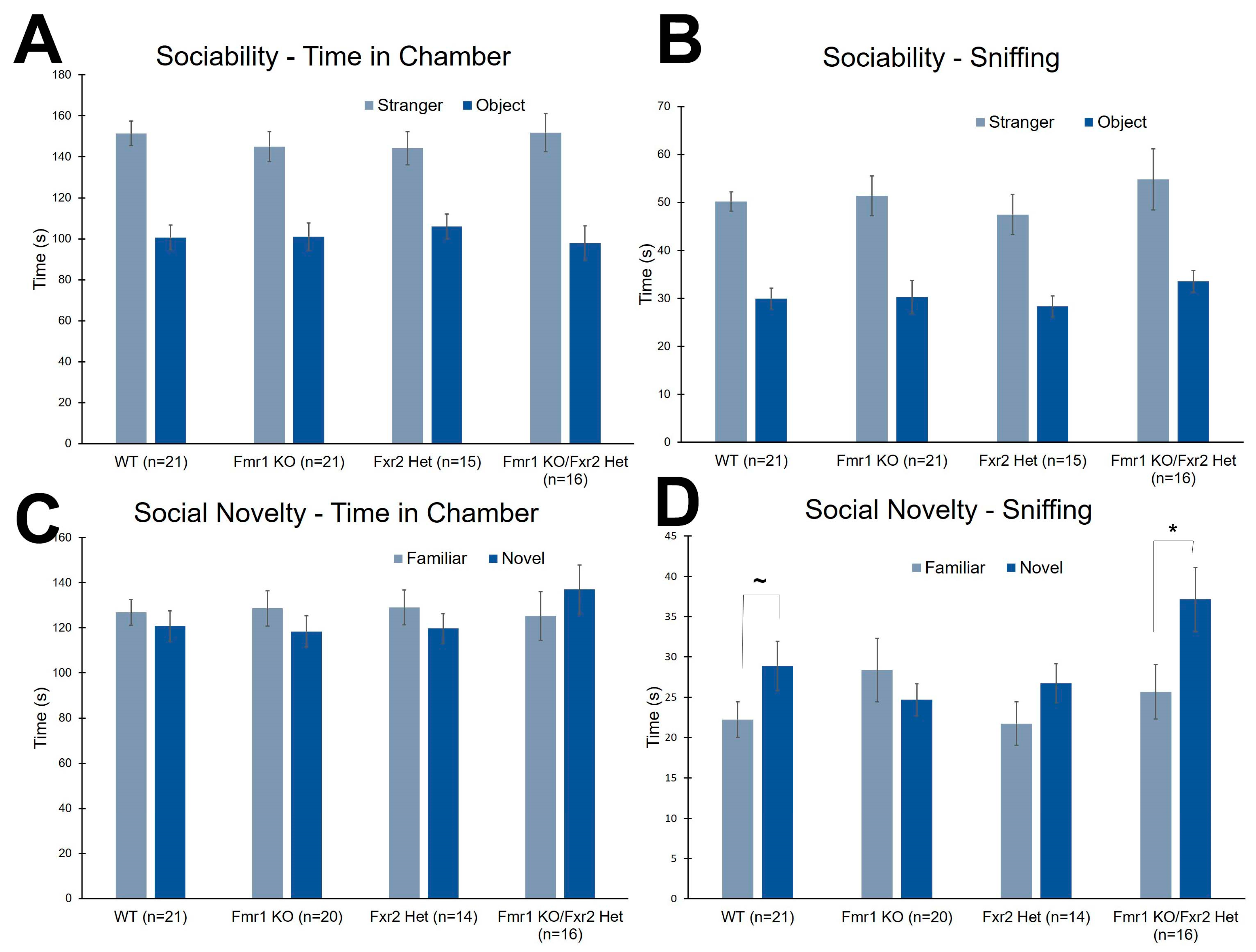
3.5. Social Behavior
3.6. Learning and Memory
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Turner, G.; Webb, T.; Wake, S.; Robinson, H. Prevalence of fragile X syndrome. Am. J. Med. Genet. 1996, 64, 196–197. [Google Scholar] [CrossRef]
- Garber, K.B.; Visootsak, J.; Warren, S.T. Fragile X syndrome. Eur. J. Hum. Genet. EJHG 2008, 16, 666–672. [Google Scholar] [CrossRef]
- Verheij, C.; Bakker, C.E.; de Graaff, E.; Keulemans, J.; Willemsen, R.; Verkerk, A.J.; Galjaard, H.; Reuser, A.J.; Hoogeveen, A.T.; Oostra, B.A. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature 1993, 363, 722–724. [Google Scholar] [CrossRef]
- Darnell, J.C.; Van Driesche, S.J.; Zhang, C.; Hung, K.Y.; Mele, A.; Fraser, C.E.; Stone, E.F.; Chen, C.; Fak, J.J.; Chi, S.W.; et al. Fmrp stalls ribosomal translocation on mrnas linked to synaptic function and autism. Cell 2011, 146, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Darnell, J.C.; Klann, E. The translation of translational control by Fmrp: Therapeutic targets for fxs. Nat. Neurosci. 2013, 16, 1530–1536. [Google Scholar] [CrossRef]
- Qin, M.; Kang, J.; Burlin, T.V.; Jiang, C.; Smith, C.B. Postadolescent changes in regional cerebral protein synthesis: An in vivo study in the Fmr1 null mouse. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 5087–5095. [Google Scholar] [CrossRef] [PubMed]
- Bardoni, B.; Schenck, A.; Mandel, J.L. The fragile X mental retardation protein. Brain Res. Bull. 2001, 56, 375–382. [Google Scholar] [CrossRef]
- Ferron, L. Fragile x mental retardation protein controls ion channel expression and activity. J. Physiol. 2016, 594, 5861–5867. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, F.; Bi, X.; Zhang, Y.Q. Drosophila fmrp participates in the DNA damage response by regulating g2/m cell cycle checkpoint and apoptosis. Hum. Mol. Genet. 2012, 21, 4655–4668. [Google Scholar] [CrossRef] [PubMed]
- Bakker, C.E.; Verheij, C.; Willemsen, R.; van der Helm, R.; Oerlemans, F.; Vermey, M.; Bygrave, A.; Hoogeveen, A.; Oostra, B.A.; Reyniers, E.; et al. Fmr1 knockout mice: A model to study fragile X mental retardation. The dutch-belgian fragile X consortium. Cell 1994, 78, 23–33. [Google Scholar]
- Sare, R.M.; Harkless, L.; Levine, M.; Torossian, A.; Sheeler, C.A.; Smith, C.B. Deficient sleep in mouse models of fragile X syndrome. Front. Mol. Neurosci. 2017, 10, 280. [Google Scholar] [CrossRef]
- Kazdoba, T.M.; Leach, P.T.; Silverman, J.L.; Crawley, J.N. Modeling fragile X syndrome in the Fmr1 knockout mouse. Intractable Rare Dis. Res. 2014, 3, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; O’Connor, J.P.; Siomi, M.C.; Srinivasan, S.; Dutra, A.; Nussbaum, R.L.; Dreyfuss, G. The fragile X mental retardation syndrome protein interacts with novel homologs FXR1 and FXR2. EMBO J. 1995, 14, 5358–5366. [Google Scholar] [CrossRef] [PubMed]
- Bakker, C.E.; de Diego Otero, Y.; Bontekoe, C.; Raghoe, P.; Luteijn, T.; Hoogeveen, A.T.; Oostra, B.A.; Willemsen, R. Immunocytochemical and biochemical characterization of FMRP, FXR1P, and FXR2P in the mouse. Exp. Cell Res. 2000, 258, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Bontekoe, C.J.; McIlwain, K.L.; Nieuwenhuizen, I.M.; Yuva-Paylor, L.A.; Nellis, A.; Willemsen, R.; Fang, Z.; Kirkpatrick, L.; Bakker, C.E.; McAninch, R.; et al. Knockout mouse model for Fxr2: A model for mental retardation. Hum. Mol. Genet. 2002, 11, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Giordano, L.; Palestra, F.; Giuffrida, M.G.; Molinaro, A.; Iodice, A.; Bernardini, L.; La Boria, P.; Accorsi, P.; Novelli, A. 17p13.1 microdeletion: Genetic and clinical findings in a new patient with epilepsy and comparison with literature. Am. J. Med. Genet. Part A 2014, 164A, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Schluth-Bolard, C.; Sanlaville, D.; Labalme, A.; Till, M.; Morin, I.; Touraine, R.; Edery, P. 17p13.1 microdeletion involving the TP53 gene in a boy presenting with mental retardation but no tumor. Am. J. Med. Genet. Part A 2010, 152A, 1278–1282. [Google Scholar] [CrossRef]
- Stepniak, B.; Kastner, A.; Poggi, G.; Mitjans, M.; Begemann, M.; Hartmann, A.; van der Auwera, S.; Sananbenesi, F.; Krueger-Burg, D.; Matuszko, G.; et al. Accumulated common variants in the broader fragile X gene family modulate autistic phenotypes. EMBO Mol. Med. 2015, 7, 1565–1579. [Google Scholar] [CrossRef]
- Mientjes, E.J.; Willemsen, R.; Kirkpatrick, L.L.; Nieuwenhuizen, I.M.; Hoogeveen-Westerveld, M.; Verweij, M.; Reis, S.; Bardoni, B.; Hoogeveen, A.T.; Oostra, B.A.; et al. Fxr1 knockout mice show a striated muscle phenotype: Implications for Fxr1p function in vivo. Hum. Mol. Genet. 2004, 13, 1291–1302. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, L.; Klann, E.; Nelson, D.L. Altered hippocampal synaptic plasticity in the Fmr1 gene family knockout mouse models. J. Neurophysiol. 2009, 101, 2572–2580. [Google Scholar] [CrossRef]
- Spencer, C.M.; Serysheva, E.; Yuva-Paylor, L.A.; Oostra, B.A.; Nelson, D.L.; Paylor, R. Exaggerated behavioral phenotypes in Fmr1/Fxr2 double knockout mice reveal a functional genetic interaction between fragile X-related proteins. Hum. Mol. Genet. 2006, 15, 1984–1994. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, Z.; Jud, C.; Vansteensel, M.J.; Kaasik, K.; Lee, C.C.; Albrecht, U.; Tamanini, F.; Meijer, J.H.; Oostra, B.A.; et al. Fragile X-related proteins regulate mammalian circadian behavioral rhythms. Am. J. Hum. Genet. 2008, 83, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Nadler, J.J.; Moy, S.S.; Dold, G.; Trang, D.; Simmons, N.; Perez, A.; Young, N.B.; Barbaro, R.P.; Piven, J.; Magnuson, T.R.; et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004, 3, 303–314. [Google Scholar] [CrossRef]
- Sare, R.M.; Song, A.; Loutaev, I.; Cook, A.; Maita, I.; Lemons, A.; Sheeler, C.; Smith, C.B. Negative effects of chronic rapamycin treatment on behavior in a mouse model of fragile X syndrome. Front. Mol. Neurosci. 2017, 10, 452. [Google Scholar] [CrossRef]
- Corr, P.J. J.A. Gray’s reinforcement sensitivity theory: Tests of the joint subsystems hypothesis of anxiety and impulsivity. Personal. Individ. Differ. 2002, 33, 511–532. [Google Scholar] [CrossRef]
- Moon, J.; Beaudin, A.E.; Verosky, S.; Driscoll, L.L.; Weiskopf, M.; Levitsky, D.A.; Crnic, L.S.; Strupp, B.J. Attentional dysfunction, impulsivity, and resistance to change in a mouse model of fragile X syndrome. Behav. Neurosci. 2006, 120, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Martin, L.J.; Isbester, K.A.; Sotocinal, S.G.; Rosen, S.; Tuttle, A.H.; Wieskopf, J.S.; Acland, E.L.; Dokova, A.; Kadoura, B.; et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nature Methods 2014, 11, 629–632. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Rivera, S.M.; Hagerman, P.J. The fragile X family of disorders: A model for autism and targeted treatments. Curr. Pediatr. Rev. 2008, 4, 40–52. [Google Scholar] [CrossRef]
- Christie, S.B.; Akins, M.R.; Schwob, J.E.; Fallon, J.R. The fxg: A presynaptic fragile X granule expressed in a subset of developing brain circuits. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 1514–1524. [Google Scholar] [CrossRef]
- Chyung, E.; LeBlanc, H.F.; Fallon, J.R.; Akins, M.R. Fragile X granules are a family of axonal ribonucleoprotein particles with circuit-dependent protein composition and mrna cargos. J. Comp. Neurol. 2018, 526, 96–108. [Google Scholar] [CrossRef]
- Lumaban, J.G.; Nelson, D.L. The fragile X proteins Fmrp and Fxr2p cooperate to regulate glucose metabolism in mice. Hum. Mol. Genet. 2015, 24, 2175–2184. [Google Scholar] [CrossRef] [PubMed]


| ANOVA Results | ||||
|---|---|---|---|---|
| Behavior | Interaction | Main Effect | F(df,error) Value | p-Value |
| Open Field | ||||
| Distance | Genotype × epoch | F(14,300) = 0.796 | 0.671 | |
| Genotype | F(3,66) = 7.477 | <0.001 * | ||
| Epoch | F(5,300) = 105.254 | <0.001 * | ||
| Center/Total Distance | Genotype × epoch | F(13,279) = 2.390 | 0.005 * | |
| Genotype | F(3,66) = 13.296 | <0.001 * | ||
| Epoch | F(4,279) = 2.034 | 0.074 ~ | ||
| Zero Maze | Genotype | F(3,92) = 2.283 | 0.084 ~ | |
| Marble Burying | Genotype | F(3,89) = 2.675 | 0.052 ~ | |
| Sociability | ||||
| Time in Chamber | Genotype × chamber | F(3,69) = 0.252 | 0.860 | |
| Genotype | F(3,69) = 0.236 | 0.871 | ||
| Chamber | F(1,69) = 47.857 | <0.001 * | ||
| Sniffing Time | Genotype × chamber | F(3,67) = 0.026 | 0.994 | |
| Genotype | F(3,67) = 1.186 | 0.322 | ||
| Chamber | F(1,67) = 53.519 | <0.001 * | ||
| Social Novelty | ||||
| Time in Chamber | Genotype × chamber | F(3,69) = 0.427 | 0.734 | |
| Genotype | F(3,69) = 1.981 | 0.125 | ||
| Chamber | F(1,69) = 0.206 | 0.651 | ||
| Sniffing Time | Genotype × chamber | F(3,67) = 2.871 | 0.043 * | |
| Genotype | F(3,67) = 1.516 | 0.218 | ||
| Chamber | F(1,67) = 6.344 | 0.014 * | ||
| NOR | Genotype | F(3,70) = 0.519 | 0.671 | |
| Passive Avoidance | Genotype | F(3,72) = 3.421 | 0.022 * | |
| p-Values for Post Hoc Pairwise Comparisons—Center: Total Distance Ratio Open Field | ||||||
|---|---|---|---|---|---|---|
| Epoch | WT × Fmr1 | WT × Fxr2 | WT × Fmr1/Fxr2 | Fmr1 × Fxr2 | Fmr1 × Fmr1/Fxr2 | Fxr2 × Fmr1/Fxr2 |
| 1 | 0.973 | 1.000 | 0.085 ~ | 1.000 | 1.000 | 1.000 |
| 2 | 0.036 * | 1.000 | <0.001 * | 0.312 | 0.567 | 0.006 * |
| 3 | 0.261 | 1.000 | <0.001 * | 1.000 | 0.031 * | 0.003 * |
| 4 | 0.014 * | 1.000 | 0.001 * | 0.710 | 1.000 | 0.128 |
| 5 | 0.001 * | 1.000 | 0.003 * | 0.006 * | 1.000 | 0.010 * |
| 6 | <0.001 * | 1.000 | <0.001 * | 0.005 * | 1.000 | 0.005 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saré, R.M.; Figueroa, C.; Lemons, A.; Loutaev, I.; Beebe Smith, C. Comparative Behavioral Phenotypes of Fmr1 KO, Fxr2 Het, and Fmr1 KO/Fxr2 Het Mice. Brain Sci. 2019, 9, 13. https://doi.org/10.3390/brainsci9010013
Saré RM, Figueroa C, Lemons A, Loutaev I, Beebe Smith C. Comparative Behavioral Phenotypes of Fmr1 KO, Fxr2 Het, and Fmr1 KO/Fxr2 Het Mice. Brain Sciences. 2019; 9(1):13. https://doi.org/10.3390/brainsci9010013
Chicago/Turabian StyleSaré, Rachel Michelle, Christopher Figueroa, Abigail Lemons, Inna Loutaev, and Carolyn Beebe Smith. 2019. "Comparative Behavioral Phenotypes of Fmr1 KO, Fxr2 Het, and Fmr1 KO/Fxr2 Het Mice" Brain Sciences 9, no. 1: 13. https://doi.org/10.3390/brainsci9010013
APA StyleSaré, R. M., Figueroa, C., Lemons, A., Loutaev, I., & Beebe Smith, C. (2019). Comparative Behavioral Phenotypes of Fmr1 KO, Fxr2 Het, and Fmr1 KO/Fxr2 Het Mice. Brain Sciences, 9(1), 13. https://doi.org/10.3390/brainsci9010013





