A Pilot Study Investigating a Novel Non-Linear Measure of Eyes Open versus Eyes Closed EEG Synchronization in People with Alzheimer’s Disease and Healthy Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Magnetic Resonance Imaging Acquisition
2.4. Magnetic Resonance Imaging Analysis
2.5. EEG Recordings
2.6. Neuropsychological Assessment
2.7. Statistical Methods
3. Results
3.1. MRI Results
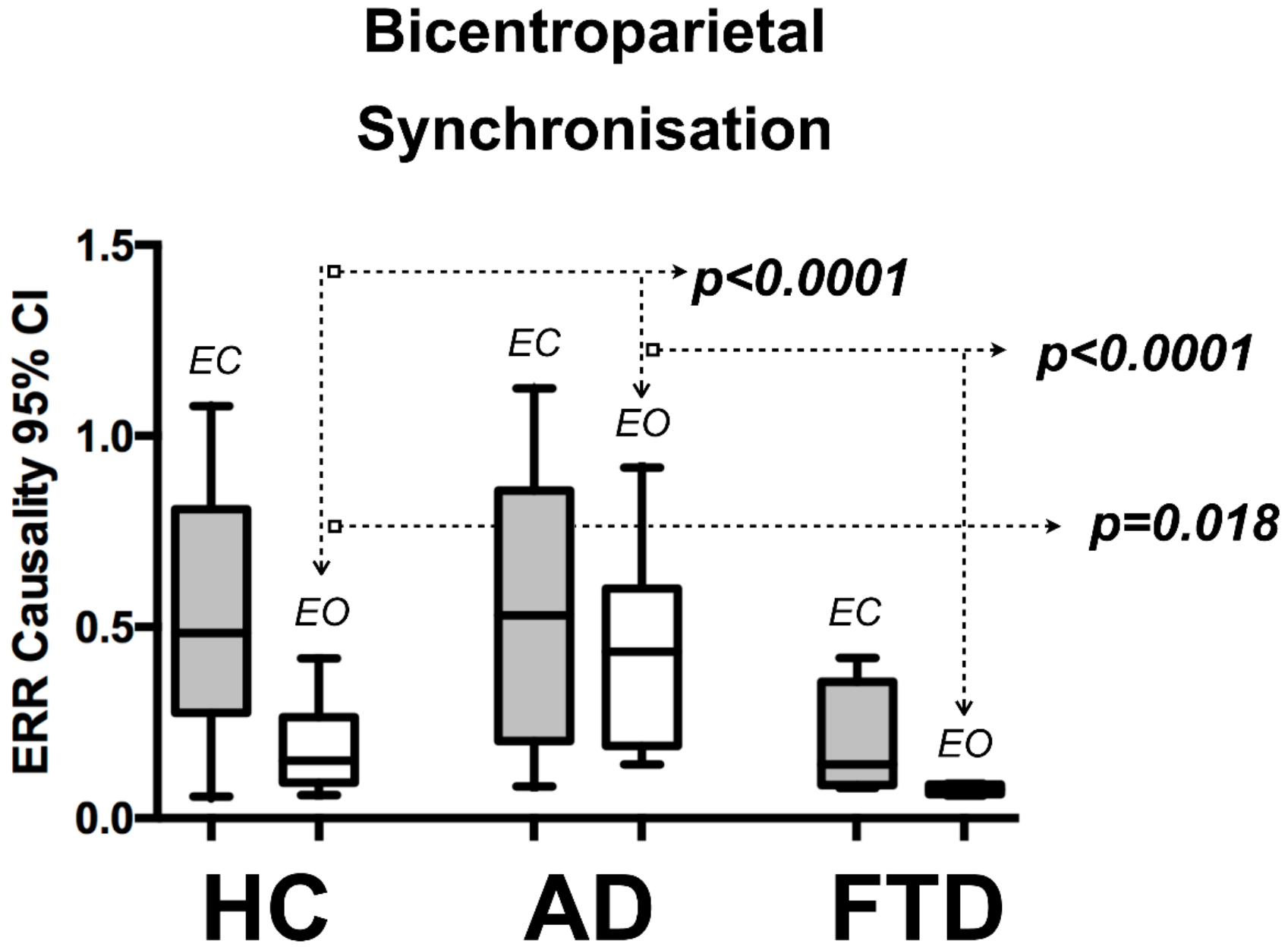
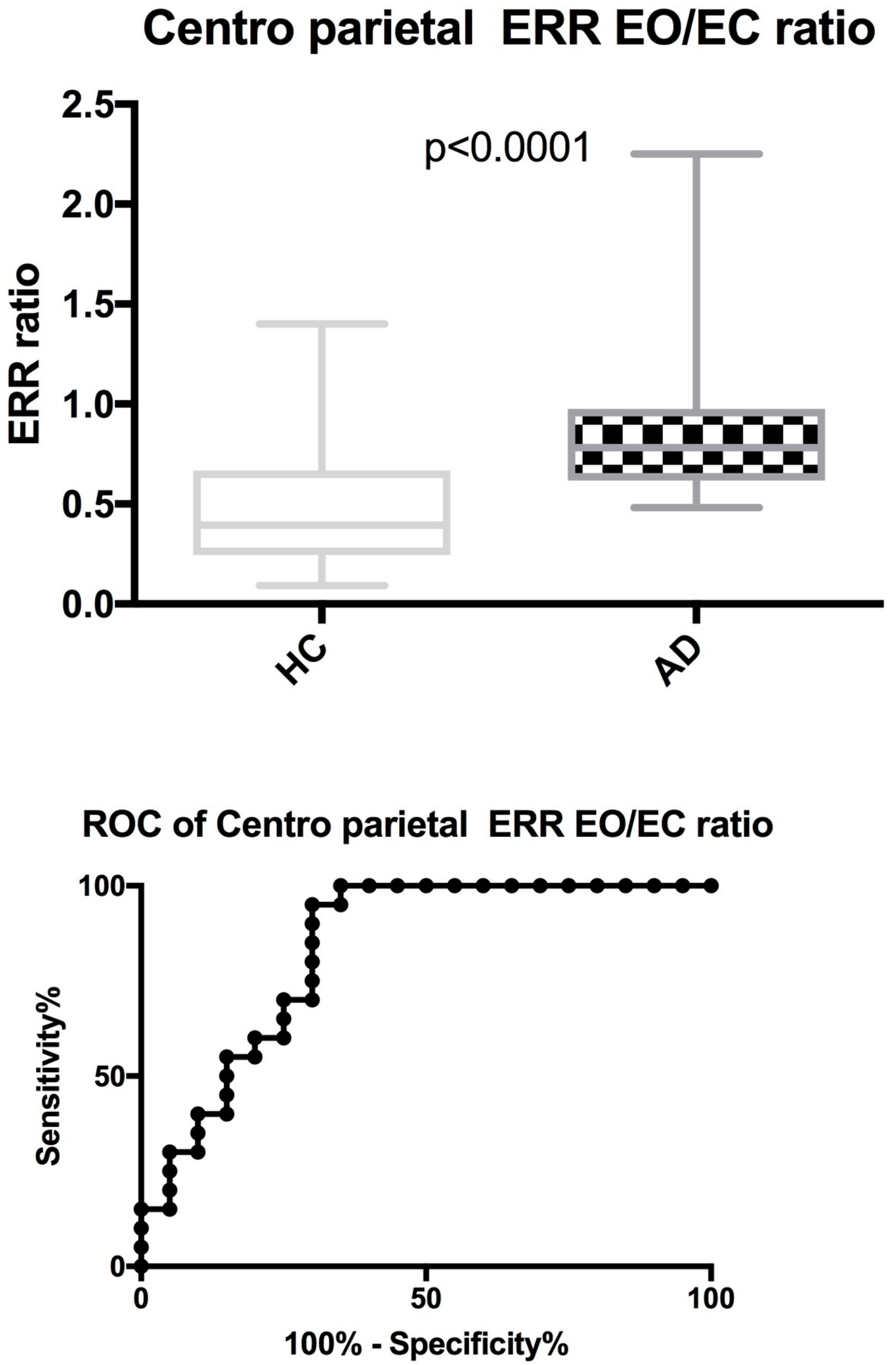
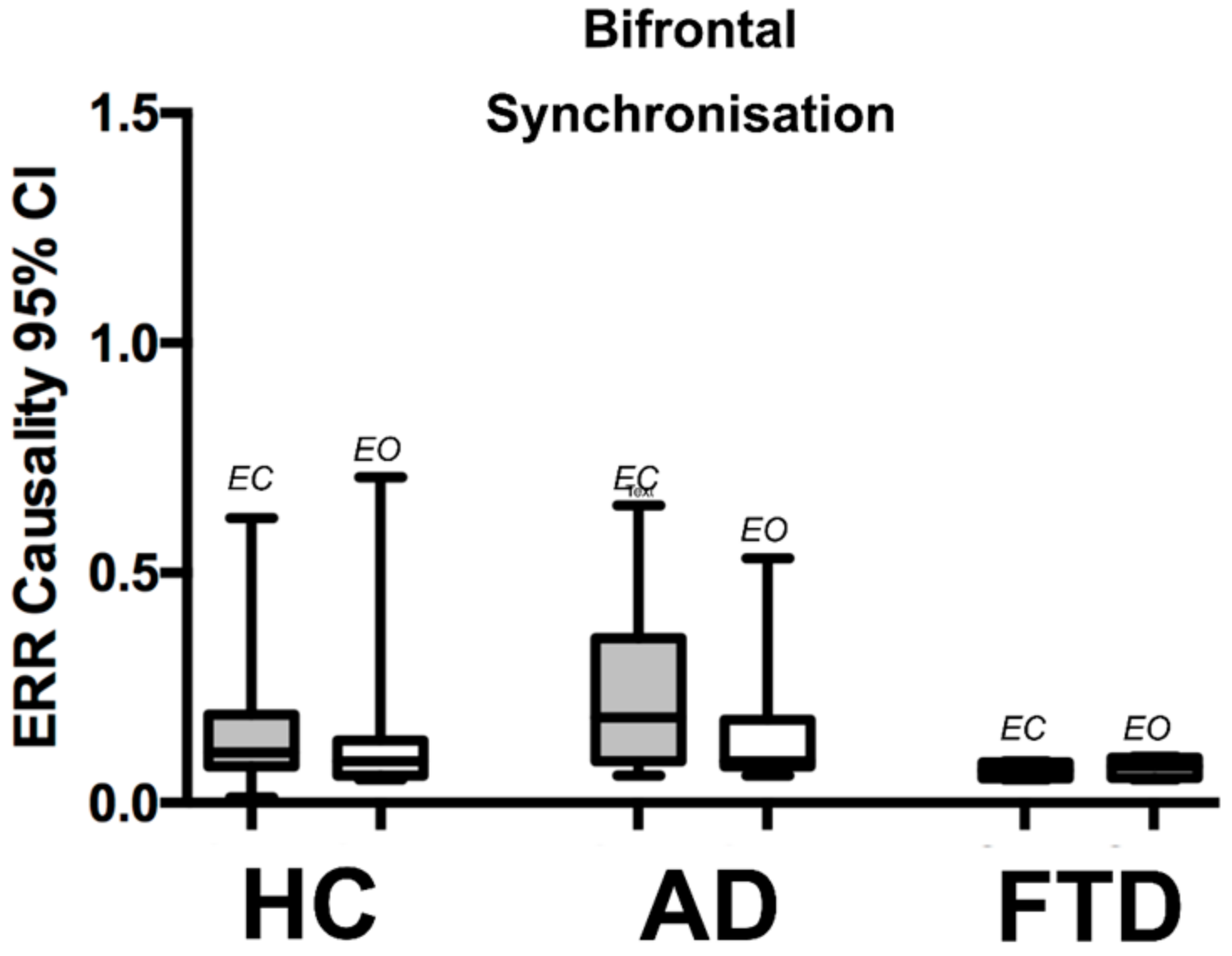
3.2. QEEG Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A. The Error Reduction Ratio (ERR) Causality tEst
References
- Laske, C.; Sohrabi, H.R.; Frost, S.M.; López-de-Ipiña, K.; Garrard, P.; Buscema, M.; Dauwels, J.; Soekadar, S.R.; Mueller, S.; Linnemann, C.; et al. Innovative diagnostic tools for early detection of alzheimer’s disease. Alzheimers Dement. 2015, 11, 561–578. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J. Eeg dynamics in patients with alzheimer’s disease. Clin. Neurophysiol. 2004, 115, 1490–1505. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.P.; Ulrich, R.F.; Spiker, D.G.; Sclabassi, R.J.; Reynolds, C.F., 3rd.; Marin, R.S.; Boller, F. Computerized eeg spectral analysis in elderly normal, demented and depressed subjects. Electroencephalogr. Clin. Neurophysiol. 1986, 64, 483–492. [Google Scholar] [CrossRef]
- Pievani, M.; de Haan, W.; Wu, T.; Seeley, W.W.; Frisoni, G.B. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011, 10, 829–843. [Google Scholar] [CrossRef]
- Stam, C.J. Modern network science of neurological disorders. Nat. Rev. Neurosci. 2014, 15, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Besthorn, C.; Forstl, H.; Geiger-Kabisch, C.; Sattel, H.; Gasser, T.; Schreiter-Gasser, U. Eeg coherence in alzheimer disease. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 242–245. [Google Scholar] [CrossRef]
- Dunkin, J.J.; Leuchter, A.F.; Newton, T.F.; Cook, I.A. Reduced eeg coherence in dementia: State or trait marker? Biol. Psychiatry 1994, 35, 870–879. [Google Scholar] [CrossRef]
- Locatelli, T.; Cursi, M.; Liberati, D.; Franceschi, M.; Comi, G. Eeg coherence in alzheimer’s disease. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 229–237. [Google Scholar] [CrossRef]
- Stam, C.J.; Dijk, B.W.V. Synchronization likelihood: An unbiased measure of generalized synchronization in multivariate data sets. Phys. D 2002, 163, 236–251. [Google Scholar] [CrossRef]
- Stam, C.J.; van der Made, Y.; Pijnenburg, Y.A.; Scheltens, P. Eeg synchronization in mild cognitive impairment and alzheimer’s disease. Acta Neurol. Scand. 2003, 108, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J.; Jones, B.F.; Nolte, G.; Breakspear, M.; Scheltens, P. Small-world networks and functional connectivity in alzheimer’s disease. Cereb. Cortex 2007, 17, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J.; Montez, T.; Jones, B.F.; Rombouts, S.A.; van der Made, Y.; Pijnenburg, Y.A.; Scheltens, P. Disturbed fluctuations of resting state eeg synchronization in alzheimer’s disease. Clin. Neurophysiol. 2005, 116, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Koenig, T.; Prichep, L.; Dierks, T.; Hubl, D.; Wahlund, L.O.; John, E.R.; Jelic, V. Decreased eeg synchronization in alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2005, 26, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shu, H.; Ward, B.D.; Antuono, P.G.; Zhang, Z.; Li, S.J.; Initiative, A.S.D.N. Staging alzheimer’s disease risk by sequencing brain function and structure, cerebrospinal fluid, and cognition biomarkers. J. Alzheimers Dis. 2016, 54, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, A.; Gross, J. Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci. 2005, 6, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L.; et al. Functional network organization of the human brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.M.; Del Percio, C.; Pasqualetti, P.; Cassetta, E.; Binetti, G.; Dal Forno, G.; Ferreri, F.; Frisoni, G.; Chiovenda, P.; Miniussi, C.; et al. Conversion from mild cognitive impairment to alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience 2006, 143, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Delbeuck, X.; Van der Linden, M.; Collette, F. Alzheimer’s disease as a disconnection syndrome? Neuropsychol. Rev. 2003, 13, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Arigita, E.J.; Schoonheim, M.M.; Damoiseaux, J.S.; Rombouts, S.A.; Maris, E.; Barkhof, F.; Scheltens, P.; Stam, C.J. Loss of ‘small-world’ networks in alzheimer’s disease: Graph analysis of fmri resting-state functional connectivity. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, H.Y.; Xie, C.; Zhang, Z.J.; Teng, G.J.; Li, S.J. Modular reorganization of brain resting state networks and its independent validation in alzheimer’s disease patients. Front. Hum. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- de Haan, W.; van der Flier, W.M.; Koene, T.; Smits, L.L.; Scheltens, P.; Stam, C.J. Disrupted modular brain dynamics reflect cognitive dysfunction in alzheimer’s disease. Neuroimage 2012, 59, 3085–3093. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Kazui, H.; Tanaka, T.; Ishii, R.; Canuet, L.; Pascual-Marqui, R.D.; Aoki, Y.; Ikeda, S.; Kanemoto, H.; Yoshiyama, K.; et al. Functional connectivity assessed by resting state eeg correlates with cognitive decline of alzheimer’s disease—An eloreta study. Clin. Neurophysiol. 2016, 127, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Abásolo, D.; Hornero, R.; Gómez, C.; García, M.; López, M. Analysis of eeg background activity in alzheimer’s disease patients with lempel-ziv complexity and central tendency measure. Med. Eng. Phys. 2006, 28, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Abásolo, D.; Hornero, R.; Espino, P.; Alvarez, D.; Poza, J. Entropy analysis of the eeg background activity in alzheimer’s disease patients. Physiol. Meas. 2006, 27, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, S.Y.; Han, S.H. Non-linear dynamical analysis of the eeg in alzheimer’s disease with optimal embedding dimension. Electroencephalogr. Clin. Neurophysiol. 1998, 106, 220–228. [Google Scholar] [CrossRef]
- Pritchard, W.S.; Duke, D.W.; Coburn, K.L.; Moore, N.C.; Tucker, K.A.; Jann, M.W.; Hostetler, R.M. Eeg-based, neural-net predictive classification of alzheimer’s disease versus control subjects is augmented by non-linear eeg measures. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 118–130. [Google Scholar] [CrossRef]
- Stam, C.J. Nonlinear dynamical analysis of eeg and meg: Review of an emerging field. Clin. Neurophysiol. 2005, 116, 2266–2301. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, W.S.; Duke, D.W.; Coburn, K.L. Altered eeg dynamical responsivity associated with normal aging and probable alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 1991, 2, 102–105. [Google Scholar] [CrossRef]
- Miraglia, F.; Vecchio, F.; Bramanti, P.; Rossini, P.M. Eeg characteristics in “Eyes-open” Versus “Eyes-closed” Conditions: Small-world network architecture in healthy aging and age-related brain degeneration. Clin. Neurophysiol. 2016, 127, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Billings, S.A.; Wei, H.L.; Sarrigiannis, P.G. A parametric method to measure time-varying linear and nonlinear causality with applications to eeg data. IEEE Trans. Biomed. Eng. 2013, 60, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Anchisi, D.; Borroni, B.; Franceschi, M.; Kerrouche, N.; Kalbe, E.; Beuthien-Beumann, B.; Cappa, S.; Lenz, O.; Ludecke, S.; Marcone, A.; et al. Heterogeneity of brain glucose metabolism in mild cognitive impairment and clinical progression to alzheimer disease. Arch. Neurol. 2005, 62, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- McGeown, W.J.; Shanks, M.F.; Forbes-McKay, K.E.; Venneri, A. Patterns of brain activity during a semantic task differentiate normal aging from early alzheimer’s disease. Psychiatry Res. 2009, 173, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, J.; Zhao, Z.; Min, B.; Lu, J.; Li, K.; He, Y.; Jia, J. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: A resting-state fmri study. Neuroimage 2011, 55, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Machulda, M.M.; Vemuri, P.; McDade, E.M.; Zeng, G.; Senjem, M.L.; Gunter, J.L.; Przybelski, S.A.; Avula, R.T.; Knopman, D.S.; et al. Age-related changes in the default mode network are more advanced in alzheimer disease. Neurology 2011, 77, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Sankari, Z.; Adeli, H.; Adeli, A. Intrahemispheric, interhemispheric, and distal eeg coherence in alzheimer’s disease. Clin. Neurophysiol. 2011, 122, 897–906. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.J.; Melbourne, A.; Kendall, G.S.; Modat, M.; Robertson, N.J.; Marlow, N.; Ourselin, S. Adapt: An adaptive preterm segmentation algorithm for neonatal brain mri. Neuroimage 2013, 65, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Song, X.W.; Dong, Z.Y.; Long, X.Y.; Li, S.F.; Zuo, X.N.; Zhu, C.Z.; He, Y.; Yan, C.G.; Zang, Y.F. Rest: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, V.D.; Adali, T.; Pearlson, G.D.; Pekar, J.J. A method for making group inferences from functional mri data using independent component analysis. Hum. Brain Mapp. 2001, 14, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Billings, S. Representations of non-linear systems: the NARMAX model. Int. J. Control 1989, 49, 1013–1032. [Google Scholar] [CrossRef]
- Billings, S.A.; Wei, H.L. An adaptive orthogonal search algorithm for model subset selection and non-linear system identification. Int. J. Control 2008, 81, 714–724. [Google Scholar] [CrossRef]
- Wakefield, S.J.; McGeown, W.J.; Shanks, M.F.; Venneri, A. Differentiating normal from pathological brain ageing using standard neuropsychological tests. Curr. Alzheimer Res. 2014, 11, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult inteLligence Scale-III; The Psychological Corporation: San Antonio, TX, USA, 1997. [Google Scholar]
- Rey, A. L’Examen Clinique en Psychologie; Presses Universitaires de France: Paris, France, 1964. [Google Scholar]
- Raven, J. Coloured Progressive Matrices Sets a, ab, b. Manual Sections 1 & 2 Oxford; Psychologists Press: Oxford, UK, 1995. [Google Scholar]
- Stroop, J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- De Renzi, E.; Faglioni, P. Normative data and screening power of a shortened version of the token test. Cortex 1978, 14, 41–49. [Google Scholar] [CrossRef]
- Lancaster, J.L.; Woldorff, M.G.; Parsons, L.M.; Liotti, M.; Freitas, C.S.; Rainey, L.; Kochunov, P.V.; Nickerson, D.; Mikiten, S.A.; Fox, P.T. Automated talairach atlas labels for functional brain mapping. Hum. Brain Mapp. 2000, 10, 120–131. [Google Scholar] [CrossRef]
- Sarrigiannis, P.G.; Zhao, Y.; Wei, H.L.; Billings, S.A.; Fotheringham, J.; Hadjivassiliou, M. Quantitative eeg analysis using error reduction ratio-causality test; validation on simulated and real eeg data. Clin. Neurophysiol. 2014, 125, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, J.; Li, S.; Yu, H.; Deng, B.; Wei, X. Multiple feature extraction and classification of electroencephalograph signal for alzheimers’ with spectrum and bispectrum. Chaos 2015, 25. [Google Scholar] [CrossRef] [PubMed]
- Hendler, N.; Cimini, C.; Ma, T.; Long, D. A comparison of cognitive impairment due to benzodiazepines and to narcotics. Am. J. Psychiatry 1980, 137, 828–830. [Google Scholar] [PubMed]
- Babiloni, C.; Del Percio, C.; Bordet, R.; Bourriez, J.L.; Bentivoglio, M.; Payoux, P.; Derambure, P.; Dix, S.; Infarinato, F.; Lizio, R.; et al. Effects of acetylcholinesterase inhibitors and memantine on resting-state electroencephalographic rhythms in alzheimer’s disease patients. Clin. Neurophysiol. 2013, 124, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, R.; Shankle, W.R.; Hara, J.; Rodriquez, A.; Hoffman, D.; Saha, U. Qeeg monitoring of alzheimer’s disease treatment: A preliminary report of three case studies. Clin. EEG Neurosci. 2006, 37, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Garn, H.; Waser, M.; Deistler, M.; Benke, T.; Dal-Bianco, P.; Ransmayr, G.; Schmidt, H.; Sanin, G.; Santer, P.; Caravias, G.; et al. Quantitative eeg markers relate to alzheimer’s disease severity in the prospective dementia registry austria (prodem). Clin. Neurophysiol. 2015, 126, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for alzheimer’s disease: The iwg-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Pijnenburg, Y.A.; van de Made, Y.; van Cappellen van Walsum, A.M.; Knol, D.L.; Scheltens, P.; Stam, C.J. Eeg synchronization likelihood in mild cognitive impairment and alzheimer’s disease during a working memory task. Clin. Neurophysiol. 2004, 115, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Miraglia, F.; Marra, C.; Quaranta, D.; Vita, M.G.; Bramanti, P.; Rossini, P.M. Human brain networks in cognitive decline: A graph theoretical analysis of cortical connectivity from eeg data. J. Alzheimers Dis. 2014, 41, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Hatz, F.; Benz, N.; Hardmeier, M.; Zimmermann, R.; Rueegg, S.; Schindler, C.; Miserez, A.R.; Gschwandtner, U.; Monsch, A.U.; Fuhr, P. Quantitative eeg and apolipoprotein e-genotype improve classification of patients with suspected alzheimer’s disease. Clin. Neurophysiol. 2013, 124, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, R.F.; Knepper, H.; Nolte, G.; Strüber, D.; Rach, S.; Herrmann, C.S.; Schneider, T.R.; Engel, A.K. Selective modulation of interhemispheric functional connectivity by HD-tACS shapes perception. PLoS Biol 2014, 12, e1002031. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Billings, S.A.; Wei, H.; Sarrigiannis, P.G. Tracking time-varying causality and directionality of information flow using an error reduction ratio test with applications to electroencephalography data. Phys. Rev. E 2012, 86, 051919. [Google Scholar] [CrossRef] [PubMed]

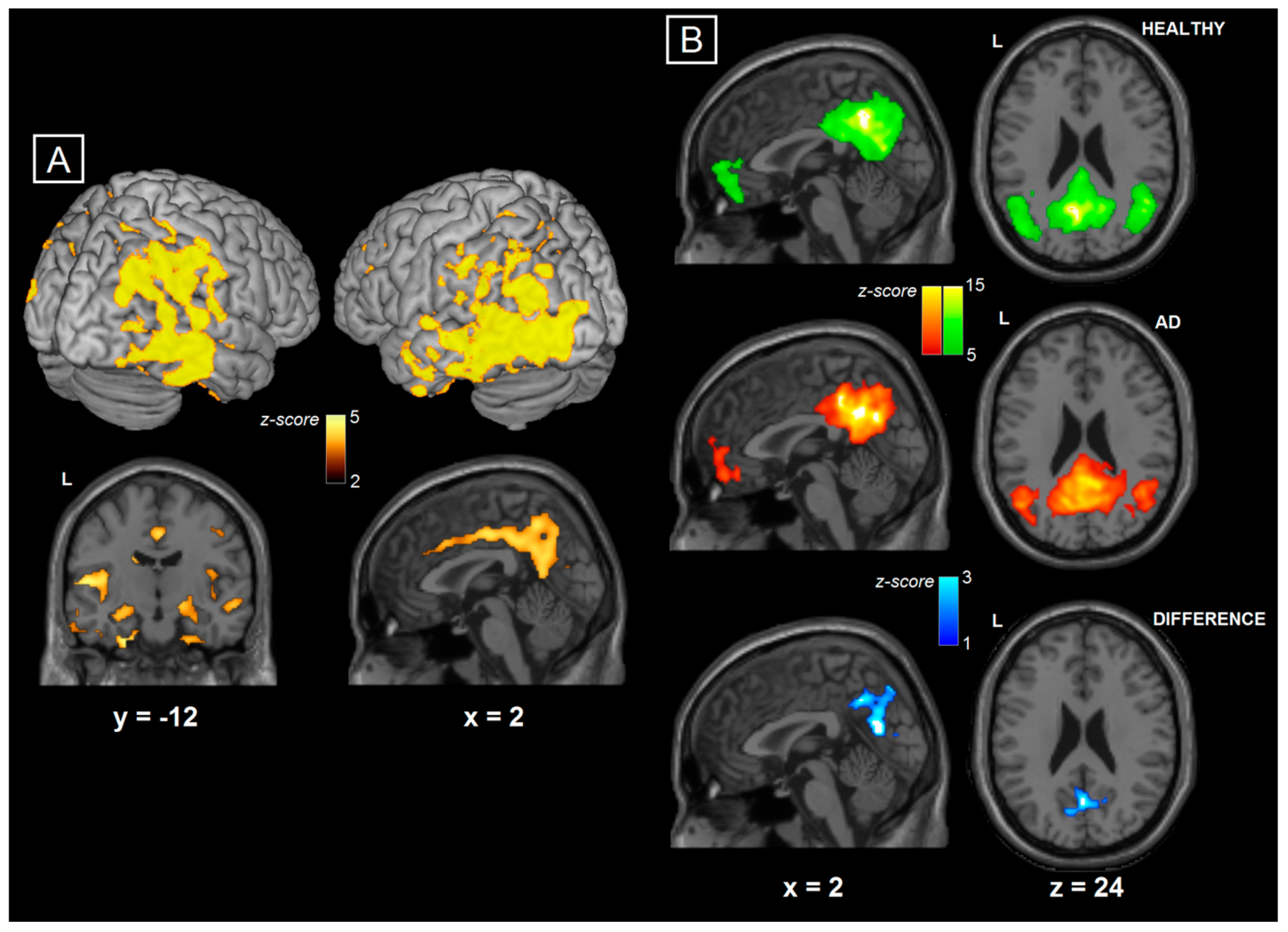
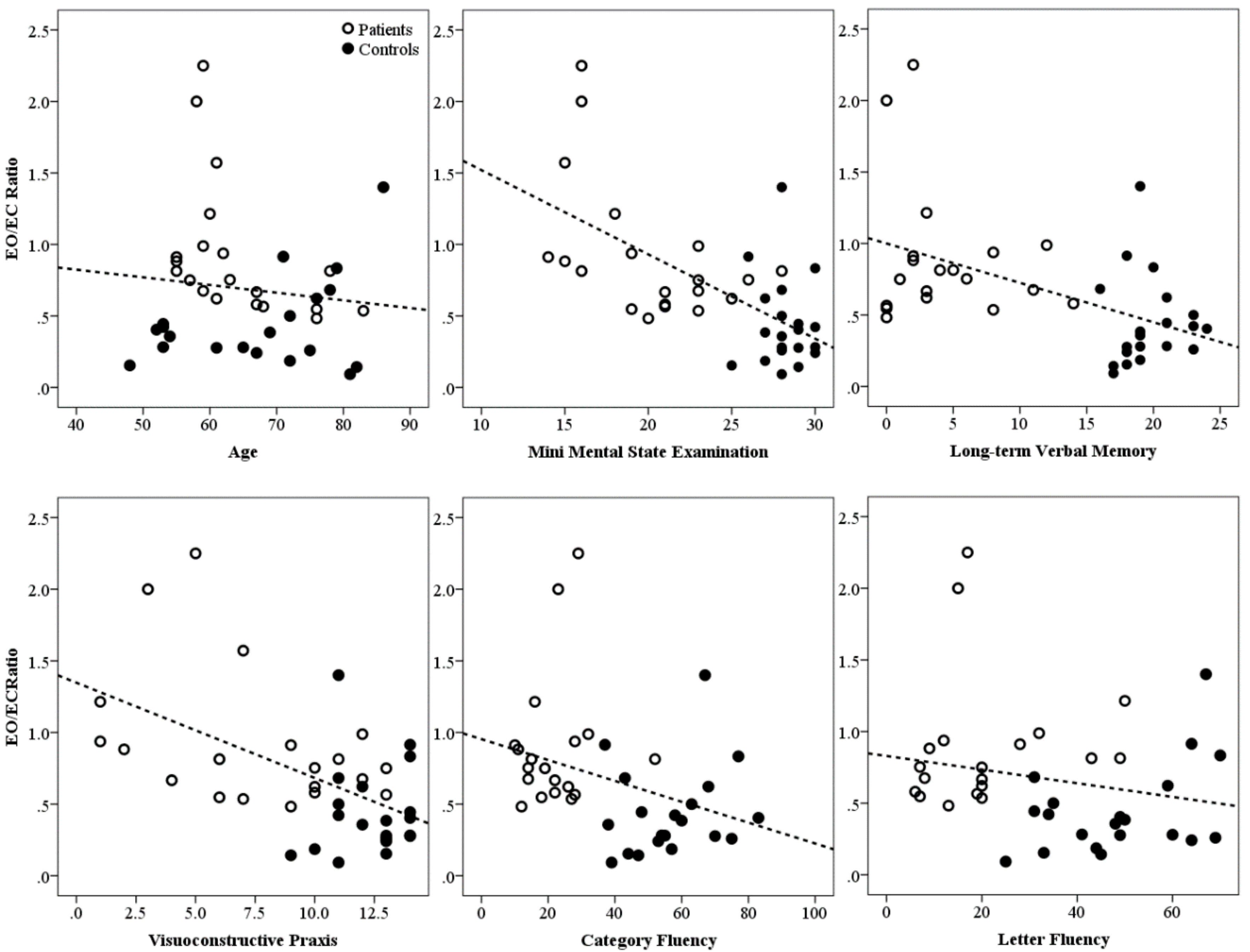
| Variable | AD Patients | Healthy Controls | p |
|---|---|---|---|
| Demographics | |||
| Age at MRI scan (years) | 63.95 (8.34) | 67.35 (11.79) | 0.429 |
| Education (years) | 11.75 (2.05) | 15.20 (2.98) | <0.001 |
| Gender (f/m) | 10/10 | 11/9 | 0.752 |
| Mini Mental State Examination (max. 30) | 20.10 (4.00) | 28.20 (1.36) | <0.001 |
| Brain Structure-Absolute Volumes | |||
| Left Hippocampus (mL) | 2.08 (0.48) | 2.59 (0.24) | <0.001 |
| Right Hippocampus (mL) | 2.21 (0.48) | 2.61 (0.34) | 0.007 |
| Grey Matter (mL) | 550.24 (83.00) | 627.19 (61.11) | 0.003 |
| White Matter (mL) | 396.76 (58.17) | 418.40 (39.01) | 0.192 |
| Brain Structure-Fractional Volumes | |||
| Grey Matter | 0.38 (0.05) | 0.43 (0.05) | 0.002 |
| White Matter | 0.28 (0.04) | 0.29 (0.02) | 0.121 |
| Cognition | |||
| Letter Fluency Test * | 20.79 (13.77) | 48.40 (14.21) | <0.001 |
| Category Fluency Test * | 22.00 (9.90) | 56.80 (13.56) | <0.001 |
| Prose Memory Test-Immediate Recall (max. 24) * | 4.37 (3.62) | 15.15 (3.53) | <0.001 |
| Prose Memory Test-Delayed Recall (max. 24) * | 4.42 (4.31) | 19.65 (2.28) | <0.001 |
| Stroop Test-Time Interference * | 29.61 (21.09) | 13.70 (4.20) | 0.016 |
| Stroop Test-Error Interference * | 12.22 (10.59) | 0.00 (0.00) | <0.001 |
| Visuoconstructional Praxis Test (max. 14) | 7.55 (3.94) | 12.35 (1.53) | <0.001 |
| EEG Index | AD Median (IQR) | FTD Median (IQR) | HC Median (IQR) | AD vs. HC | AD vs. FTD | HC vs. FTD |
|---|---|---|---|---|---|---|
| Bi-centroparietal (C3–P3 to C4–P4) | ||||||
| Eyes closed 95% CI top level | 0.53 (0.071) | 0.14 (0.27) | 0.485 (0.53) | p = 0.968 | p = 0.037 | p = 0.018 |
| Eyes open 95% CI top level | 0.435 (0.41) | 0.075 (0.03) | 0.15 (0.17) | p < 0.0001 | p < 0.0001 | p = 0.018 |
| EO/EC ratio | 0.784 (0.386) | 0.586 (0.386) | 0.371 (0.346) | p < 0.0001 | p = 0.241 | p = 0.575 |
| Bi frontal (F3–F7 to F4–F8) | ||||||
| Eyes closed 95% CI top level | 0.185 (0.27) | 0.07 (0.04) | 0.11 (0.11) | p = 0.149 | p = 0.018 | p = 0.081 |
| Eyes open 95% CI top level | 0.09 (0.1) | 0.08 (0.04) | 0.09 (0.08) | p = 0.64 | p = 0.431 | p = 0.431 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blackburn, D.J.; Zhao, Y.; De Marco, M.; Bell, S.M.; He, F.; Wei, H.-L.; Lawrence, S.; Unwin, Z.C.; Blyth, M.; Angel, J.; et al. A Pilot Study Investigating a Novel Non-Linear Measure of Eyes Open versus Eyes Closed EEG Synchronization in People with Alzheimer’s Disease and Healthy Controls. Brain Sci. 2018, 8, 134. https://doi.org/10.3390/brainsci8070134
Blackburn DJ, Zhao Y, De Marco M, Bell SM, He F, Wei H-L, Lawrence S, Unwin ZC, Blyth M, Angel J, et al. A Pilot Study Investigating a Novel Non-Linear Measure of Eyes Open versus Eyes Closed EEG Synchronization in People with Alzheimer’s Disease and Healthy Controls. Brain Sciences. 2018; 8(7):134. https://doi.org/10.3390/brainsci8070134
Chicago/Turabian StyleBlackburn, Daniel J., Yifan Zhao, Matteo De Marco, Simon M. Bell, Fei He, Hua-Liang Wei, Sarah Lawrence, Zoe C. Unwin, Michelle Blyth, Jenna Angel, and et al. 2018. "A Pilot Study Investigating a Novel Non-Linear Measure of Eyes Open versus Eyes Closed EEG Synchronization in People with Alzheimer’s Disease and Healthy Controls" Brain Sciences 8, no. 7: 134. https://doi.org/10.3390/brainsci8070134
APA StyleBlackburn, D. J., Zhao, Y., De Marco, M., Bell, S. M., He, F., Wei, H.-L., Lawrence, S., Unwin, Z. C., Blyth, M., Angel, J., Baster, K., Farrow, T. F. D., Wilkinson, I. D., Billings, S. A., Venneri, A., & Sarrigiannis, P. G. (2018). A Pilot Study Investigating a Novel Non-Linear Measure of Eyes Open versus Eyes Closed EEG Synchronization in People with Alzheimer’s Disease and Healthy Controls. Brain Sciences, 8(7), 134. https://doi.org/10.3390/brainsci8070134







