What Is Going On? The Process of Generating Questions about Emotion and Social Cognition in Bipolar Disorder and Schizophrenia with Cartoon Situations and Faces
Abstract
1. Introduction
1.1. Best Practices?
“There must be no barriers for freedom of inquiry. There is no place for dogma in science. The scientist is free, and must be free to ask any question, to doubt any assertion, to seek for any evidence, to correct any errors.”[1]
“Take chances, make mistakes, and get messy.”[2]
1.2. The Challenge of Studying Social Neuroscience
1.3. Dissecting the Pertinent Components
1.4. Studying Patient Populations
1.5. Previous Research
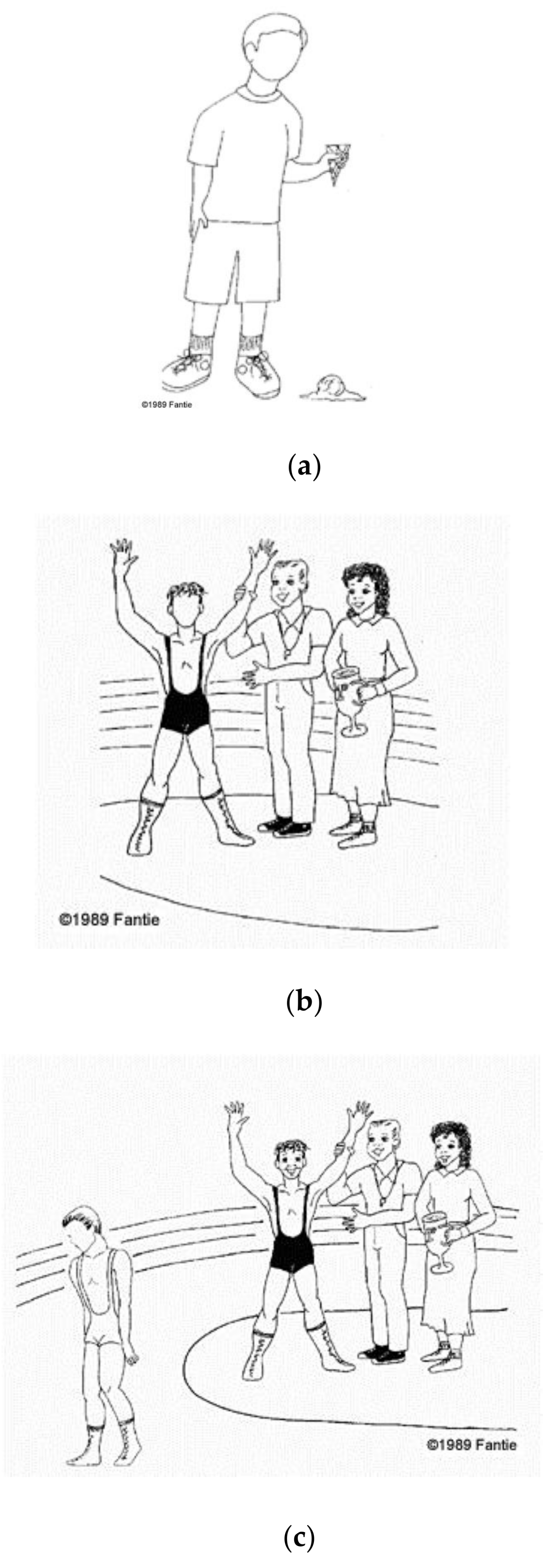
1.6. Matching Photos of Emotional Facial Expressions to Situations Depicted in Cartoons
1.7. Aim and Hypotheses of the Present Study
2. Materials and Methods
2.1. Participants
2.2. Procedure
3. Results
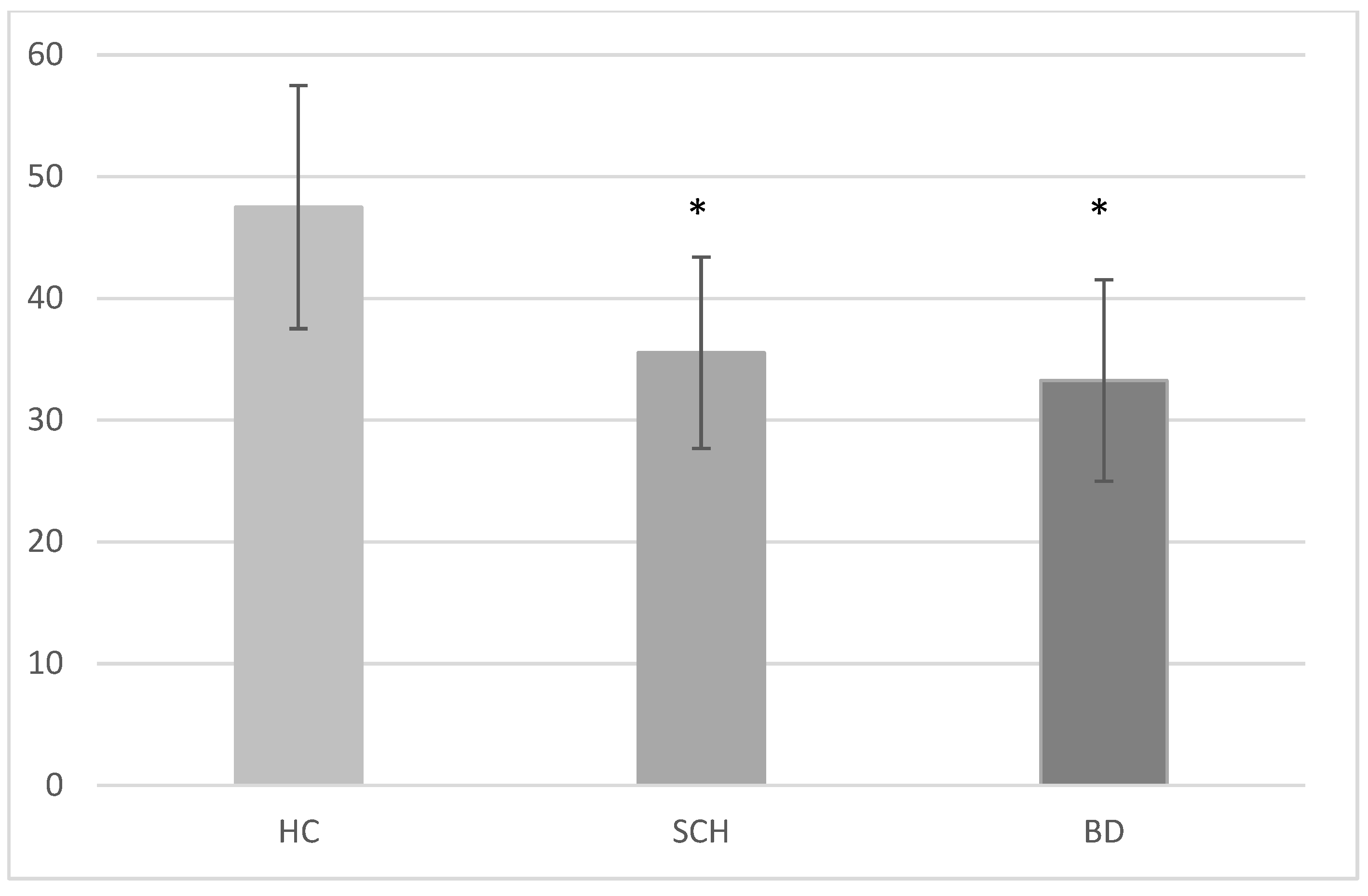
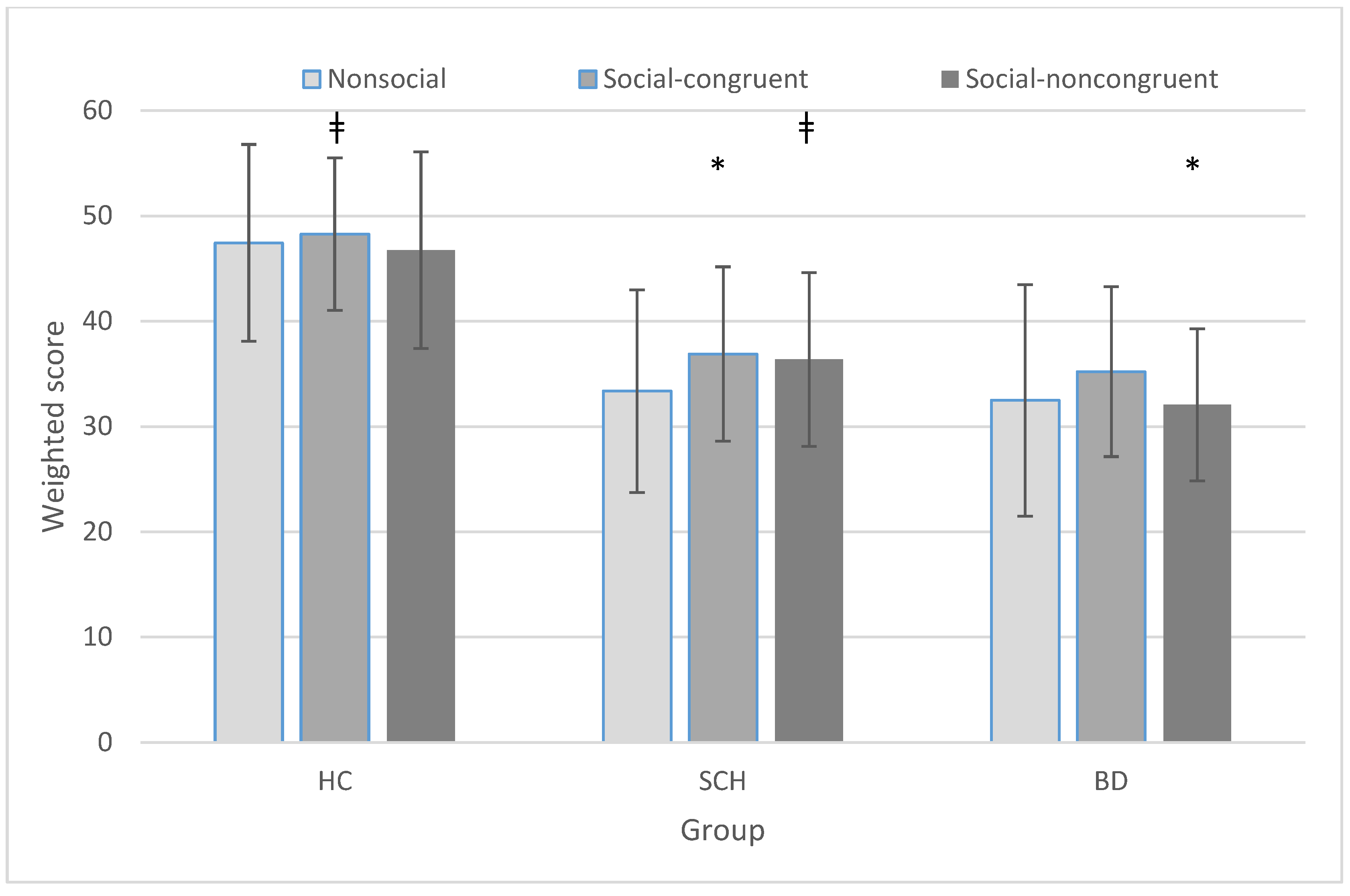
3.1. Scoring Procedure, Variables, and Statistical Analyses
3.2. Group × Condition
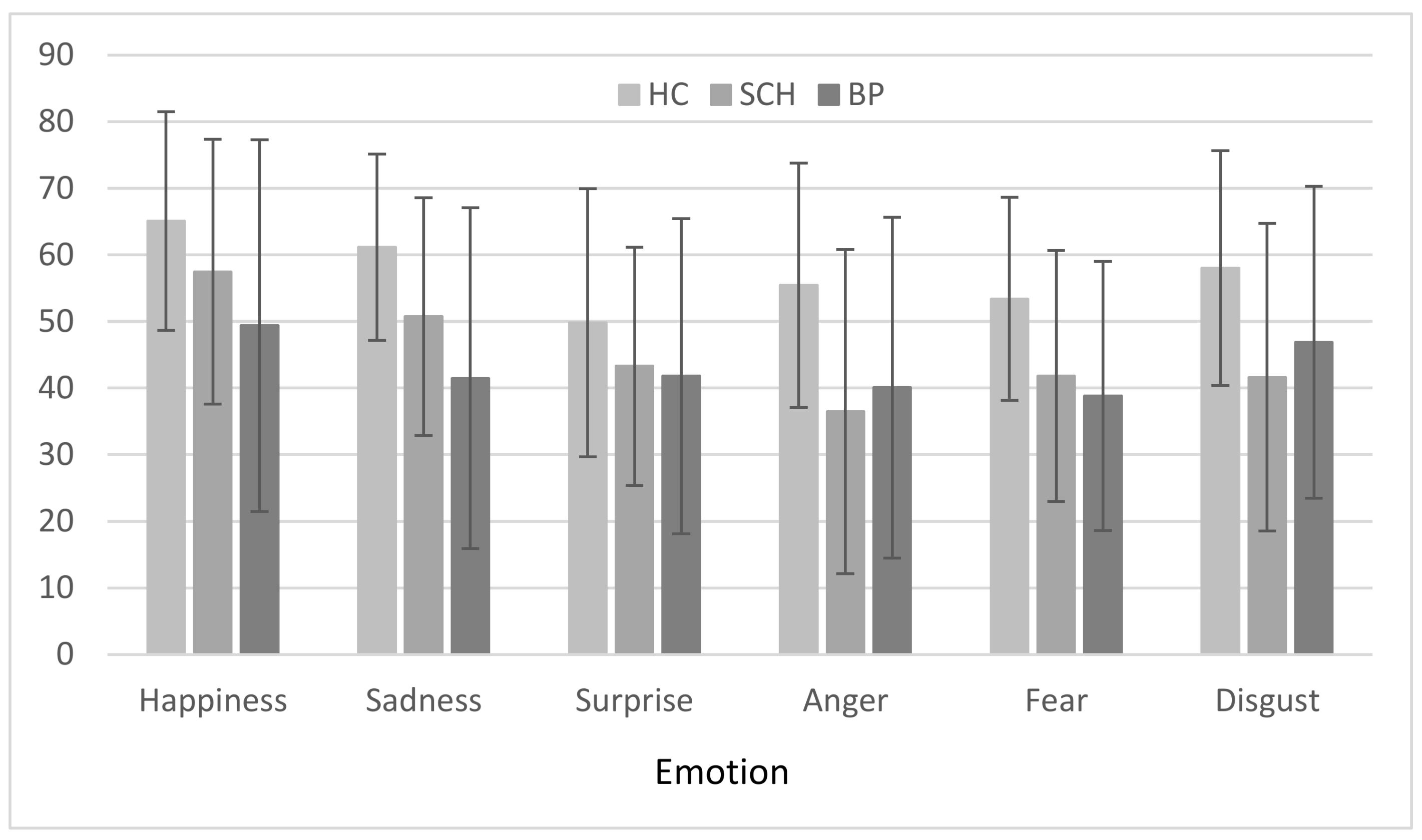
3.3. Group × Emotion
3.4. Correlations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oppenheimer, J.R. Life Magazine; 10 October; Time Inc.: New York, NY, USA, 1949. [Google Scholar]
- Frizzle, V.F. The Magic School Bus. 1994. Available online: https://en.wikipedia.org/wiki/The_Magic_School_Bus (accessed on 28 December 2017).
- Tottenham, N.; Tanaka, J.W.; Leon, A.C.; McCarry, T.; Nurse, M.; Hare, T.A.; Nelson, C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009, 168, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual; University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Parsons, C.E.; Young, K.S.; Craske, M.G.; Stein, A.L.; Kringelbach, M.L. Introducing the Oxford Vocal (OxVoc) Sounds database: A validated set of non-acted affective sounds from human infants, adults, and domestic animals. Front. Psychol. 2014, 5, 562. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Bauer, P.J.; Gershon, R.C. Cognition assessment using the NIH Toolbox. Neurology 2013, 80 (Suppl. 3), S54–S64. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.F. The theory of constructed emotion: An active inference account of interoception and categorization. Soc. Cognit. Affect. Neurosci. 2017, 12, 1833. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.M.; Subramanian, S.V.; Christakis, N.A. Social Networks and Health: A Systematic Review of Sociocentric Network Studies in Low- and Middle-Income Countries. Soc. Sci. Med. 2015, 125, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Clay, H.B.; Sillivan, S.; Konradi, C. Mitochondrial dysfunction and pathology in bipolar disorder and schizophrenia. Int. J. Dev. Neurosci. 2011, 29, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.; Barguil, M.; Contreras, J.; Montero, P.; Dassori, A.; Escamilla, M.A.; Raventós, H. Social and clinical comparison between schizophrenia and bipolar disorder type I with psychosis in Costa Rica. Soc. Psychiatry Psychiatr. Epidemiol. 2010, 45, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Addington, J.; Addington, D. Facial affect recognition and information processing in schizophrenia and bipolar disorder. Schizophr. Res. 1998, 32, 171–181. [Google Scholar] [CrossRef]
- Baez, S.; Herrera, E.; Villarin, L.; Theil, D.; Gonzalez-Gadea, M.L.; Gomez, P.; Ibañez, A.M. Contextual Social Cognition Impairments in Schizophrenia and Bipolar Disorder. PLoS ONE 2013, 8, e57664. [Google Scholar] [CrossRef] [PubMed]
- Bozikas, V.P.; Tonia, T.; Fokas, K.; Karavatos, A.; Kosmidis, M.H. Impaired emotion processing in remitted patients with bipolar disorder. J. Affect. Disord. 2006, 91, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Daros, A.R.; Ruocco, A.C.; Reilly, J.L.; Harris, M.S.H.; Sweeney, J.A. Facial emotion recognition in first-episode schizophrenia and bipolar disorder with psychosis. Schizophr. Res. 2014, 153, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Jackson, H.J.; Pattison, P.E. Emotion recognition via facial expression and affective prosody in schizophrenia: A methodological review. Clin. Psychol. Rev. 2002, 22, 789–832. [Google Scholar] [CrossRef]
- Edwards, J.; Jackson, H.J.; Pattison, P.E. Erratum to “Emotion recognition via facial expression and affective prosody in schizophrenia: A methodological review” [Clinical Psychology Review 22 (2002) 789–832]. Clin. Psychol. Rev. 2002, 22, 1267–1285. [Google Scholar] [CrossRef]
- Edwards, J.; Pattison, P.E.; Jackson, H.J.; Wales, R.J. Facial affect and affective prosody recognition in first-episode schizophrenia. Schizophr. Res. 2001, 48, 235–253. [Google Scholar] [CrossRef]
- Goghari, V.M.; Sponheim, S.R. More pronounced deficits in facial emotion recognition for schizophrenia than bipolar disorder. Compr. Psychiatry 2013, 54, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Harmer, C.J.; Grayson, L.; Goodwin, G.M. Enhanced recognition of disgust in bipolar illness. Biol. Psychiatry 2002, 51, 298–304. [Google Scholar] [CrossRef]
- Kohler, C.G.; Hoffman, L.J.; Eastman, L.B.; Healey, K.; Moberg, P.J. Facial emotion perception in depression and bipolar disorder: A quantitative review. Psychiatry Res. 2011, 188, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, M.H.; Bozikas, V.P.; Giannakou, M.; Anezoulaki, D.; Fantie, B.D.; Karavatos, A. Impaired emotion perception in schizophrenia: A differential deficit. Psychiatry Res. 2007, 149, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Lembke, A.; Ketter, T.A. Impaired Recognition of Facial Emotion in Mania. Am. J. Psychiatry 2002, 159, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Meehan, K.B.; De Panfilis, C.; Cain, N.M.; Antonucci, C.; Soliani, A.; Clarkin, J.F.; Sambataro, F. Facial emotion recognition and borderline personality pathology. Psychiatry Res. 2017, 255, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Minzenberg, M.J.; Poole, J.H.; Vinogradov, S. Social-emotion recognition in borderline personality disorder. Compr. Psychiatry 2006, 47, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, A.C.; Reilly, J.L.; Rubin, L.H.; Daros, A.R.; Gershon, E.S.; Tamminga, C.A.; Sweeney, J.A. Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Schizophr. Res. 2014, 158, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.; Steger-Wuchse, D.; Kryspin-Exner, I.; Gur, R.C.; Katschnig, H. Facial recognition deficits and cognition in schizophrenia. Schizophr. Res. 2004, 68, 27–35. [Google Scholar] [CrossRef]
- Venn, H.R.; Gray, J.M.; Montagne, B.; Murray, L.K.; Burt, D.M.; Frigerio, E.; Young, A.H. Perception of facial expressions of emotion in bipolar disorder. Bipolar Disord. 2004, 6, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Whishaw, I.Q. Production and Perception of Facial Expression: A Comparison of Focal Brain Lesions and Schizophrenia; Experimental Psychology Society: St. Andrews, UK, 1982. [Google Scholar]
- Bozikas, V.P.; Kosmidis, M.H.; Anezoulaki, D.; Giannakou, M.; Andreou, C.; Karavatos, A. Impaired perception of affective prosody in schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2006, 18, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Bozikas, V.P.; Kosmidis, M.H.; Tonia, T.; Andreou, C.; Focas, K.; Karavatos, A. Impaired perception of affective prosody in remitted patients with bipolar disorder. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 436–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leitman, D.I.; Foxe, J.J.; Butler, P.D.; Saperstein, A.; Revheim, N.; Javitt, D.C. Sensory Contributions to Impaired Prosodic Processing in Schizophrenia. Biol. Psychiatry 2005, 58, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Leitman, D.I.; Laukka, P.; Juslin, P.N.; Saccente, E.; Butler, P.; Javitt, D.C. Getting the Cue: Sensory Contributions to Auditory Emotion Recognition Impairments in Schizophrenia. Schizophr. Bull. 2010, 36, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Leitman, D.I.; Wolf, D.H.; Laukka, P.; Ragland, J.D.; Valdez, J.N.; Turetsky, B.I.; Gur, R.C. Not Pitch Perfect: Sensory Contributions to Affective Communication Impairment in Schizophrenia. Biol. Psychiatry 2011, 70, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Bellack, A.S.; Blanchard, J.J.; Mueser, K.T. Cue availability and affect perception in schizophrenia. Schizophr. Bull. 1996, 22, 535–544. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cramer, P.; Bowen, J.; Oneill, M. Schizophrenics and Social Judgement: Why Do Schizophrenics Get It Wrong? Br. J. Psychiatry 1992, 160, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.L.A.; Sturgeon, D.A.; Leff, J. The Perception of Emotion by Schizophrenic Patients. Br. J. Psychiatry 1992, 161, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, M.H.; Aretouli, E.; Bozikas, V.P.; Giannakou, M.; Ioannidis, P. Studying social cognition in patients with schizophrenia and patients with frontotemporal dementia: Theory of mind and the perception of sarcasm. Behav. Neurol. 2008, 19, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, P.W.; Toomey, R. Interpersonal problem-solving and information-processing in schizophrenia. Schizophr. Bull. 1995, 21, 395–403. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fantie, B.D. Fantie Cartoon Task. Unpublished work. 1995. [Google Scholar]
- Ekman, P.; Oster, H. Facial Expressions of Emotion. Annu. Rev. Psychol. 1979, 30, 527–554. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Lykouras, L.; Oulis, P.; Psarros, K.; Daskalopoulou, E.; Botsis, A.; Christodoulou, G.N.; Stefanis, C. Five-factor model of schizophrenic psychopathology: How valid is it? Eur. Arch. Psychiatry Clin. Neurosci. 2000, 250, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Pfungst, O. Clever Hans (The Horse of Mr. von Osten) a Contribution to Experimental Animal and Human Psychology; H. Holt and company: New York, NY, USA, 1911. [Google Scholar]
- Johnston, P.J.; Devir, H.; Karayanidis, F. Facial emotion processing in schizophrenia: No evidence for a deficit specific to negative emotions in a differential deficit design. Psychiatry Res. 2006, 143, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.J.; McCabe, K.; Schall, U. Differential susceptibility to performance degradation across categories of facial emotion—A model confirmation. Biol. Psychol. 2003, 63, 45–58. [Google Scholar] [CrossRef]
| BD | SCH | HC | ||||
|---|---|---|---|---|---|---|
| M (SD) | Range | M (SD) | Range | M (SD) | Range | |
| Age (years) | 39.32 (10.72) | 24–57 | 37.00 (9.84) | 21–57 | 35.55 (9.61) | 21–61 |
| Education (years) | 12.16 (2.83) | 6–18 | 10.72 (3.19) | 6–16 | 11.55 (2.10) | 6–16 |
| Duration of illness (years) | 13.16 (10.24) | 2–36 | 10.85 (8.51) | 0.5–36 | - | - |
| MADRS | 1.53 (2.61) | 0–8 | - | - | - | - |
| YMRS | 3.16 (2.48) | 0–8 | - | - | - | - |
| PANSS | ||||||
| Positive symptoms | -- | -- | 14.66 (8.49) | 7–30 | - | - |
| Negative symptoms | -- | -- | 20.38 (6.10) | 10–35 | - | - |
| General psychopathology | -- | -- | 27.56 (5.90) | 17–42 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantie, B.D.; Kosmidis, M.H.; Giannakou, M.; Moza, S.; Karavatos, A.; Bozikas, V.P. What Is Going On? The Process of Generating Questions about Emotion and Social Cognition in Bipolar Disorder and Schizophrenia with Cartoon Situations and Faces. Brain Sci. 2018, 8, 68. https://doi.org/10.3390/brainsci8040068
Fantie BD, Kosmidis MH, Giannakou M, Moza S, Karavatos A, Bozikas VP. What Is Going On? The Process of Generating Questions about Emotion and Social Cognition in Bipolar Disorder and Schizophrenia with Cartoon Situations and Faces. Brain Sciences. 2018; 8(4):68. https://doi.org/10.3390/brainsci8040068
Chicago/Turabian StyleFantie, Bryan D., Mary H. Kosmidis, Maria Giannakou, Sotiria Moza, Athanasios Karavatos, and Vassilis P. Bozikas. 2018. "What Is Going On? The Process of Generating Questions about Emotion and Social Cognition in Bipolar Disorder and Schizophrenia with Cartoon Situations and Faces" Brain Sciences 8, no. 4: 68. https://doi.org/10.3390/brainsci8040068
APA StyleFantie, B. D., Kosmidis, M. H., Giannakou, M., Moza, S., Karavatos, A., & Bozikas, V. P. (2018). What Is Going On? The Process of Generating Questions about Emotion and Social Cognition in Bipolar Disorder and Schizophrenia with Cartoon Situations and Faces. Brain Sciences, 8(4), 68. https://doi.org/10.3390/brainsci8040068





