Effects of Dietary Vitamin E Supplementation in Bladder Function and Spasticity during Spinal Cord Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
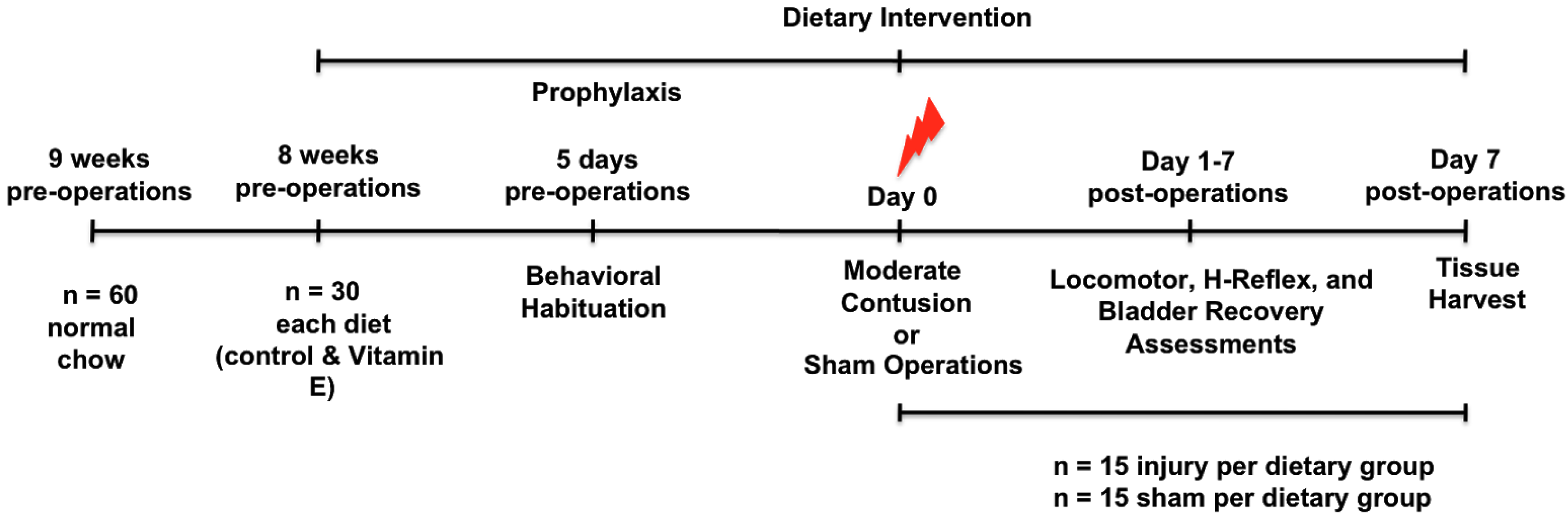
2.2. Study Design
2.3. Diets
2.4. Surgical and Post-Operative Procedures
2.5. Behavioral Evaluation of Spontaneous Locomotion
2.6. H-Reflex Recording
2.7. Autonomic Bladder Control Recovery
2.8. Immunohistochemistry Studies and Microscopy
2.9. Statistical Analyses
3. Results
3.1. Dietary Vitamin E Improves Locomotor Recovery after SCI
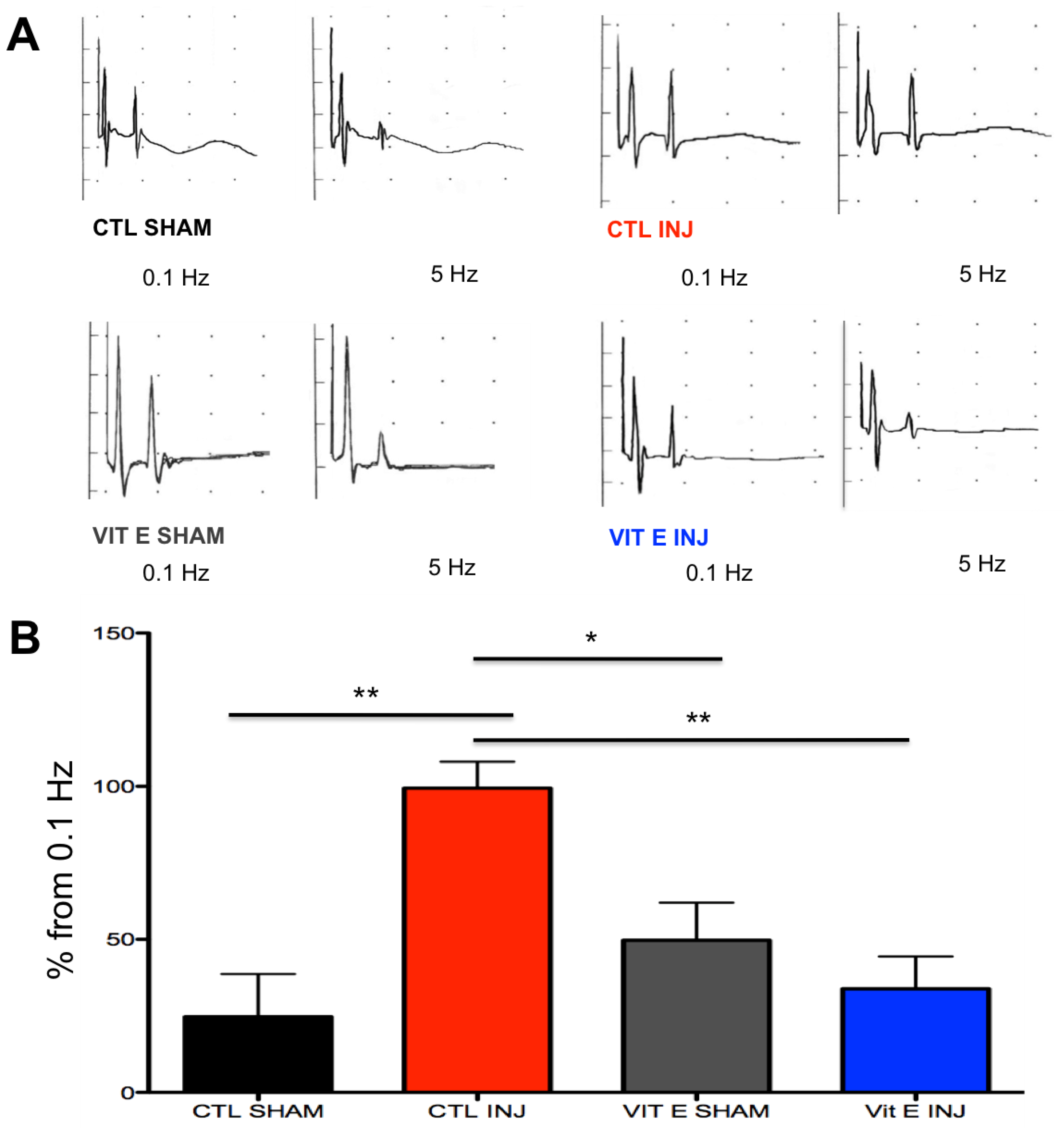
3.2. Dietary Vitamin E Prophylaxis Restores H-Reflex Depression at 7 dpi at 5 Hz.
3.3. Beneficial Effects of Dietary Vitamin E Prophylaxis on Autonomic Function after Contusion Injury
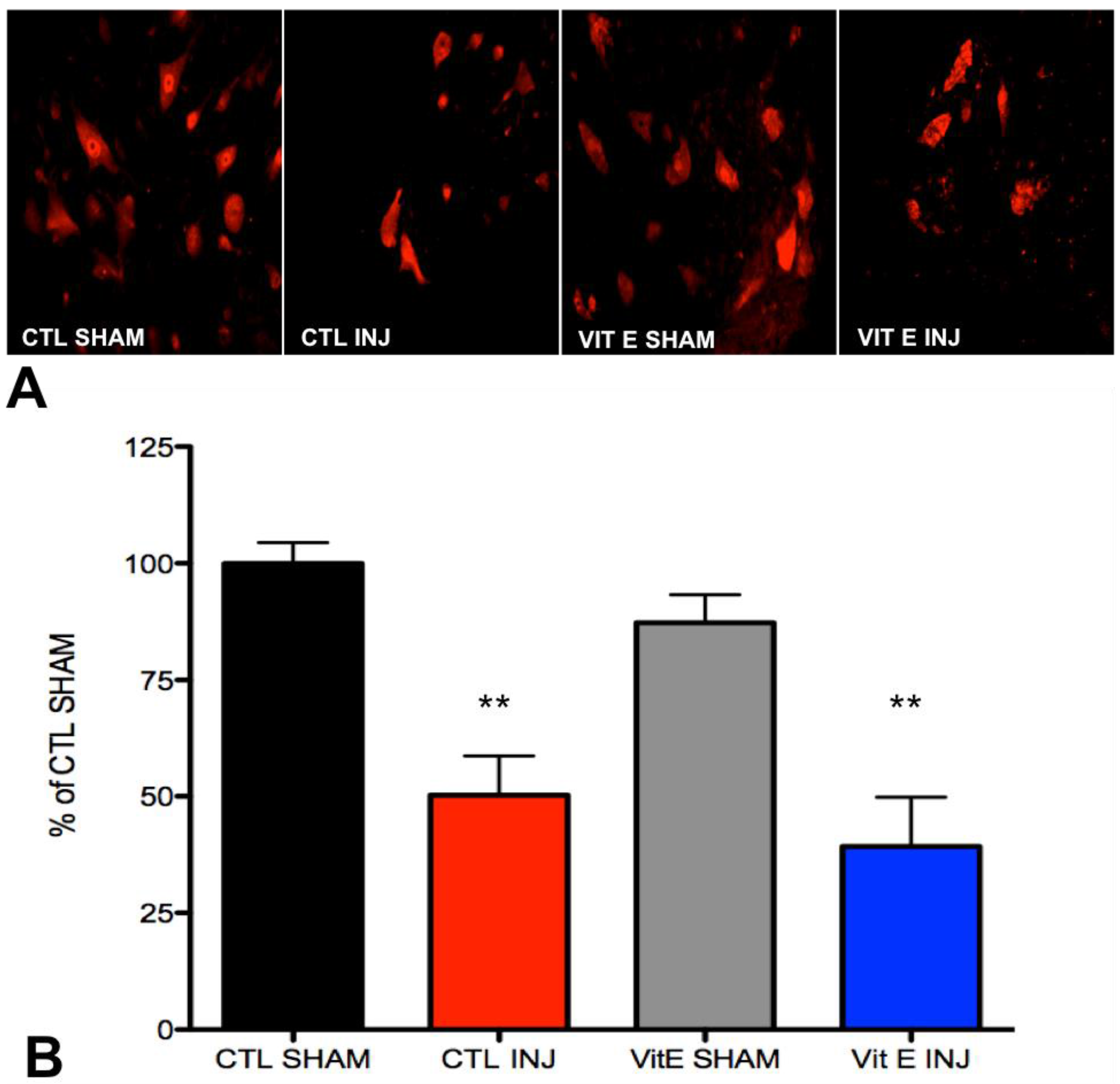
3.4. Dietary Vitamin E Does Not Preserve Neurons at 1 Week after Spinal Cord Injury (SCI)
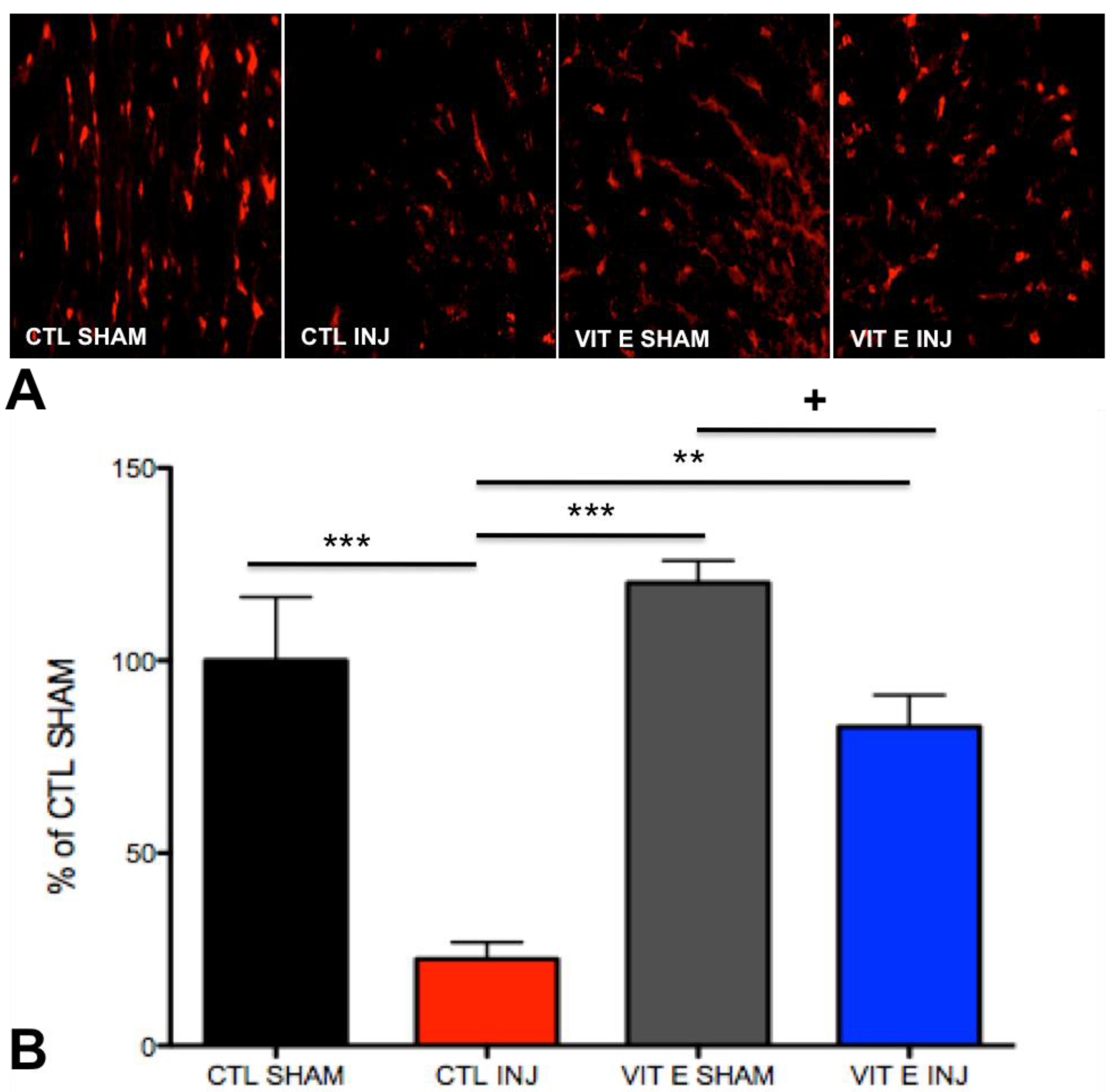
3.5. Dietary Vitamin E Preserves Oligodendrocytes Following SCI
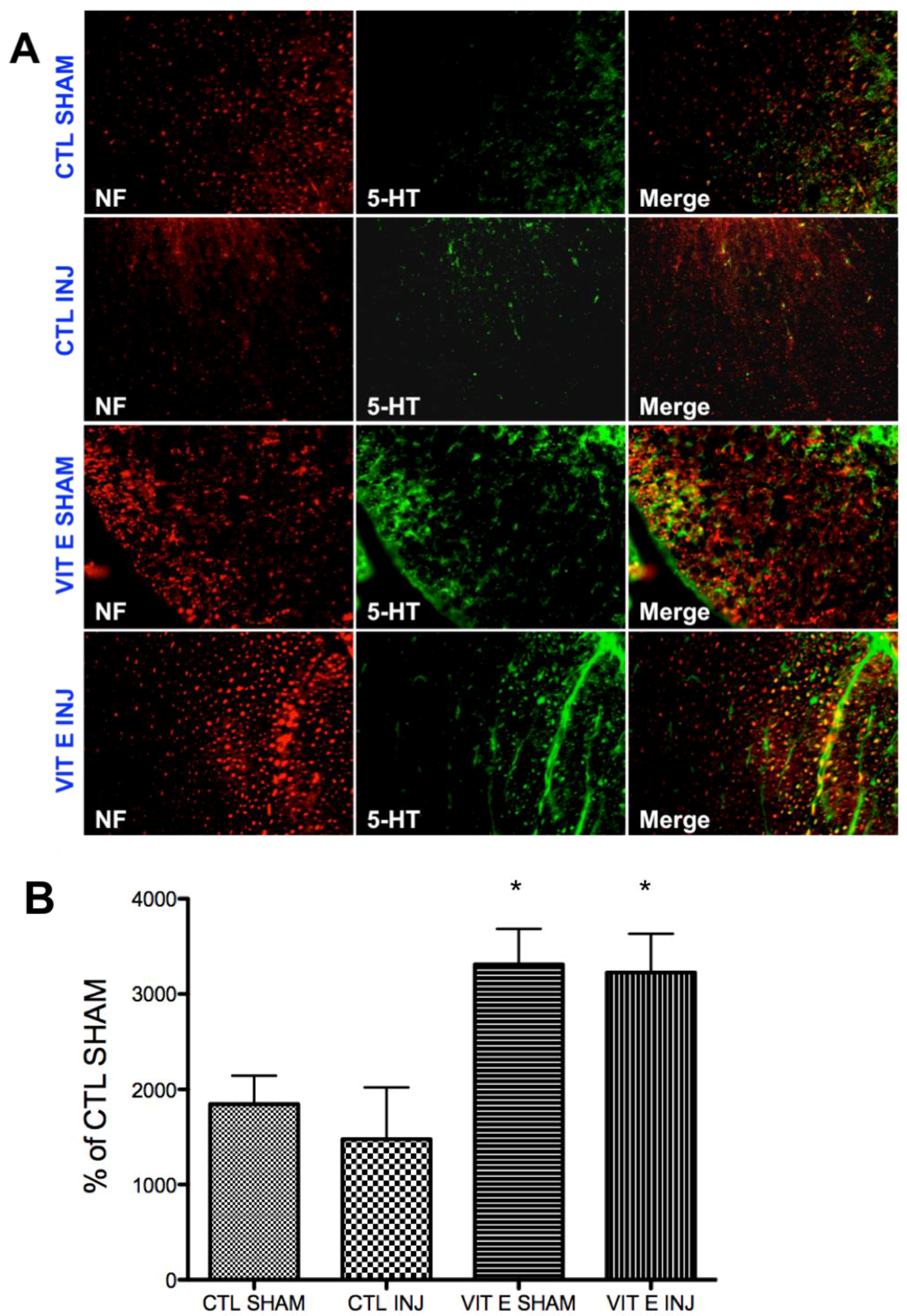
3.6. Dietary Vitamin E Upregulates Serotonin Immunoreactivity Following SCI
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dallmeijer, A.J.; van der Woude, L.H.; van Kamp, G.J.; Hollander, A.P. Changes in lipid, lipoprotein and apolipoprotein profiles in persons with spinal cord injuries during the first 2 years post-injury. Spinal Cord 1999, 37, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Demediuk, P.; Saunders, R.D.; Anderson, D.K.; Means, E.D.; Horrocks, L.A. Membrane lipid changes in laminectomized and traumatized cat spinal cord. Proc. Natl. Acad. Sci. USA 1985, 82, 7071–7075. [Google Scholar] [CrossRef] [PubMed]
- Demediuk, P.; Saunders, R.D.; Anderson, D.K.; Means, E.D.; Horrocks, L.A. Early membrane lipid changes in laminectomized and traumatized cat spinal cord. Neurochem. Pathol. 1987, 7, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Demediuk, P.; Saunders, R.D.; Clendenon, N.R.; Means, E.D.; Anderson, D.K.; Horrocks, L.A. Changes in lipid metabolism in traumatized spinal cord. Prog. Brain Res. 1985, 63, 211–226. [Google Scholar] [PubMed]
- Bauman, W.A.; Spungen, A.M. Metabolic changes in persons after spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2000, 11, 109–140. [Google Scholar] [PubMed]
- Kearns, P.J.; Thompson, J.D.; Werner, P.C.; Pipp, T.L.; Wilmot, C.B. Nutritional and metabolic response to acute spinal-cord injury. JPEN J. Parenter. Enteral Nutr. 1992, 16, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Murai, H.; Itoh, C.; Wagai, N.; Nakamura, T.; Yamaura, A.; Makino, H. Ocal spinal cord glucose utilization and extracellular potassium activity changes after spinal cord injury in rats. No To Shinkei 1991, 43, 337–342. [Google Scholar] [PubMed]
- Vink, R.; Noble, L.J.; Knoblach, S.M.; Bendall, M.R.; Faden, A.I. Metabolic changes in rabbit spinal cord after trauma: Magnetic resonance spectroscopy studies. Ann. Neurol. 1989, 25, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Schwartzman, R.J.; Eidelberg, E.; Alexander, G.M.; Yu, J. Regional metabolic changes in the spinal cord related to spinal shock and later hyperreflexia in monkeys. Ann. Neurol. 1983, 14, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ling, X.; Wen, J.; Liu, J. The role of reactive nitrogen species in secondary spinal cord injury: Formation of nitric oxide, peroxynitrite, and nitrated protein. J. Neurochem. 2000, 75, 2144–2154. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Kroner, A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Hulsebosch, C.E. Recent advances in pathophysiology and treatment of spinal cord injury. Adv. Physiol Educ 2002, 26, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Olivas, A.D.; Noble-Haeusslein, L.J. Inflammation and Spinal Cord Injury: Infiltrating Leukocytes as Determinants of Injury and Repair Processes. Clin. Neurosci. Res. 2006, 6, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Popovich, P.G.; Wei, P.; Stokes, B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997, 377, 443–464. [Google Scholar] [CrossRef]
- Bartholdi, D.; Schwab, M.E. Expression of pro-inflammatory cytokine and chemokine mRNA upon experimental spinal cord injury in mouse: An in situ hybridization study. Eur J. Neurosci. 1997, 9, 1422–1438. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Brechtel, K.; Nguyen, T.D.; Schluesener, H.J. Persistent accumulation of cyclooxygenase-1 (COX-1) expressing microglia/macrophages and upregulation by endothelium following spinal cord injury. J. Neuroimmunol. 2000, 111, 122–130. [Google Scholar] [CrossRef]
- Resnick, D.K.; Graham, S.H.; Dixon, C.E.; Marion, D.W. Role of cyclooxygenase 2 in acute spinal cord injury. J. Neurotrauma 1998, 15, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Li, Q.; Kim, G.M.; Xu, J.; Hsu, C.Y.; Xu, X.M. Cellular localization of tumor necrosis factor-alpha following acute spinal cord injury in adult rats. J. Neurotrauma 2001, 18, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Blight, A.R. Macrophages and inflammatory damage in spinal cord injury. J. Neurotrauma 1992, 9, S83–S91. [Google Scholar] [PubMed]
- Schwab, M.E.; Bartholdi, D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 1996, 76, 319–370. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.D.; Hiester, E.D.; Bunge, R.P. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 2005, 192, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Bunge, R.P.; Puckett, W.R.; Becerra, J.L.; Marcillo, A.; Quencer, R.M. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 1993, 59, 75–89. [Google Scholar] [PubMed]
- Bunge, R.P.; Puckett, W.R.; Hiester, E.D. Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv. Neurol. 1997, 72, 305–315. [Google Scholar] [PubMed]
- Totoiu, M.O.; Keirstead, H.S. Spinal cord injury is accompanied by chronic progressive demyelination. J. Comp. Neurol. 2005, 486, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Crowe, M.J.; Bresnahan, J.C.; Shuman, S.L.; Masters, J.N.; Beattie, M.S. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997, 3, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Eldadah, B.A.; Faden, A.I. Caspase pathways, neuronal apoptosis, and CNS injury. J. Neurotrauma 2000, 17, 811–829. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.; Aldana, P.; Bunge, M.B.; Puckett, W.; Srinivasan, A.; Keane, R.W.; Bethea, J.; Levi, A.D. Apoptosis after traumatic human spinal cord injury. J. Neurosurg. 1998, 89, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Xu, X.M.; Hu, R.; Du, C.; Zhang, S.X.; McDonald, J.W.; Dong, H.X.; Wu, Y.J.; Fan, G.S.; Jacquin, M.F.; et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 1997, 17, 5395–5406. [Google Scholar] [PubMed]
- Beattie, M.S.; Farooqui, A.A.; Bresnahan, J.C. Review of current evidence for apoptosis after spinal cord injury. J. Neurotrauma 2000, 17, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ashwell, K.W.; Waite, P. Advances in secondary spinal cord injury: Role of apoptosis. Spine 2000, 25, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Yamamoto, T.; Sugiyama, Y.; Watanabe, T.; Saito, N.; Kayama, H.; Kumagai, T. Apoptotic cells associated with Wallerian degeneration after experimental spinal cord injury: A possible mechanism of oligodendroglial death. J. Neurotrauma 1999, 16, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D.; Smith, J.; Marcillo, A. The pathology of human spinal cord injury: Defining the problems. J. Neurotrauma 2004, 21, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Rabchevsky, A.G.; Hall, E.D. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J. Neurochem. 2007, 100, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Bastani, N.E.; Kostovski, E.; Sakhi, A.K.; Karlsen, A.; Carlsen, M.H.; Hjeltnes, N.; Blomhoff, R.; Iversen, P.O. Reduced antioxidant defense and increased oxidative stress in spinal cord injured patients. Arch. Phys. Med. Rehabil. 2012, 93, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Hillard, V.H.; Peng, H.; Zhang, Y.; Das, K.; Murali, R.; Etlinger, J.D.; Zeman, R.J. Tempol, a nitroxide antioxidant, improves locomotor and histological outcomes after spinal cord contusion in rats. J. Neurotrauma 2004, 21, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.D.; Cordero, K.; Baldeosingh, K.; Torrado, A.I.; Walker, R.L.; Miranda, J.D.; Leon, M.D. Docosahexaenoic acid pretreatment confers protection and functional improvements after acute spinal cord injury in adult rats. J. Neurotrauma 2012, 29, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.D.; Cordero, K.; Llán, M.S.; De Leon, M. Dietary omega-3 polyunsaturated fatty acids improve the neurolipidome and restore the DHA status while promoting functional recovery after experimental spinal cord injury. J. Neurotrauma 2013, 30, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.D.; Cordero, K.; Serrano-Illan, M.; Almeyda, A.; Baldeosingh, K.; Almaguel, F.G.; De Leon, M. Metabolomics uncovers dietary omega-3 fatty acid-derived metabolites implicated in anti-nociceptive responses after experimental spinal cord injury. Neuroscience 2013, 255, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.D.; Dugan, L.L.; Demediuk, P.; Means, E.D.; Horrocks, L.A.; Anderson, D.K. Effects of methylprednisolone and the combination of alpha-tocopherol and selenium on arachidonic acid metabolism and lipid peroxidation in traumatized spinal cord tissue. J. Neurochem. 1987, 49, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Wolf, D.L. A pharmacological analysis of the pathophysiological mechanisms of posttraumatic spinal cord ischemia. J. Neurosurg. 1986, 64, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.K.; Waters, T.R.; Means, E.D. Pretreatment with alpha tocopherol enhances neurologic recovery after experimental spinal cord compression injury. J. Neurotrauma 1988, 5, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, K.; Ikata, T.; Fukuzawa, K. Protective effect of vitamin E on spinal cord injury by compression and concurrent lipid peroxidation. Free Radic. Biol. Med. 1989, 6, 599–606. [Google Scholar] [CrossRef]
- Al Jadid, M.S.; Robert, A.; Al-Mubarak, S. The efficacy of alpha-tocopherol in functional recovery of spinal cord injured rats: An experimental study. Spinal Cord 2009, 47, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.A.; Zamzami, M.; Sam, A.E.; Al Jadid, M.; Al Mubarak, S. The efficacy of antioxidants in functional recovery of spinal cord injured rats: An experimental study. Neurol. Sci. 2012, 33, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Bozbuga, M.; Izgi, N.; Canbolat, A. The effects of chronic alpha-tocopherol administration on lipid peroxidation in an experimental model of acute spinal cord injury. Neurosurg. Rev. 1998, 21, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Taoka, Y.; Ikata, T.; Fukuzawa, K. Influence of dietary vitamin E deficiency on compression injury of rat spinal cord. J. Nutr. Sci. Vitaminol. 1990, 36, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Ernesto, C.; Thomas, R.G.; Klauber, M.R.; Schafer, K.; Grundman, M.; Woodbury, P.; Growdon, J.; Cotman, C.W.; Pfeiffer, E.; et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer's Disease Cooperative Study. N. Engl. J. Med. 1997, 336, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Morris, M.C.; Rajan, K.B. Vitamin E, memantine, and Alzheimer disease. JAMA 2014, 311, 29–30. [Google Scholar] [CrossRef] [PubMed]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Gruner, J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma 1992, 9, 123–126, discussion 126–128. [Google Scholar] [CrossRef] [PubMed]
- Pikov, V.; Gillis, R.A.; Jasmin, L.; Wrathall, J.R. Assessment of lower urinary tract functional deficit in rats with contusive spinal cord injury. J. Neurotrauma 1998, 15, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.N.; Belton, A.L.; de Groat, W.C. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am. J. Physiol. 1993, 264, R1157–R1163. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.M. Management of bladder dysfunction in cord injury. Rocky Mt. Med. J. 1961, 58, 30–31. [Google Scholar] [PubMed]
- Cruz, C.D.; Cruz, F. Spinal cord injury and bladder dysfunction: New ideas about an old problem. Sci. World J. 2011, 11, 214–234. [Google Scholar] [CrossRef] [PubMed]
- Jousse, A.T.; Geisler, W.O.; Wynne-Jones, M.; MacKay, I. The management of the spastic contracted bladder in spinal cord dysfunction. Proc. Annu. Clin. Spinal Cord Inj. Conf. 1966, 15, 140–151. [Google Scholar] [PubMed]
- Shevtsov, I.P. On the problem of emptying the urinary bladder in patients with spinal cord injury. Voen. Med. Zhurnal 1967, 3, 80–81. [Google Scholar]
- Campbell, E.W. Bladder dysfunction related to lesions of the spinal cord. South. Med. J. 1967, 60, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Damanski, M. Recovery of bladder function in paraplegia. Br. J. Surg. 1967, 54, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, K. Experimental studies on the physiopathology of neurogenic bladder due to spinal cord injury, with special reference to the effect of drugs on the smooth muscle of the bladder in the acute stage. Hinyokika Kiyo 1969, 15, 321–336. [Google Scholar] [PubMed]
- Hofman, P. Bladder behaviour following lesions of the cervical spinal cord. Acta Neurochir 1970, 22, 265–269. [Google Scholar] [PubMed]
- Lee, J.K.; Emch, G.S.; Johnson, C.S.; Wrathall, J.R. Effect of spinal cord injury severity on alterations of the H-reflex. Exp. Neurol. 2005, 196, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Antri, M.; Mouffle, C.; Orsal, D.; Barthe, J.Y. 5-HT1A receptors are involved in short- and long-term processes responsible for 5-HT-induced locomotor function recovery in chronic spinal rat. Eur. J. Neurosci. 2003, 18, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Antri, M.; Barthe, J.Y.; Mouffle, C.; Orsal, D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci. Lett. 2005, 384, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, H.; Rossignol, S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991, 546, 250–260. [Google Scholar] [CrossRef]
- Eaton, M.J.; Pearse, D.D.; McBroom, J.S.; Berrocal, Y.A. The combination of human neuronal serotonergic cell implants and environmental enrichment after contusive SCI improves motor recovery over each individual strategy. Behav. Brain Res. 2008, 194, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Fouad, K.; Rank, M.M.; Vavrek, R.; Murray, K.C.; Sanelli, L.; Bennett, D.J. Locomotion after spinal cord injury depends on constitutive activity in serotonin receptors. J. Neurophysiol. 2010, 104, 2975–2984. [Google Scholar] [CrossRef] [PubMed]
- Gerin, C.G.; Hill, A.; Hill, S.; Smith, K.; Privat, A. Serotonin release variations during recovery of motor function after a spinal cord injury in rats. Synapse 2010, 64, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Harris-Warrick, R.M.; Cohen, A.H. Serotonin modulates the central pattern generator for locomotion in the isolated lamprey spinal cord. J. Exp. Biol. 1985, 116, 27–46. [Google Scholar] [PubMed]
- Hains, B.C.; Johnson, K.M.; Eaton, M.J.; Willis, W.D.; Hulsebosch, C.E. Serotonergic neural precursor cell grafts attenuate bilateral hyperexcitability of dorsal horn neurons after spinal hemisection in rat. Neuroscience 2003, 116, 1097–1110. [Google Scholar] [CrossRef]
- Hains, B.C.; Willis, W.D.; Hulsebosch, C.E. Serotonin receptors 5-HT1A and 5-HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Exp. Brain Res. 2003, 149, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Y.; Wienecke, J.; Chen, M.; Hultborn, H.; Zhang, M. The time course of serotonin 2A receptor expression after spinal transection of rats: An immunohistochemical study. Neuroscience 2011, 177, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.C.; Stephens, M.J.; Ballou, E.W.; Heckman, C.J.; Bennett, D.J. Motoneuron excitability and muscle spasms are regulated by 5-HT2B and 5-HT2C receptor activity. J. Neurophysiol. 2011, 105, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.C.; Stephens, M.J.; Rank, M.; D’Amico, J.; Gorassini, M.A.; Bennett, D.J. Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5-HT1B and 5-HT1F receptors. J. Neurophysiol. 2011, 106, 925–943. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.N.; Bray, L.A.; de Groat, W.C. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J. Auton. Nerv. Syst. 1995, 54, 215–224. [Google Scholar] [CrossRef]
- Karsenty, G.; Reitz, A.; Wefer, B.; Boy, S.; Schurch, B. Understanding detrusor sphincter dyssynergia--significance of chronology. Urology 2005, 66, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, G.; Schröder, A.; Moore, K.; Genois, L.; Lamontagne, P.; Hamel, M.; Pellerin, E.; Bolduc, S. Double anticholinergic therapy for refractory neurogenic and nonneurogenic detrusor overactivity in children: Long-term results of a prospective open-label study. Can. Urol. Assoc. J. 2014, 8, 175–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salcedo, C.; Davalillo, S.; Cabellos, J.; Lagunas, C.; Balsa, D.; Pérez-Del-Pulgar, S.; Ballarín, M.; Fernández, A. In vivo and in vitro pharmacological characterization of SVT-40776, a novel M3 muscarinic receptor antagonist, for the treatment of overactive bladder. Br. J. Pharmacol. 2009, 156, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.I.; Palea, S.; Guilloteau, V.; Guerard, M.; Lluel, P.; Korstanje, C. Modulation of non-voiding activity by the muscarinergic antagonist tolterodine and the beta(3)-adrenoceptor agonist mirabegron in conscious rats with partial outflow obstruction. BJU Int. 2012, 110, E132–E142. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.S.; Noronha-Blob, L. Effects of selective cholinergic antagonists and alpha,beta-methylene ATP on guinea-pig urinary bladder contractions in vivo following pelvic nerve stimulation. J. Auton. Pharmacol. 1989, 9, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Amend, B.; Hennenlotter, J.; Schäfer, T.; Horstmann, M.; Stenzl, A.; Sievert, K.D. Effective treatment of neurogenic detrusor dysfunction by combined high-dosed antimuscarinics without increased side-effects. Eur. Urol. 2008, 53, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, M.; Schaefer, T.; Aguilar, Y.; Stenzl, A.; Sievert, K.D. Neurogenic bladder treatment by doubling the recommended antimuscarinic dosage. Neurourol. Urodyn. 2006, 25, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Ethans, K.D.; Nance, P.W.; Bard, R.J.; Casey, A.R.; Schryvers, O.I. Efficacy and safety of tolterodine in people with neurogenic detrusor overactivity. J. Spinal Cord Med. 2004, 27, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Rivas, D.A.; Shenot, P.J.; Green, B.; Kennelly, M.; Erickson, J.R.; O’Leary, M.; Yoshimura, N.; Chancellor, M.B. Intravesical resiniferatoxin for refractory detrusor hyperreflexia: A multicenter, blinded, randomized, placebo-controlled trial. J. Spinal Cord Med. 2003, 26, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Yuan, J. Experimental study of the effect of capsaicin on the urinary bladder function in rats. J. Tongji Med. Univ. 2000, 20, 116–119. [Google Scholar] [PubMed]
- Geirsson, G.; Fall, M.; Sullivan, L. Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyperreflexia. J. Urol 1995, 154, 1825–1829. [Google Scholar] [CrossRef]
- Das, A.; Chancellor, M.B.; Watanabe, T.; Sedor, J.; Rivas, D.A. Intravesical capsaicin in neurologic impaired patients with detrusor hyperreflexia. J. Spinal Cord Med. 1996, 19, 190–193. [Google Scholar] [CrossRef] [PubMed]
- De Seze, M.; Wiart, L.; Joseph, P.A.; Dosque, J.P.; Mazaux, J.M.; Barat, M. Capsaicin and neurogenic detrusor hyperreflexia: A double-blind placebo-controlled study in 20 patients with spinal cord lesions. Neurourol. Urodyn. 1998, 17, 513–523. [Google Scholar] [CrossRef]
- Cruz, F. Desensitization of bladder sensory fibers by intravesical capsaicin or capsaicin analogs. A new strategy for treatment of urge incontinence in patients with spinal detrusor hyperreflexia or bladder hypersensitivity disorders. Int. Urogynecol. J. 1998, 9, 214–220. [Google Scholar] [CrossRef]
- De Seze, M.; Wiart, L.; de Sèze, M.P.; Joseph, P.A.; Brochet, B.; Ferrière, J.M.; Mazaux, J.M.; Barat, M. Reiterated intravesical instillation of capsaicin in neurogenic detrusor hyperreflexia: A 5-years experience of 100 instillations. Ann. Readapt. Med. Phys. 2001, 44, 514–524. [Google Scholar] [PubMed]
- Linsenmeyer, T.A.; Horton, J.; Benevento, J. Impact of alpha1-blockers in men with spinal cord injury and upper tract stasis. J. Spinal Cord Med. 2002, 25, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K. alpha1-adrenoceptors and bladder function. Eur. Urol. 1999, 36, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Z.; Tillig, B.; Perkash, I.; Constantinou, C.E. Effect of alpha1 adrenoceptor antagonist on the urodynamics of the upper and lower urinary tract of the male rat. Neurourol. Urodyn. 1998, 17, 213–229. [Google Scholar] [CrossRef]
- Markiewicz, W.; Jasiecka, A.; Barski, D.; Janiuk, J.; Bossowska, A.; Jaroszewski, J.J. The influence of doxazosin, an alpha1-adrenergic receptor antagonist on the urinary bladder contractility in pigs. Pol. J. Vet. Sci. 2014, 17, 527–529. [Google Scholar] [PubMed]
- Usta, C.; Kukul, E.; Yalcinkaya, M. Doxazosin effects on cholinergic and adrenergic responses in rat isolated detrusor smooth muscle preparations from obstructed bladder. J. Pharmacol. Sci. 2004, 95, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Leggett, R.E.; Whitbeck, C.; Eagen, G.; Levin, R.M. Effect of doxazosin on rat urinary bladder function after partial outlet obstruction. Neurourol. Urodyn. 2002, 21, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Serels, S.; Stein, M. Prospective study comparing hyoscyamine, doxazosin, and combination therapy for the treatment of urgency and frequency in women. Neurourol. Urodyn. 1998, 17, 31–36. [Google Scholar] [CrossRef]
- Miyazato, M.; Sugaya, K.; Nishijima, S.; Kadekawa, K.; Ashimine, S.; Ogawa, Y. Intrathecal or dietary glycine inhibits bladder and urethral activity in rats with spinal cord injury. J. Urol. 2005, 174, 2397–2400. [Google Scholar] [CrossRef] [PubMed]
- Magora, F.; Shazar, N.; Drenger, B. Urodynamic studies after intrathecal administration of baclofen and morphine in dogs. J. Urol. 1989, 141, 143–147. [Google Scholar] [CrossRef]
- Steers, W.D.; Meythaler, J.M.; Haworth, C.; Herrell, D.; Park, T.S. Effects of acute bolus and chronic continuous intrathecal baclofen on genitourinary dysfunction due to spinal cord pathology. J. Urol. 1992, 148, 1849–1855. [Google Scholar] [CrossRef]
- Kilicarslan, H.; Ayan, S.; Vuruskan, H.; Gokce, G.; Gultekin, E.Y. Treatment of detrusor sphincter dyssynergia with baclofen and doxazosin. Int. Urol. Nephrol. 2006, 38, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Miyazato, M.; Sasatomi, K.; Hiragata, S.; Sugaya, K.; Chancellor, M.B.; de Groat, W.C.; Yoshimura, N. Suppression of detrusor-sphincter dysynergia by GABA-receptor activation in the lumbosacral spinal cord in spinal cord-injured rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R336–R342. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Araki, I.; Mochizuki, T.; Nakagomi, H.; Kobayashi, H.; Sawada, N.; Zakohji, H. The forefront for novel therapeutic agents based on the pathophysiology of lower urinary tract dysfunction: Pathophysiology of voiding dysfunction and pharmacological therapy. J. Pharmacol. Sci. 2010, 112, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kalinichev, M.; Palea, S.; Haddouk, H.; Royer-Urios, I.; Guilloteau, V.; Lluel, P.; Schneider, M.; Saporito, M.; Poli, S. ADX71441, a novel, potent and selective positive allosteric modulator of the GABA(B) receptor, shows efficacy in rodent models of overactive bladder. Br. J. Pharmacol. 2014, 171, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Leippold, T.; Reitz, A.; Schurch, B. Botulinum toxin as a new therapy option for voiding disorders: Current state of the art. Eur. Urol. 2003, 44, 165–174. [Google Scholar] [CrossRef]
- Gallien, P.; Robineau, S.; Verin, M.; Le Bot, M.P.; Nicolas, B.; Brissot, R. Treatment of detrusor sphincter dyssynergia by transperineal injection of botulinum toxin. Arch. Phys. Med. Rehabil. 1998, 79, 715–717. [Google Scholar] [CrossRef]
- Giannantoni, A.; Mearini, E.; Del Zingaro, M.; Porena, M. Six-year follow-up of botulinum toxin A intradetrusorial injections in patients with refractory neurogenic detrusor overactivity: Clinical and urodynamic results. Eur. Urol. 2009, 55, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Chancellor, M.B. Emerging role of botulinum toxin in the management of voiding dysfunction. J. Urol. 2004, 171, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Schurch, B. Botulinum toxin for the management of bladder dysfunction. Drugs 2006, 66, 1301–1318. [Google Scholar] [CrossRef] [PubMed]
- Petit, H.; Wiart, L.; Gaujard, E.; Le Breton, F.; Ferrière, J.M.; Lagueny, A.; Joseph, P.A.; Barat, M. Botulinum A toxin treatment for detrusor-sphincter dyssynergia in spinal cord disease. Spinal Cord 1998, 36, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Cevik, O.; Erşahin, M.; Sener, T.E.; Tinay, I.; Tarcan, T.; Cetinel, S.; Sener, A.; Toklu, H.Z.; Sener, G. Beneficial effects of quercetin on rat urinary bladder after spinal cord injury. J. Surg. Res. 2013, 183, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.J.; Hess, P.E.; Sullivan, M.R.; Nee, M.; Yalla, S.V. Evaluation of cranberry tablets for the prevention of urinary tract infections in spinal cord injured patients with neurogenic bladder. Spinal Cord 2008, 46, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Back, S.A.; Gan, X.; Li, Y.; Rosenberg, P.A.; Volpe, J.J. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J. Neurosci. 1998, 18, 6241–6253. [Google Scholar] [PubMed]
- Yonezawa, M.; Back, S.A.; Gan, X.; Rosenberg, P.A.; Volpe, J.J. Cystine deprivation induces oligodendroglial death: Rescue by free radical scavengers and by a diffusible glial factor. J. Neurochem. 1996, 67, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Nury, T.; Zarrouk, A.; Mackrill, J.J.; Samadi, M.; Durand, P.; Riedinger, J.M.; Doria, M.; Vejux, A.; Limagne, E.; Delmas, D. Induction of oxiapoptophagy on 158N murine oligodendrocytes treated by 7-ketocholesterol-, 7beta-hydroxycholesterol-, or 24(S)-hydroxycholesterol: Protective effects of alpha-tocopherol and docosahexaenoic acid (DHA; C22:6 n-3). Steroids 2015, 99, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Nury, T.; Zarrouk, A.; Vejux, A.; Doria, M.; Riedinger, J.M.; Delage-Mourroux, R.; Lizard, G. Induction of oxiapoptophagy, a mixed mode of cell death associated with oxidative stress, apoptosis and autophagy, on 7-ketocholesterol-treated 158N murine oligodendrocytes: Impairment by alpha-tocopherol. Biochem. Biophys. Res. Commun. 2014, 446, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Ragot, K.; Delmas, D.; Athias, A.; Nury, T.; Baarine, M.; Lizard, G. alpha-Tocopherol impairs 7-ketocholesterol-induced caspase-3-dependent apoptosis involving GSK-3 activation and Mcl-1 degradation on 158N murine oligodendrocytes. Chem. Phys. Lipids 2011, 164, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H.; Martens, U.; Prall, F.; Uhr, M. Relevance of endogenous ascorbate and tocopherol for brain cell vitality indicated by photon emission. J. Neurochem. 1994, 62, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Bjartmar, C.; Trapp, B.D. Axonal and neuronal degeneration in multiple sclerosis: Mechanisms and functional consequences. Curr. Opin. Neurol. 2001, 14, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Blight, A.R. Remyelination, revascularization, and recovery of function in experimental spinal cord injury. Adv. Neurol. 1993, 59, 91–104. [Google Scholar] [PubMed]
- Valdovinos, B.D.; Jimenez, J.M.D.; Estrada, I.J.; Pineda, J.B.; Rodriguez, N.E.F.; Ruiz, J.R.L.; Carrasco, L.P.O.; Arellano, A.C.; Jimenez, S.H.D. Tamoxifen Promotes Axonal Preservation and Gait Locomotion Recovery after Spinal Cord Injury in Cats. J. Vet. Med. 2016, 2016, 9561968. [Google Scholar]
- Jeffery, N.D.; Blakemore, W.F. Locomotor deficits induced by experimental spinal cord demyelination are abolished by spontaneous remyelination. Brain 1997, 120, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, N.D.; Crang, A.J.; O’leary, M.T.; Hodge, S.J.; Blakemore, W.F. Behavioural consequences of oligodendrocyte progenitor cell transplantation into experimental demyelinating lesions in the rat spinal cord. Eur. J. Neurosci. 1999, 11, 1508–1514. [Google Scholar] [CrossRef]
- Qiu, X.C.; Jin, H.; Zhang, R.Y.; Ding, Y.; Zeng, X.; Lai, B.Q.; Ling, E.A.; Wu, J.L.; Zeng, Y.S. Donor mesenchymal stem cell-derived neural-like cells transdifferentiate into myelin-forming cells and promote axon regeneration in rat spinal cord transection. Stem Cell Res. Ther. 2015, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lin, C.Y.; Robertson, R.T.; Yu, J.; Deng, X.; Hsiao, I.; Lin, V.W. Re-growth of catecholaminergic fibers and protection of cholinergic spinal cord neurons in spinal repaired rats. Eur J. Neurosci. 2006, 23, 693–702. [Google Scholar] [CrossRef] [PubMed]
- DePaul, M.A.; Lin, C.Y.; Silver, J.; Lee, Y.S. Peripheral Nerve Transplantation Combined with Acidic Fibroblast Growth Factor and Chondroitinase Induces Regeneration and Improves Urinary Function in Complete Spinal Cord Transected Adult Mice. PLoS ONE 2015, 10, e0139335. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, T.; Neuhuber, B.; Fischer, I. Acute administration of AMPA/Kainate blocker combined with delayed transplantation of neural precursors improves lower urinary tract function in spinal injured rats. Brain Res. 2011, 1418, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Cheng, C.L.; Chen, J.J.; de Groat, W.C. Serotonergic drugs and spinal cord transections indicate that different spinal circuits are involved in external urethral sphincter activity in rats. Am. J. Physiol. Ren. Physiol. 2007, 292, F1044–F1053. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Vyas, D.; Bieger, D. Serotonin immunoreactivity in spinal cord axons and terminals of rodents with experimental allergic encephalomyelitis. Neuroscience 1985, 16, 701–709. [Google Scholar] [CrossRef]
- Sandyk, R. Serotonergic neuronal atrophy with synaptic inactivation, not axonal degeneration, are the main hallmarks of multiple sclerosis. Int. J. Neurosci. 1998, 95, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Fukuda, N. Contribution of serotonin neurons to the functional recovery after spinal cord injury in rats. Brain Res. 1991, 539, 263–270. [Google Scholar] [CrossRef]
- Saruhashi, Y.; Young, W.; Perkins, R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp. Neurol. 1996, 139, 203–213. [Google Scholar] [CrossRef] [PubMed]


| Ingredient | AIN-93G Control Diet (%) | AIN-93G Vitamin E |
|---|---|---|
| Soybean Oil | 7 | 7 |
| Vitamin E, IU kg | 0.0816 | 51 |
| Total Saturated Fat | 1.13 g/100 g | 1.00 g/100 g |
| Total Monosaturated Fat | 1.61 g/100 g | 1.49 g/100 g |
| Total Polysaturated Fat | 4.09 g/100 g | 4.34 g/100 g |
| Percentage kcal Carbohydrates | 64.7 | 60.5 |
| Percentages kcal Protein | 18.8 | 21.1 |
| Percentage kcal Fat | 16.5 | 18.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordero, K.; Coronel, G.G.; Serrano-Illán, M.; Cruz-Bracero, J.; Figueroa, J.D.; De León, M. Effects of Dietary Vitamin E Supplementation in Bladder Function and Spasticity during Spinal Cord Injury. Brain Sci. 2018, 8, 38. https://doi.org/10.3390/brainsci8030038
Cordero K, Coronel GG, Serrano-Illán M, Cruz-Bracero J, Figueroa JD, De León M. Effects of Dietary Vitamin E Supplementation in Bladder Function and Spasticity during Spinal Cord Injury. Brain Sciences. 2018; 8(3):38. https://doi.org/10.3390/brainsci8030038
Chicago/Turabian StyleCordero, Kathia, Gemma G. Coronel, Miguel Serrano-Illán, Jennifer Cruz-Bracero, Johnny D. Figueroa, and Marino De León. 2018. "Effects of Dietary Vitamin E Supplementation in Bladder Function and Spasticity during Spinal Cord Injury" Brain Sciences 8, no. 3: 38. https://doi.org/10.3390/brainsci8030038
APA StyleCordero, K., Coronel, G. G., Serrano-Illán, M., Cruz-Bracero, J., Figueroa, J. D., & De León, M. (2018). Effects of Dietary Vitamin E Supplementation in Bladder Function and Spasticity during Spinal Cord Injury. Brain Sciences, 8(3), 38. https://doi.org/10.3390/brainsci8030038






