Update on Insomnia after Mild Traumatic Brain Injury
Abstract
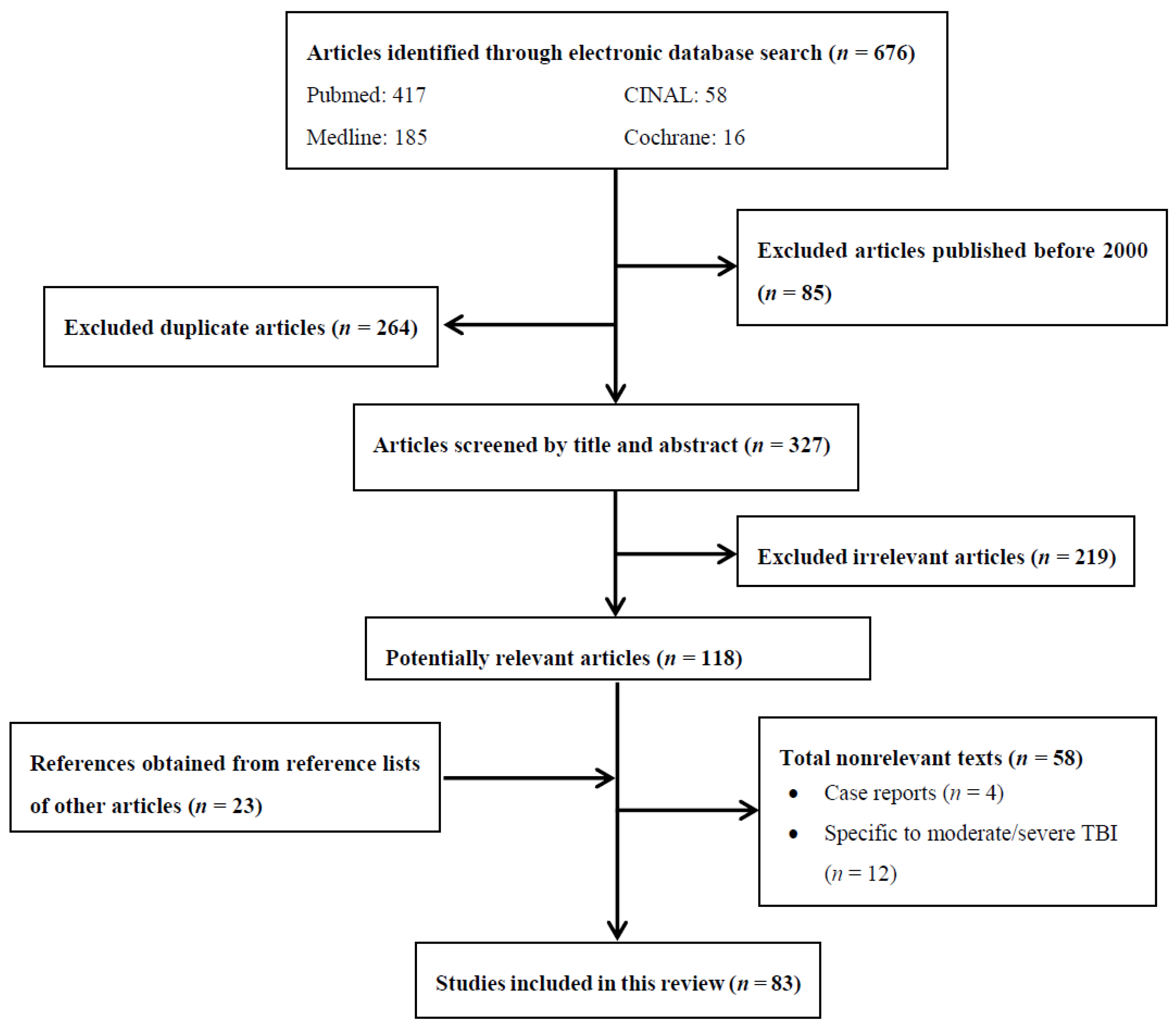
1. Methods
2. Introduction
3. Pathophysiology of Sleep Disturbance after TBI
Sleep Architecture Changes Seen after mTBI
4. Insomnia and mTBI
5. Epidemiology of Insomnia after mTBI
5.1. Repeat TBI and Blast Injury
5.2. Implications of TBI Severity
6. Prognosis
Consequences of Sleep Impairment
7. Differential Diagnosis of Sleep Disturbance after TBI
7.1. Pain
7.2. Sleep Apnea
7.3. Post-Traumatic Stress Disorder
7.4. Circadian Rhythm
8. Treatment
8.1. Sleep Hygiene
8.2. Cognitive Behavioral Therapy & Digital CBT
9. Pharmacologic Options
9.1. Benzodiazepines/Z-Drugs
9.2. Trazodone
9.3. Melatonin/Melatonin Agonists
10. Summary and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, C.A.; Bell, J.M.; Breiding, M.J.; Xu, L. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 2017, 66, 1–16. [Google Scholar] [CrossRef]
- Cassidy, J.D.; Carroll, L.J.; Peloso, P.M.; Borg, J.; von Holst, H.; Holm, L.; Kraus, J.; Coronado, V. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, 36, 28–60. [Google Scholar] [CrossRef]
- Powell, J.M.; Ferraro, J.V.; Dikmen, S.S.; Temkin, N.R.; Bell, K.R. Accuracy of mild traumatic brain injury diagnosis. Arch. Phys. Med. Rehabil. 2008, 89, 1550–1555. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Williams, S.G.; Roth, T.; Capaldi, V.F.; Jaffe, M.; Moline, M.; Motamedi, G.K.; Morgan, G.W.; Mysliwiec, V.; Germain, A.; et al. Sleep, Sleep Disorders, and Mild Traumatic Brain Injury. What We Know and What We Need to Know: Findings from a National Working Group. Neurotherapeutics 2016, 13, 403–417. [Google Scholar] [CrossRef]
- Mollayeva, T.; Mollayeva, S.; Colantonio, A. The Risk of Sleep Disorder among Persons with Mild Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2016, 16, 55. [Google Scholar] [CrossRef]
- Vermaelen, J.; Greiffenstein, P.; deBoisblanc, B.P. Sleep in traumatic brain injury. Crit. Care Clinic. 2015, 31, 551–561. [Google Scholar] [CrossRef]
- Kraus, M.F.; Susmaras, T.; Caughlin, B.P.; Walker, C.J.; Sweeney, J.A.; Little, D.M. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 2007, 130, 2508–2519. [Google Scholar] [CrossRef]
- Viola-Saltzman, M.; Watson, N.F. Traumatic Brain Injury and Sleep Disorders. Neurol. Clin. 2012, 30, 1299–1312. [Google Scholar] [CrossRef]
- Arciniegas, D.B.; Topkoff, J.; Silver, J.M. Neuropsychiatric aspects of traumatic brain injury. Curr. Treat. Options Neurol. 2000, 2, 169–186. [Google Scholar] [CrossRef]
- Orff, H.J.; Ayalon, L.; Drummond, S.P. Traumatic brain injury and sleep disturbance: A review of current research. J. Head Trauma Rehabil. 2009, 24, 155–165. [Google Scholar] [CrossRef]
- Viola-Saltzman, M.; Musleh, C. Traumatic brain injury-induced sleep disorders. Neuropsychiatr. Dis. Treat. 2016, 12, 339–348. [Google Scholar] [CrossRef]
- Gilbert, K.S.; Kark, S.M.; Gehrman, P.; Bogdanova, Y. Sleep Disturbances, TBI and PTSD: Implications for Treatment and Recovery. Clin. Psychol. Rev. 2015, 40, 195–212. [Google Scholar] [CrossRef]
- Yaeger, K.; Alhilali, L.; Fakhran, S. Evaluation of tentorial length and angle in sleep-wake disturbances after mild traumatic brain injury. Am. J. Roentgenol. 2014, 202, 614–618. [Google Scholar] [CrossRef]
- Fakhran, S. Symptomatic white matter changes in mild traumatic brain injury resemble pathologic features of early alzheimer dementia. Radiology 2013, 269, 249–257. [Google Scholar] [CrossRef]
- Hazra, A. Delayed thalamic astrocytosis and disrupted sleep-wake patterns in a preclinical model of traumatic brain injury. J. Neurosci Res. 2014, 92, 1434–1445. [Google Scholar] [CrossRef]
- Shekleton, J.; Parcell, D.L.; Redman, J.R.; Phipps-Nelson, J.; Ponsford, J.L.; Rajaratnam, S.M.W. Sleep disturbance and melatonin levels following traumatic brain injury. Neurology 2010, 74, 1732–1738. [Google Scholar] [CrossRef]
- Wilkinson, C.W.; Pagulayan, K.F.; Petrie, E.C.; Mayer, C.L.; Colasurdo, E.A.; Shofer, J.B.; Hart, K.L.; Hoff, D.; Tarabochia, M.A.; Peskind, E.R. High Prevalence of Chronic Pituitary and Target-Organ Hormone Abnormalities after Blast-Related Mild Traumatic Brain Injury. Front. Neurol. 2012, 3, 11. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Friedman, L.; O’Hara, R. Insomnia in the context of traumatic brain injury. J. Rehabil. Res. Dev. 2009, 46, 827–836. [Google Scholar] [CrossRef]
- Sabir, M.; Gaudreault, P.O.; Freyburger, M.; Massart, R.; Blanchet-Cohen, A.; Jaber, M.; Gosselin, N.; Mongrain, V. Impact of traumatic brain injury on sleep structure, electrocorticographic activity and transcriptome in mice. Brain Behav. Immun. 2015, 47, 118–130. [Google Scholar] [CrossRef]
- Hong, C.T.; Wong, C.S.; Ma, H.P.; Wu, D.; Huang, Y.H.; Wu, C.C.; Lin, C.M.; Su, Y.K.; Liao, K.H.; Ou, J.C.; et al. PERIOD3 polymorphism is associated with sleep quality recovery after a mild traumatic brain injury. J. Neurol. Sci. 2015, 358, 385–389. [Google Scholar] [CrossRef]
- El Shakankiry, H.M. Sleep physiology and sleep disorders in childhood. Nat. Sci. Sleep 2011, 3, 101–114. [Google Scholar] [CrossRef]
- Schreiber, S.; Barkai, G.; Gur-Hartman, T.; Peles, E.; Tov, N.; Dolberg, O.T.; Pick, C.G. Long-lasting sleep patterns of adult patients with minor traumatic brain injury (mTBI) and non-mTBI subjects. Sleep Med. 2008, 9, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Lucke-Wold, B.P.; Smith, K.E.; Nguyen, L.; Turner, R.C.; Logsdon, A.F.; Jackson, G.J.; Huber, J.D.; Rosen, C.L.; Miller, D.B. Sleep disruption and the sequelae associated with traumatic brain injury. Neurosci. Biobehav. Rev. 2015, 55, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, Y.; Tzischinsky, O.; Epstein, R.; Etzioni, A.; Lavie, P.; Pillar, G. Long-term sleep disturbances in adolescents after minor head injury. Pediatr. Neurol. 2001, 24, 129–134. [Google Scholar] [CrossRef]
- Ouellet, M.C.; Morin, C.M. Subjective and objective measures of insomnia in the context of traumatic brain injury: A preliminary study. Sleep Med. 2006, 7, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Waldron-Perrine, B.; McGuire, A.P.; Spencer, R.J.; Drag, L.L.; Pangilinan, P.H.; Bieliauskas, L.A. The influence of sleep and mood on cognitive functioning among veterans being evaluated for mild traumatic brain injury. Mil. Med. 2012, 177, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Bolge, S.C.; Doan, J.F.; Kannan, H.; Baran, R.W. Association of insomnia with quality of life, work productivity, and activity impairment. Qual. Life Res. 2009, 18, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.; Alvaro, P. Prevalence of sleep disturbances, disorders and problems following traumatic brain injury: A meta-analysis. Sleep Med. 2017, 13, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.C.; Beaulieu-Bonneau, S.; Morin, C.M. Insomnia in patients with traumatic brain injury: Frequency, characteristics, and risk factors. J. Head Trauma Rehabil. 2006, 21, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.A.; Berndt, S.L.; Edmed, S.L.; Smith, S.S.; Allan, A.C. Poor sleep predicts subacute postconcussion symptoms following mild traumatic brain injury. Appl. Neuropsychol. Adult 2016, 23, 426–435. [Google Scholar] [CrossRef]
- Ouellet, M.C.; Savard, J.; Morin, C.M. Insomnia following Traumatic Brain Injury: A Review. Neurorehabil. Neural Repair 2004, 18, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Fichtenberg, N.L.; Millis, S.R.; Mann, N.R.; Zafonte, R.D.; Millard, A.E. Factors associated with insomnia among post-acute traumatic brain injury survivors. Brain Inj. 2000, 14, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Tham, S.W.; Fales, J.; Palermo, T.M. Subjective and objective assessment of sleep in adolescents with mild traumatic brain injury. J. Neurotrauma 2015, 32, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Hunt, C.; Ouchterlony, D. Is Age Associated With the Severity of Post-Mild Traumatic Brain Injury Symptoms? Can. J. Neurol. Sci. 2017, 44, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, A.L.; Plog, B.A.; Dayawansa, S.; Chen, M.; Dashnaw, M.L.; Czerniecka, K.; Huang, J.H. The Spectrum of Neurobehavioral Sequelae after Repetitive Mild Traumatic Brain Injury: A Novel Mouse Model of Chronic Traumatic Encephalopathy. J. Neurotrauma 2014, 31, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Bryan, C.J. Repetitive traumatic brain injury (or concussion) increases severity of sleep disturbance among deployed military personnel. Sleep 2013, 36, 941–946. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; Robinson, M.E. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014, 10, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Hicks, R.R.; Fertig, S.J.; Desrocher, R.E.; Koroshetz, W.J.; Pancrazio, J.J. Neurological effects of blast injury. J. Trauma 2010, 68, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Sayer, N.A.; Chiros, C.E.; Sigford, B.; Scott, S.; Clothier, B.; Pickett, T.; Lew, H.L. Characteristics and Rehabilitation Outcomes among Patients with Blast and Other Injuries Sustained During the Global War on Terror. Arch. Phys. Med. Rehabil. 2008, 89, 163–170. [Google Scholar] [CrossRef]
- Linton, S.J.; Bryngelsson, I.L. Insomnia and its relationship to work and health in a working-age population. J. Occup. Rehabil. 2000, 10, 169–183. [Google Scholar] [CrossRef]
- Baumann, C.R.; Werth, E.; Stocker, R.; Ludwig, S.; Bassetti, C.L. Sleep-wake disturbances 6 months after traumatic brain injury: A prospective study. Brain 2007, 130, 1873–1883. [Google Scholar] [CrossRef]
- Farrell-Carnahan, L.; Barnett, S.; Lamberty, G.; Hammond, F.M.; Kretzmer, T.S.; Franke, L.M.; Geiss, M.; Howe, L.; Nakase-Richardson, R. Insomnia symptoms and behavioural health symptoms in veterans 1 year after traumatic brain injury. Brain Inj. 2015, 29, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Losoi, H.; Silverberg, N.D.; Wäljas, M.; Turunen, S.; Rosti-Otajärvi, E.; Helminen, M.; Luoto, T.M.; Julkunen, J.; Öhman, J.; Iverson, G.L. Recovery from Mild Traumatic Brain Injury in Previously Healthy Adults. J. Neurotrauma 2016, 33, 766–776. [Google Scholar] [CrossRef] [PubMed]
- McMahon, P.J.; Hricik, A.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Lingsma, H.F.; Vassar, M.J. Symptomatology and functional outcome in mild traumatic brain injury: Results from the prospective TRACK-TBI study. J. Neurotrauma 2014, 31, 26–33. [Google Scholar] [CrossRef]
- Ma, H.P.; Ou, J.C.; Yeh, C.T.; Wu, D.; Tsai, S.H.; Chiu, W.T.; Hu, C.J. Recovery from sleep disturbance precedes that of depression and anxiety following mild traumatic brain injury: A 6-week follow-up study. BMJ Open 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Martindale, S.L.; Farrell-Carnahan, L.V.; Ulmer, C.S.; Kimbrel, N.A.; McDonald, S.D.; Rowland, J.A. VA Mid-Atlantic MIRECC Registry Workgroup. Sleep Quality in Returning Veterans: The Influence of Mild Traumatic Brain Injury. Rehabil. Psychol. 2017, 62, 563–570. [Google Scholar] [CrossRef]
- Theadom, A.; Starkey, N.; Jones, K.; Cropley, M.; Parmar, P.; Barker-Collo, S.; Feigin, V.L. Sleep difficulties and their impact on recovery following mild traumatic brain injury in children. Brain Inj. 2016, 30, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Pillar, G.; Averbooch, E.; Katz, N.; Peled, N.; Kaufman, Y.; Shahar, E. Prevalence and risk of sleep disturbances in adolescents after minor head injury. Pediatr. Neurol. 2003, 29, 131–135. [Google Scholar] [CrossRef]
- Theadom, A.; Parag, V.; Dowell, T.; McPherson, K.; Starkey, N.; Barker-Collo, S.; Jones, K.; Ameratunga, S.; Feigin, V.L. Persistent problems 1 year after mild traumatic brain injury: A longitudinal population study in New Zealand. Br. J. Gen. Pract. 2015, 66, 16–23. [Google Scholar] [CrossRef]
- Duclos, C.; Beauregard, M.P.; Bottari, C.; Ouellet, M.C.; Gosselin, N. The impact of poor sleep on cognition and activities of daily living after traumatic brain injury: A review. Aust. Occup. Ther. J. 2015, 62, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Theadom, A.; Cropley, M.; Parmar, P.; Barker-Collo, S.; Starkey, N.; Jones, K.; Feigin, V.L. BIONIC Research Group. Sleep difficulties one year following mild traumatic brain injury in a population-based study. Sleep Med. 2015, 16, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, S.B.; Ouellet, M.C. Fatigue in the first year after traumatic brain injury: Course, relationship with injury severity, and correlates. Neuropsychol. Rehabil. 2017, 27, 983–1001. [Google Scholar] [CrossRef] [PubMed]
- Wylie, G.R.; Flashman, L.A. Understanding the interplay between mild traumatic brain injury and cognitive fatigue: Models and treatments. Concussion 2017, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Norre, J.; Heitger, M.; Leathem, J.; Anderson, T.; Jones, R.; Flett, R. Mild traumatic brain injury and fatigue: A prospective longitudinal study. Brain Inj. 2010, 24, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Stulemeijer, M.; van der Werf, S.; Bleijenberg, G.; Biert, J.; Brauer, J.; Vos, P. Recovery from mild traumatic brain injury: A focus on fatigue. J. Neurol. 2006, 253, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Albicini, M.S.; Lee, J.; McKinlay, A. Ongoing daytime behavioural problems in university students following childhood mild traumatic brain injury. Int. J. Rehabil. Res. 2016, 39, 77–83. [Google Scholar] [CrossRef]
- Mollayeva, T.; Pratt, B.; Mollayeva, S.; Shapiro, C.M.; Cassidy, J.D.; Colantonio, A. The relationship between insomnia and disability in workers with mild traumatic brain injury/concussion: Insomnia and disability in chronic mild traumatic brain injury. Sleep Med. 2016, 20, 157–166. [Google Scholar] [CrossRef]
- Lavigne, G.; Khoury, S.; Chauny, J.M.; Desautels, A. Pain and sleep in post-concussion/mild traumatic brain injury. Pain 2015, 156, 75–85. [Google Scholar] [CrossRef]
- Suzuki, Y.; Khoury, S.; El-Khatib, H.; Chauny, J.M.; Paquet, J.; Giguère, J.F.; Denis, R.; Gosselin, N.; Lavigne, G.J.; Arbour, C. Individuals with pain need more sleep in the early stage of mild traumatic brain injury. Sleep Med. 2017, 33, 36–42. [Google Scholar] [CrossRef]
- MacGregor, A.J.; Dougherty, A.L.; Tang, J.J.; Galarneau, M.R. Postconcussive symptom reporting among US combat veterans with mild traumatic brain injury from Operation Iraqi Freedom. J. Head Trauma Rehabil. 2013, 28, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Sherman, K.B.; Goldberg, M.; Bell, K.R. Traumatic Brain Injury and Pain. Phys. Med. Rehabil. Clin. N. Am. 2006, 17, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Sandsmark, D.K.; Elliott, J.E.; Lim, M.M. Sleep-Wake Disturbances after Traumatic Brain Injury: Synthesis of Human and Animal Studies. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Tregear, S.; Reston, J.; Schoelles, K.; Phillips, B. Obstructive sleep apnea and risk of motor vehicle crash: Systematic review and meta-analysis. J. Clin. Sleep Med. 2009, 5, 573–581. [Google Scholar] [PubMed]
- Howlett, J.R.; Stein, M.B. Post-Traumatic Stress Disorder: Relationship to Traumatic Brain Injury and Approach to Treatment. In Translation Research in Traumatic Brain Injury (Frontiers in Neuroscience), 1st ed.; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016; Volume 57, Chapter 16; ISBN 978-1-4665-8491-4. [Google Scholar]
- Tanev, K.S.; Pentel, K.Z.; Kredlow, M.A.; Chamey, M.E. PTSD and TBI co-morbidity: Scope, clinical presentation and treatment options. Brain Inj. 2014, 28, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Capaldi, V.F.; Guerrero, M.L.; Killgore, W.D. Sleep disruptions among returning combat veterans from Iraq and Afghanistan. Mil. Med. 2011, 176, 879–888. [Google Scholar] [CrossRef]
- Ayalon, L.; Borodkin, K.; Dishon, L.; Kanety, H.; Dagan, Y. Circadian rhythm sleep disorders following mild traumatic brain injury. Neurology 2007, 68, 1136–1140. [Google Scholar] [CrossRef]
- Roth, T. Prevalence, associated risks, and treatment patterns of insomnia. J. Clin. Psychiatry 2005, 6, 10–13. [Google Scholar]
- Capaldi, V.F.; Kim, J.R.; Grillakis, A.A.; Taylor, M.R.; York, C.M. Insomnia in the Military: Application and Effectiveness of Cognitive and Pharmacologic Therapies. Curr. Psychiatry Rep. 2015, 17, 85. [Google Scholar] [CrossRef]
- Blinman, T.A.; Houseknecht, E.; Snyder, C.; Wiebe, D.J.; Nance, M.L. Postconcussive symptoms in hospitalized pediatric patients after mild traumatic brain injury. J. Pediatr. Surg. 2009, 44, 1223–1228. [Google Scholar] [CrossRef]
- Bogdanov, S.; Naismith, S.; Lah, S. Sleep outcomes following sleep-hygiene-related interventions for individuals with traumatic brain injury: A systematic review. Brain Inj. 2017, 31, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.C.; Morin, C.M. Efficacy of cognitive-behavioral therapy for insomnia associated with traumatic brain injury: A single-case experimental design. Arch. Phys. Med. Rehabil. 2007, 88, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; McKay, A.; Wong, D.; Rajaratnam, S.M.; Spitz, G.; Williams, G.; Mansfield, D.; Ponsford, J.L. Cognitive Behavior Therapy to Treat Sleep Disturbance and Fatigue After Traumatic Brain Injury: A Pilot Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Luik, A.I.; Kyle, S.D.; Espie, C.A. Digital Cognitive Behavioral Therapy (dCBT) for Insomnia: A State-of-the-Science Review. Curr. Sleep Med. Rep. 2017, 3, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Espie, C.A.; Luik, A.I.; Cape, J.; Drake, C.L.; Siriwardena, A.N.; Ong, J.C.; Gordon, C.; Bostock, S.; Bostock, S.; Hames, P.; et al. Digital Cognitive Behavioural Therapy for Insomnia versus sleep hygiene education: The impact of improved sleep on functional health, quality of life and psychological well-being. Study protocol for a randomised controlled trial. Trials 2016, 17, 257. [Google Scholar] [CrossRef]
- Sirdifield, C.; Chipchase, S.Y.; Owen, S.; Siriwardena, A.N. A Systematic Review and Meta-Synthesis of Patients’ Experiences and Perceptions of Seeking and Using Benzodiazepines and Z-Drugs: Towards Safer Prescribing. Patient 2017, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Larson, E.B.; Zollman, F.S. The effect of sleep medications on cognitive recovery from traumatic brain injury. J. Head Trauma Rehabil. 2010, 25, 61–67. [Google Scholar] [CrossRef]
- Hajak, G.; Muller, W.E.; Wittchen, H.U.; Pittrow, D.; Kirch, W. Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: A review of case reports and epidemiological data. Addiction 2003, 98, 1371–1378. [Google Scholar] [CrossRef]
- Li Pi Shan, R.S.; Ashworth, N.L. Comparison of lorazapam and zopicloine for insomnia in patients with stroke and brain injury. Am. J. Phys. Med. Rehabil. 2004, 83, 421–427. [Google Scholar] [CrossRef]
- Glenn, M.B.; Wroblewski, B. Twenty years of pharmacology. J. Head Trauma Rehabil. 2005, 20, 51–61. [Google Scholar] [CrossRef]
- Grima, N.A.; Rajaratam, S.M.W.; Mansfield, D.; Sletten, T.L.; Spitz, G.; Ponsford, J.L. Efficacy of melatonin for sleep disturbance following traumatic brain injury: A randomized controlled trial. BMC Med. 2018, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Leguerica, A.; Jasey, N.; Portelli Tremont, J.N.; Chiaravalloti, N.D. Pilot study on the effect of ramelteon on sleep disturbance after traumatic brain injury: Preliminary evidence from a clinical trial. Arch. Phys. Med. Rehabil. 2015, 96, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Design | Participants | Sleep Outcome Measure | Results | Limitations |
|---|---|---|---|---|---|
| Ma et al. (2014) [46] | Prospective cohort | mTBI group:
| Pittsburgh Sleep Quality Index (PSQI) |
|
|
| McMahon et al. (2014) [45] | Prospective cohort | mTBI study population:
| Postconcussion Syndrome (PCS) Symptom Checklist |
|
|
| Martindale et al. (2017) [47] | Cross-sectional | mTBI study population:
| Pittsburgh Sleep Quality Index (PSQI) |
|
|
| Theadom and Parag et al. (2016) [50] | Longitudinal population study | mTBI study population:
| Rivermead Post Concussion Symptoms Questionnaire (RPQ) |
|
|
| Theadom and Starkey et al. (2016) [48] | Prospective cohort | mTBI group:
| Pittsburgh Sleep Quality Index (PSQI) |
|
|
| Pillar et al. (2003) [49] | Cross-sectional | mTBI group:
| Study designed questionnaire |
|
|
| Intervention | Pros | Cons |
|---|---|---|
| Sleep Hygiene Counseling |
|
|
| Cognitive Behavioral Therapy (CBT) |
|
|
| Benzodiazepines (i.e., flurazepam, lorazepam, estazolam) |
|
|
| Z-drugs (i.e., zaleplon, zolpidem, zopiclone) |
|
|
| Trazodone |
|
|
| Melatonin & Melatonin Agonists (i.e., ramelteon, tasimelteon) |
|
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Greenwald, B.D. Update on Insomnia after Mild Traumatic Brain Injury. Brain Sci. 2018, 8, 223. https://doi.org/10.3390/brainsci8120223
Zhou Y, Greenwald BD. Update on Insomnia after Mild Traumatic Brain Injury. Brain Sciences. 2018; 8(12):223. https://doi.org/10.3390/brainsci8120223
Chicago/Turabian StyleZhou, Yi, and Brian D. Greenwald. 2018. "Update on Insomnia after Mild Traumatic Brain Injury" Brain Sciences 8, no. 12: 223. https://doi.org/10.3390/brainsci8120223
APA StyleZhou, Y., & Greenwald, B. D. (2018). Update on Insomnia after Mild Traumatic Brain Injury. Brain Sciences, 8(12), 223. https://doi.org/10.3390/brainsci8120223





