Facial Sadness Recognition is Modulated by Estrogen Receptor Gene Polymorphisms in Healthy Females
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Genotyping
2.2.1. ESR1 Gene Polymorphisms
2.2.2. ESR2 Gene Polymorphisms
2.3. Facial Emotion Recognition Task
2.4. Hormone Quantifications
2.5. Questionnaires
2.5.1. Mood Questionnaire
2.5.2. Mini Mental Test
2.6. Statistical Analysis
3. Results
3.1. Features of Females
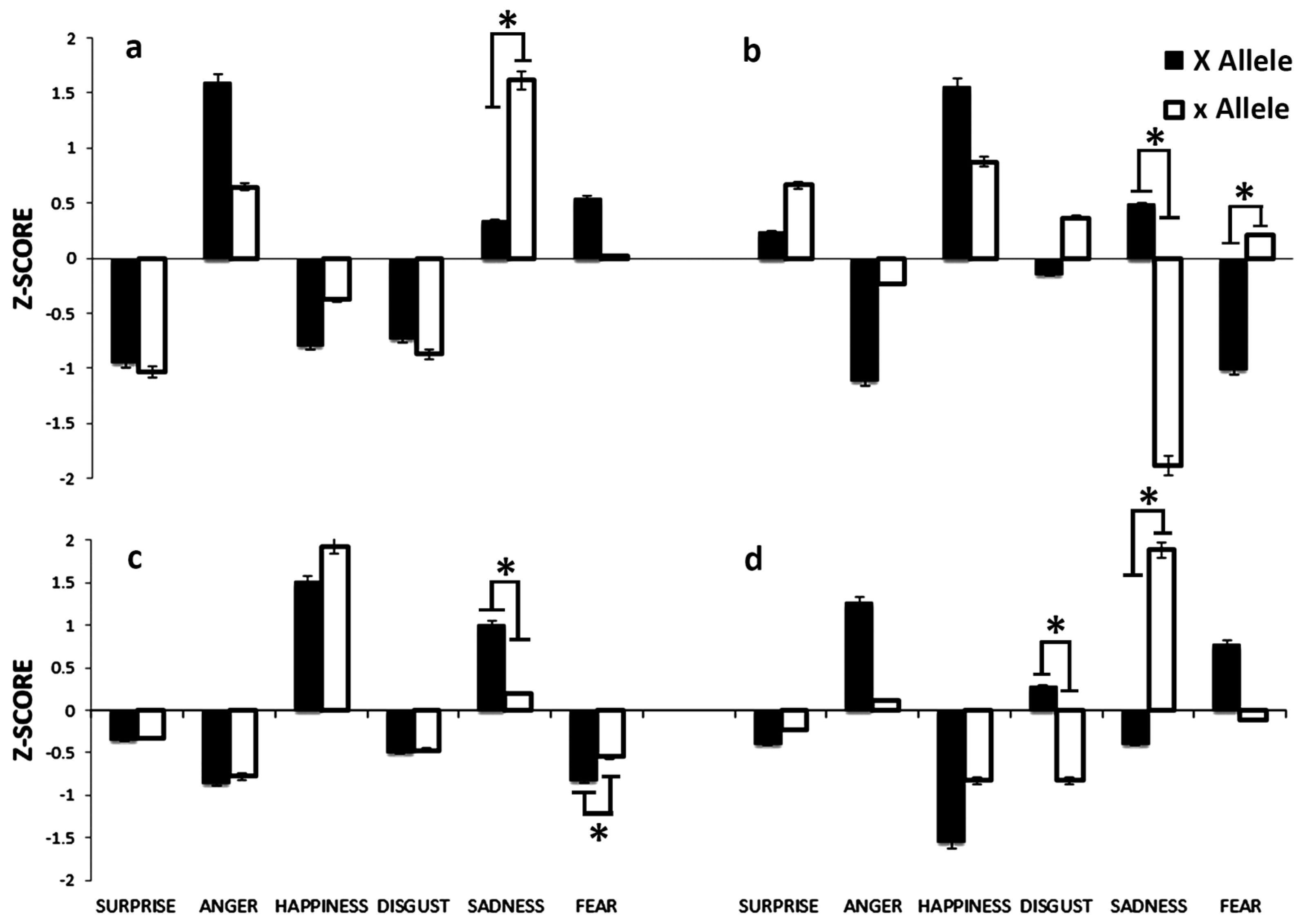
3.2. ESR1 Gene Polymorphisms
3.2.1. PvuII Polymorphism
3.2.2. Xbal Polymorphism
3.3. ESR2 rs1256030 Polymorphism
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karlsson, S.; Henningsson, S.; Hovey, D.; Zettergren, A.; Jonsson, L.; Cortes, D.S.; Melke, J.; Laukka, P.; Fischer, H.; Westberg, L. Social memory associated with estrogen receptor polymorphisms in women. Soc. Cogn. Affect. Neurosci. 2016, 11, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Ekman, P.; Friesen, W.V. Pictures of Facial Affect; Consulting Psychologist Press: Palo Alto, CA, USA, 1976. [Google Scholar]
- Phelps, E.A. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004, 14, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, M.; Singh-Manoux, A.; Batty, G.D.; Ebmeier, K.P.; Jokela, M.; Harmer, C.J.; Kivimäki, M. The level of cognitive function and recognition of emotions in older adults. PLoS ONE 2017, 12, e0185513. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Whalen, P.J. The amygdala: Vigilance and emotion. Mol. Psychiatry 2001, 6, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E.A.; LeDoux, J.E. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 2005, 48, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.J.; Lawrence, A.D.; Young, A.W. The neuropsychology of fear and loathing. Nat. Rev. Neurosci. 2001, 2, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.S.; Frith, C.D.; Perrett, D.I.; Rowland, D.; Young, A.W.; Calder, A.J.; Dolan, R.J. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 1996, 383, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhao, J.; Zhang, M.; Cui, Q.; Fu, X. Neural responses to rapid facial expressions of fear and surprise. Front. Psychol. 2017, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.L.; Young, A.W.; Senior, C.; Brammer, M.; Andrew, C.; Calder, A.J.; Bullmore, E.T.; Perrett, D.I.; Rowland, D.; Williams, S.C.; et al. A specific neural substrate for perceiving facial expressions of disgust. Nature 1997, 389, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A. Insula and disgust. Rinsho Shinkeigaku 2010, 50, 1000–1002. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.E.; Voyer, D. Sex differences in the ability to recognise non-verbal displays of emotion: A meta-analysis. Cogn. Emot. 2014, 28, 1164–1195. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Matsumoto, D. Gender differences in judgments of multiple emotions from facial expressions. Emotion 2004, 4, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Sawada, R.; Sato, W.; Kochiyama, T.; Uono, S.; Kubota, Y.; Yoshimura, S.; Toichi, M. Sex differences in the rapid detection of emotional facial expressions. PLoS ONE 2014, 9, e94747. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, N.; Javdani, S.; Finy, M.S.; Verona, E. Gender differences in emotional risk for self- and other-directed violence among externalizing adults. J. Consult. Clin. Psychol. 2011, 79, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Iliceto, P.; Pompili, M.; Lester, D.; Gonda, X.; Niolu, C.; Girardi, N.; Rihmer, Z.; Candilera, G.; Girardi, P. Relationship between temperament, depression, anxiety, and hopelessness in adolescents: A structural equation model. Depress. Res. Treat. 2011, 2011, 160175. [Google Scholar] [CrossRef] [PubMed]
- Dadomo, H.; Grecucci, A.; Giardini, I.; Ugolini, E.; Carmelita, A.; Panzeri, M. Schema therapy for emotional dysregulation: Theoretical implication and clinical applications. Front. Psychol. 2016, 7, 1987. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.M.; Goldstein-Piekarski, A.N.; Williams, L.M. Sex differences modulating serotonergic polymorphisms implicated in the mechanistic pathways of risk for depression and related disorders. J. Neurosci. Res. 2017, 95, 737–762. [Google Scholar] [CrossRef] [PubMed]
- Slopién, R.; Slopién, A.; Rozycka, A.; Warenik-Szymankiewicz, A.; Lianeri, M.; Jagodzinski, P.P. The c.1460C>T polymorphism of MAO-A is associated with the risk of depression in postmenopausal women. Sci. World J. 2012, 2012, 194845. [Google Scholar] [CrossRef]
- Serafini, G.; Pompili, M.; Innamorati, M.; Gentile, G.; Borro, M.; Lamis, D.A.; Lala, N.; Negro, A.; Simmaco, M.; Girardi, P.; et al. Gene variants with suicidal risk in a sample of subjects with chronic migraine and affective temperamental dysregulation. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1389–1398. [Google Scholar]
- Little, A.C. The influence of steroid sex hormones on the cognitive and emotional processing of visual stimuli in humans. Front. Neuroendocrinol. 2013, 34, 315–328. [Google Scholar] [CrossRef]
- Osório, F.L.; de Paula Cassis, J.M.; Machado de Sousa, J.P.; Poli-Neto, O.; Martín-Santos, R. Sex hormones and processing of facial expressions of emotion: A Systematic literature review. Front. Psychol. 2018, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.; Lewis, M.B. Fear recognition across the menstrual cycle. Horm. Behav. 2005, 47, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Gasbarri, A.; Pompili, A.; d’Onofrio, A.; Cifariello, A.; Tavares, M.C.; Tomaz, C. Working memory for emotional facial expressions: Role of the estrogen in young women. Psychoneuroendocrinology 2008, 33, 964–972. [Google Scholar] [CrossRef]
- Guapo, V.G.; Graeff, F.G.; Zani, A.C.; Labate, C.M.; dos Reis, R.M.; Del-Ben, C.M. Effects of sex hormones levels and phases of the menstrual cycle in the processing of emotional faces. Psychoneuroendocrinology 2009, 34, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, S.K.; Krol, K.M.; Curran, H.V. A specific association between facial disgust recognition and estradiol levels in naturally cycling women. PLoS ONE 2016, 10, e0122311. [Google Scholar] [CrossRef] [PubMed]
- Dalal, K.; Agarwal, M. Postmenopausal syndrome. Indian J. Psychiatry 2015, 57, S222–S232. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Epperson, C.N.; Mathews, S.B. Menopausal symptoms and their management. Endocrinol. Metab. Clin. N. Am. 2015, 44, 497–515. [Google Scholar] [CrossRef]
- Halbreich, U. Role of estrogen in postmenopausal depression. Neurology 1997, 48, 16–19. [Google Scholar] [CrossRef]
- Freeman, E.W.; Sammel, M.D.; Liu, L.; Gracia, C.R.; Nelson, D.B.; Hollander, L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch. Gen. Psychiatry 2004, 61, 62–70. [Google Scholar] [CrossRef]
- Núñez-Pizarro, J.L.; González-Luna, A.; Mezones-Holguín, E.; Blümel, J.E.; Barón, G.; Bencosme, A.; Benítez, Z.; Bravo, L.M.; Calle, A.; Flores, D.; et al. Association between anxiety and severe quality-of-life impairment in postmenopausal women: Analysis of a multicenter Latin American cross-sectional study. Menopause 2017, 24, 645–652. [Google Scholar] [CrossRef]
- Luine, V.N. Estradiol and cognitive function: Past, present and future. Horm. Behav. 2014, 66, 602–618. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.L.; Tang, N.L.; Leung, G.T.; Fung, A.W.; Lam, L.C. Estrogen receptor α polymorphisms and the risk of cognitive decline: A 2-year follow-up study. Am. J. Geriatr. Psychiatry 2014, 22, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, E.E.; Maki, P.M.; Bishop, J.R. A review of estrogen receptor alpha gene (ESR1) polymorphisms, mood, and cognition. Menopause 2010, 17, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Osterlund, H.; Keller, E.; Hurd, Y.L. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann. N. Y. Acad. Sci. 2003, 1007, 54–63. [Google Scholar] [CrossRef]
- Brailoiu, E.; Dun, S.L.; Brailoiu, G.C.; Mizuo, K.; Sklar, L.A.; Oprea, T.I.; Prossnitz, E.R.; Dun, N.J. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 2007, 193, 311–321. [Google Scholar] [CrossRef]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Estrogen signaling multiple pathways to impact gene transcription. Curr. Genomics 2006, 7, 497–508. [Google Scholar] [CrossRef]
- Menasce, L.P.; White, G.R.; Harrison, C.J.; Boyle, J.M. Localization of the estrogen receptor locus (ESR) to chromosome 6q25.1 by FISH and a simple post-FISH banding technique. Genomics 1993, 17, 263–265. [Google Scholar] [CrossRef]
- Enmark, E.; Pelto-Huikko, M.; Grandien, K.; Lagercrantz, S.; Lagercrantz, J.; Fried, G.; Nordenskjöld, M.; Gustafsson, J.A. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J. Clin. Endocrinol. Metab. 1997, 82, 4258–4265. [Google Scholar] [PubMed]
- Shughrue, P.J.; Lane, M.V.; Merchenthaler, I. Comparative distribution of estrogen receptor α- and 𝛽-mRNA in the rat central nervous system. J. Comp. Neurol. 1997, 388, 507–525. [Google Scholar] [CrossRef]
- Österlund, M.K.; Gustafsson, J.A.; Keller, E. Estrogen Receptor (ERbeta) messenger ribonucleic Acid (mRNA) expression within the human forebrain: Distinct distribution pattern to ER mRNA. J. Clin. Endocrinol. Metab. 2000, 85, 3840–3846. [Google Scholar] [PubMed]
- Gallagher, M.; Holland, P.C. The amygdala complex: Multiple roles in associative learning and attention. Proc. Natl. Acad. Sci. USA 1994, 91, 11771–11776. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.A. Estrogen receptor beta-a new dimension in estrogen mechanism of action. J. Endocrinol. 1999, 163, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Davachi, L.; Dobbin, I.G. Declarative Memory. Curr. Dir. Psychol. Sci. 2008, 17, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Foradori, C.D.; Handa, R.J. Estrogen receptor beta in the brain: From form to function. Brain Res. Rev. 2008, 57, 309–320. [Google Scholar] [CrossRef]
- Cheng, D.; Liang, B.; Hao, Y.; Zhou, W. Estrogen receptor α gene polymorphisms and risk of Alzheimer’s disease: Evidence from a meta-analysis. Clin. Interv. Aging 2014, 9, 1031–1038. [Google Scholar] [CrossRef]
- Becherini, L.; Gennari, L.; Masi, L.; Mansani, R.; Massart, F.; Morelli, A.; Falchetti, A.; Gonnelli, S.; Fiorelli, G.; Tanini, A.; et al. Evidence of a linkage disequilibrium between polymorphisms in the human estrogen receptor alpha gene and their relationship to bone mass variation in postmenopausal Italian women. Hum. Mol. Gene 2000, 9, 2043–2050. [Google Scholar] [CrossRef]
- Del Senno, L.; Aguiari, G.L.; Piva, R. Dinucleotide repeat polymorphism in the human estrogen receptor (ESR) gene. Hum. Mol. Genet. 1992, 1, 354. [Google Scholar] [CrossRef]
- Isoe, K.; Ji, Y.; Urakami, K.; Adachi, Y.; Nakashima, K. Genetic association of estrogen receptor gene polymorphisms with Alzheimer’s disease. Alzheimer’s Res. 1997, 3, 195–197. [Google Scholar]
- Brandi, M.L.; Becherini, L.; Gennar, L.; Racchi, M.; Bianchetti, A.; Nacmias, B.; Sorbi, S.; Mecocci, P.; Senin, U.; Govoni, S. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer’s disease. Biochem. Biophys. Res. Commun. 1999, 265, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Urakami, K.; Wada-Isoe, K.; Adachi, Y.; Nakashima, K. Estrogen receptor gene polymorphisms in patients with Alzheimer’s disease, vascular dementia and alcohol-associated dementia. Dement. Geriatr. Cogn. Disord. 2000, 11, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Carrière, I.; Carcaillon, L.; Dartigues, J.F.; Auriacombe, S.; Rouaud, O.; Berr, C.; Ritchie, K.; Scarabin, P.Y.; Ancelin, M.L. Estrogen receptor polymorphisms and incident dementia: The prospective 3C study. Alzheimers Dement. 2014, 10, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.; Rasmussen, H.B.; Hansen, T.; Bagger, Y.Z.; Tankó, L.B.; Qin, G.; Christiansen, C.; Werge, T. Estrogen receptor alpha and risk for cognitive impairment in postmenopausal women. Psychiatr. Genet. 2006, 16, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, H.M.; Meyer, P.M.; Seeman, T.E.; Greendale, G.A.; Sowers, M.R. Cognitive functioning and sex steroid hormone gene polymorphisms in women at midlife. Am. J. Med. 2006, 119 (Suppl. S1), S94–S102. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K.; Lui, L.Y.; Grady, D.; Stone, K.; Morin, P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biol. Psychiatry 2002, 51, 677–682. [Google Scholar] [CrossRef]
- Yaffe, K.; Lindquist, K.; Sen, S.; Cauley, J.; Ferrell, R.; Penninx, B.; Harris, T.; Li, R.; Cummings, S.R. Estrogen receptor genotype and risk of cognitive impairment in elders: Findings from the Health ABC study. Neurobiol. Aging 2009, 30, 607–614. [Google Scholar] [CrossRef]
- Chaves, A.C.; Fraga, V.G.; Guimarães, H.C.; Teixeira, A.L.; Barbosa, M.T.; Carvalho, M.D.; Mota, A.P.; Silva, I.F.; Caramelli, P.; Gomes, K.B.; et al. Estrogen receptor-alpha gene XbaI A > G polymorphism influences short-term cognitive decline in healthy oldest-old individuals. Arquivos de Neuro-Psiquiatria 2017, 75, 172–175. [Google Scholar] [CrossRef]
- Ryan, J.; Carrière, I.; Amieva, H.; Rouaud, O.; Berr, C.; Ritchie, K.; Scarabin, P.Y.; Ancelin, M.L. Prospective analysis of the association between estrogen receptor gene variants and the risk of cognitive decline in elderly women. Eur. Neuropsychopharmacol. 2013, 23, 1763–1768. [Google Scholar] [CrossRef]
- Goumidi, L.; Dahlman-Wright, K.; Tapia-Paez, I.; Matsson, H.; Pasquier, F.; Amouyel, P.; Kere, J.; Lambert, J.C.; Meirhaeghe, A. Study of estrogen receptor-α and receptor-β gene polymorphisms on Alzheimer’s disease. J. Alzheimers Dis. 2011, 26, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Solis Ortiz, S.; Pérez Luque, E.; Morado Crespo, L.; Gutiérrez Muñoz, M. Executive functions and selective attention are favored in middle aged women carriers of Val 158 allele of the catechol-o-methyltransferase gene. Behav. Brain Funct. 2010, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Gender difference in recognition memory for neutral and emotional faces. Memory 2013, 21, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini Mental State: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A. Beck Depression Inventory; The Psychological Corporation: San Antonio, TX, USA, 1993. [Google Scholar]
- World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. Bull. World Health Organ. 2001, 74, 373–374.
- Liu, W.; Shao, F.M.; Yan, L.; Cao, H.X.; Qiu, D. Polymorphisms in the gene encoding estrogen receptor alpha are associated with osteoarthritis in Han Chinese women. Int. J. Clin. Exp. Med. 2014, 7, 5772–5777. [Google Scholar] [PubMed]
- Montagne, B.; Kessels, R.P.; De Haan, E.H.; Perrett, D.I. The Emotion Recognition Task: A paradigm to measure the perception of facial emotional expressions at different intensities. Percept. Mot. Skills 2007, 104, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Paule, M.G.; Bushnell, P.J.; Maurissen, J.P.; Wenger, G.R.; Buccafusco, J.J.; Chelonis, J.J.; Elliott, R. Symposium overview: The use of delayed matching-to-sample procedures in studies of short-term memory in animals and humans. Neurotoxicol. Teratol. 1998, 20, 493–502. [Google Scholar] [CrossRef]
- Arntzen, E.; Steingrimsdottier, H.S. On the use of variations in a delayed matching-to-sample procedure in a patient with neurocognitive disorder. In Mental Disorder; Swahn, M.H., Palmier, J.B., Braunstein, S.M., Eds.; iConcept Press: Spring City, UT, USA, 2014; pp. 123–138. ISBN 978-1-922227-96-6. [Google Scholar]
- Derntl, B.; Windischberger, C.; Robinson, S.; Lamplmayr, E.; Kryspin-Exner, I.; Gur, R.C.; Moser, E.; Habel, U. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology 2008, 33, 1031–1040. [Google Scholar] [CrossRef]
- Burger, H.G.; Dudley, E.C.; Hopper, J.L.; Groome, N.; Guthrie, J.R.; Green, A.; Dennerstein, L. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J. Clin. Endocrinol. Metab. 1999, 84, 4025–4030. [Google Scholar]
- Gracia, C.R.; Sammel, M.D.; Freeman, E.W.; Lin, H.; Langan, E.; Kapoor, S.; Nelson, D.B. Defining menopause status: Creation of a new definition to identify the early changes of the menopausal transition. Menopause 2005, 12, 128–135. [Google Scholar] [CrossRef]
- Jurado, S.; Villegas, M.E.; Méndez, L.; Rodríguez, F.; Loperena, V.; Varela, R. La estandarización del inventario de la depresión de Beck para los residentes de la ciudad de México. Salud Ment. 1998, 21, 36–38. [Google Scholar]
- Desu, M.M.; Raghavarao, D. Sample Size Methodology; Academic Press: Boston, MA, USA, 1990; pp. 85–90. [Google Scholar]
- Altman, D.G. Sample size. In Practical Statistical for Medical Research; Chapman and Hall: London, UK, 1991; pp. 455–460. [Google Scholar]
- Triola, M.F. Elementary Statistics, 10th ed.; Pearson Addison Wesley: Boston, MA, USA, 2006; pp. 110–118. [Google Scholar]
- Sullivan, G.M.; Feinn, R. Using effect size or why the p value is not enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.; Kruglyak, L. Genetic dissection of complex traits: Guidelines for interpreting and reporting linkage results. Nat. Genet. 1995, 11, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N.; Randolph, J.F. Reproductive hormones and the menopause transition. Obstet. Gynecol. Clin. N. Am. 2011, 38, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Borrow, A.P.; Handa, R.J. Estrogen receptors modulation of anxiety-like behavior. Vitam. Horm. 2017, 103, 27–52. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Scali, J.; Carrière, I.; Scarabin, P.Y.; Ritchie, K.; Ancelin, M.L. Estrogen receptor gene variants are associated with anxiety disorders in older women. Psychoneuroendocrinology 2011, 36, 1582–1586. [Google Scholar] [CrossRef]
- Walf, A.A.; Koonce, C.J.; Frye, C.A. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav. Neurosc. 2008, 122, 974–981. [Google Scholar] [CrossRef]
- Viggiano, A.; Cacciola, G.; Widmer, D.A.; Viggiano, D. Anxiety as a neurodevelopmental disorder in a neuronal subpopulation: Evidence from gene expression data. Psychiatry Res. 2015, 228, 729–740. [Google Scholar] [CrossRef]
- Viggiano, D. The hyperactive syndrome: Metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav. Brain Res. 2008, 194, 1–14. [Google Scholar] [CrossRef]
- Viggiano, D.; Sadile, A.G. Hypertrophic A10 dopamine neurons in a rat model of attention-deficit hyperactivity disorder (ADHD). Neuroreport 2000, 11, 3677–3680. [Google Scholar] [CrossRef] [PubMed]
- Derntl, B.; Kryspin-Exner, I.; Fernbach, E.; Mose, E.; Habel, U. Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm. Behav. 2008, 53, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Fjeldheim, F.N.; Frydenberg, H.; Flote, V.G.; McTiernan, A.; Furberg, A.S.; Ellison, P.T.; Barrett, E.S.; Wilsgaard, T.; Jasienska, G.; Ursin, G.; et al. Polymorphisms in the estrogen receptor alpha gene (ESR1), daily cycling estrogen and mammographic density phenotypes. BMC Cancer 2016, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. Working memory. Curr. Biol. 2010, 20, R136–R140. [Google Scholar] [CrossRef] [PubMed]
- Joffe, H.; Hall, J.E.; Gruber, S.; Sarmiento, I.A.; Cohen, L.S.; Yurgelun-Todd, D.; Martin, K.A. Estrogen therapy selectively enhances prefrontal cognitive processes: A randomized, double-blind, placebo controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause 2006, 13, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Keenan, P.A.; Ezzat, W.H.; Ginsburg, K.; Moore, G.J. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology 2001, 26, 577–590. [Google Scholar] [CrossRef]
- Wang, A.C.; Hara, Y.; Janssen, W.G.; Rapp, P.R.; Morrison, J.H. Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J. Neurosci. 2010, 30, 12770–12776. [Google Scholar] [CrossRef]
- McEwen, B.S.; Milner, T.A. Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 2017, 95, 24–39. [Google Scholar] [CrossRef]
- McEwen, B.C.; Akama, K.T.; Spencer-Segal, J.L.; Milner, T.A.; Waters, E.M. Estrogen effects on the brain: Actions beyond the hypothalamus via novel mechanisms. Behav. Neurosci. 2012, 126, 4–16. [Google Scholar] [CrossRef]
- Elsabagh, S.; Hartley, D.E.; File, S.E. Cognitive function in late versus early postmenopausal stage. Maturitas 2007, 56, 84–93. [Google Scholar] [CrossRef] [PubMed]
- McCarrey, A.C.; Resnick, S.M. Postmenopausal hormone therapy and cognition. Horm. Behav. 2015, 74, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Rentz, D.M.; Weiss, B.K.; Jacobs, E.G.; Cherkerzian, S.; Klibanski, A.; Remington, A.; Aizley, H.; Goldstein, J.M. Sex differences in episodic memory in early midlife: Impact of reproductive aging. Menopause 2017, 24, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Bean, L.A.; Ianov, L.; Foster, T.C. Estrogen receptors, the hippocampus, and memory. Neuroscientist 2014, 20, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.V.; Hasten-Reiter Júnior, H.N.; Ribeiro, E.A.; Jucá, M.J.; Barbosa, F.T.; Sousa-Rodrigues, C.F. Association between visual impairment and depression in the elderly: A systematic review. Arq. Bras. Oftalmol. 2015, 78, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Codey, K. Influence of visual acuity on anxiety, panic and depression disorders among young and middle age adults in the United States. J. Affect. Disord. 2014, 167, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Renaud, J.; Bédard, E. Depression in the elderly with visual impairment and its association with quality of life. Clin. Interv. Aging 2013, 8, 931–943. [Google Scholar] [CrossRef]
- Ryan, J.; Scali, J.; Carrière, I.; Peres, K.; Rouaud, O.; Scarabin, P.Y.; Ritchie, K.; Ancelin, M.L. Estrogen receptor alpha gene variants and major depressive episodes. J. Affect. Disord. 2012, 136, 1222–1226. [Google Scholar] [CrossRef]
- Lymer, J.M.; Sheppard, P.A.S.; Kuun, T.; Blackman, A.; Jani, N.; Mahbub, S.; Choleris, E. Estrogens and their receptors in the medial amygdala rapidly facilitate social recognition in female mice. Psychoneuroendocrinology 2018, 89, 30–38. [Google Scholar] [CrossRef]
- Gasbarri, A.; Tavares, M.C.; Rodriguez, R.C.; Tomaz, C.; Pompili, A. Estrogen, cognitive functions and emotion: An overview on humans, non-human primates and rodents in reproductive years. Rev. Neurosci. 2012, 23, 587–606. [Google Scholar] [CrossRef]
- Greenspan, H.; Levitt, W.G.; Berman, J.; Hussain, A.; Waseem, S.; Nandu, B.; Nehru, V.; Jaquish, C. Worsening depression in a patient with a granulosa cell tumor. Psychiatr. Ann. 2013, 43, 377–379. [Google Scholar] [CrossRef]
- Tsai, S.J.; Wang, Y.C.; Hong, C.J.; Chiu, H.J. Association study of oestrogen receptor alpha gene polymorphism and suicidal behaviours in major depressive disorder. Psychiatr. Genet. 2003, 13, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Keyes, K.; Agnew-Blais, J.; Roberts, A.L.; Hamilton, A.; De Vivo, I.; Ranu, H.; Koenen, K. The role of allelic variation in estrogen receptor genes and major depression in the Nurses Health Study. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Różycka, A.; Słopień, R.; Słopień, A.; Dorszewska, J.; Seremak-Mrozikiewicz, A.; Lianeri, M.; Maciukiewicz, M.; Warenik-Szymankiewicz, A.; Grzelak, T.; Kurzawińska, G.; et al. The MAOA, COMT, MTHFR and ESR1 gene polymorphisms are associated with the risk of depression in menopausal women. Maturitas 2016, 84, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Wedrén, S.; Lovmar, L.; Humphreys, K.; Magnusson, C.; Melhus, H.; Syvänen, A.C.; Kindmark, A.; Landegren, U.; Fermér, M.L.; Stiger, F.; et al. Oestrogen receptor alpha gene haplotype and postmenopausal breast cancer risk: A case control study. Breast Cancer Res. 2004, 6, R437–R449. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Merlotti, D.; De Paola, V.; Calabrò, A.; Becherini, L.; Martini, G.; Nuti, R. Estrogen receptor gene polymorphisms and the genetics of osteoporosis: A HuGE review. Am. J. Epidemiol. 2005, 161, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Nelson, D.B. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch. Gen. Psychiatry 2006, 63, 375–382. [Google Scholar] [CrossRef]
- Deecher, D.; Andree, T.H.; Sloan, D.; Schechter, L.E. From menarche to menopause: Exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology 2008, 33, 3–17. [Google Scholar] [CrossRef]
- Solís-Ortiz, S.; Pérez-Luque, E.; Pacheco-Zavala, P. Resting EEG activity and ovarian hormones as predictors of depressive symptoms in postmenopausal women without a diagnosis of major depression. Psychology 2012, 3, 834–840. [Google Scholar] [CrossRef]
- Osterlund, M.K.; Witt, M.R.; Gustafsson, J.A. Estrogen action in mood and neurodegenerative disorders: Estrogenic compounds with selective properties-the next generation of therapeutics. Endocrine 2005, 28, 235–242. [Google Scholar] [CrossRef]
- Amin, Z.; Canli, T.; Epperson, C.N. Effect of estrogen-serotonin interactions on mood and cognition. Behav. Cogn. Neurosci. Rev. 2005, 4, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Borrow, A.P.; Cameron, N.M. Estrogenic mediation of serotonergic and neurotrophic systems: Implications for female mood disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 3, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kunimura, Y.; Iwata, K.; Iijima, N.; Kobayashi, M.; Ozawa, H. Effect of sex steroid hormones on the number of serotonergic neurons in rat dorsal raphe nucleus. Neurosci. Lett. 2015, 594, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Merens, W.; Willem Van der Does, A.J.; Spinhoven, P. The effects of serotonin in manipulations on emotional information processing and mood. J. Affect. Disord. 2007, 103, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, J.M. Emotional information processing in mood disorders: A review of behavioral and neuroimaging findings. Curr. Opin. Psychiatry 2006, 19, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.; Douglas, K.; Porter, R. Processing of facial emotion expression in major depression: A review. Aust. N. Z. J. Psychiatry 2010, 44, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.M.; Shippen, C.; Juhasz, G.; Chase, D.; Thomas, E.; Downey, D.; Toth, Z.G.; Lloyd-Williams, K.; Elliott, R.; Deakin, J.F. State-dependent alteration in face emotion recognition in depression. Br. J. Psychiatry 2011, 198, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Biyik, U.; Keskin, D.; Oguz, K.; Akdeniz, F.; Gonul, A.S. Facial emotion recognition in remitted depressed women. Asian J. Psychiatr. 2015, 17, 111–113. [Google Scholar] [CrossRef]
- Shafir, T.; Love, T.; Berent-Spillson, A.; Persad, C.C.; Wang, H.; Reame, N.K.; Frey, K.A.; Zubieta, J.K.; Smith, Y.R. Postmenopausal hormone use impact on emotion processing circuitry. Behav. Brain Res. 2012, 226, 147–153. [Google Scholar] [CrossRef]
- Love, T.; Smith, Y.R.; Persad, C.C.; Tkaczyk, A.; Zubieta, J.K. Short-term hormone treatment modulates emotion response circuitry in postmenopausal women. Fertil. Steril. 2010, 93, 1929–1937. [Google Scholar] [CrossRef]
- Bryant, H.U. Mechanism of action and preclinical profile of raloxifene, a selective estrogen receptor modulation. Rev. Endocr. Metab. Disord. 2001, 2, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kindler, J.; Weickert, C.S.; Schofield, P.R.; Lenroot, R.; Weickert, T.W. Raloxifene increases prefrontal activity during emotional inhibition in schizophrenia based on estrogen receptor genotype. Eur. Neuropsychopharmacol. 2016, 26, 1930–1940. [Google Scholar] [CrossRef] [PubMed]

| (n = 69) Mean ± SD | |
|---|---|
| Age (years) | 54.47 ± 4.70 |
| Years of education | 10.21 ± 3.44 |
| Menarche (years) | 12.86 ± 1.44 |
| Menopause (years) | 47.03 ± 4.85 |
| Weight (Kg) | 67.37 ± 13.20 |
| Height (cm) | 1.56 ± 0.06 |
| BMI (Kg/m2) | 27.83 ± 5.10 |
| TAS (mmHg) | 105.90 ± 13.90 |
| TAD (mmHg) | 77.34 ± 10.47 |
| FSH (mlU/mL) | 64.17 ± 24.90 |
| LH (mlU/mL) | 31.42 ± 14.97 |
| Estradiol (ng/mL) | 12.45 ± 2.04 |
| Progesterone (ng/mL) | 0.55 ± 0.11 |
| C Allele Mean ± SD | T Allele Mean ± SD | U Value | Cohen´s d | p | |
|---|---|---|---|---|---|
| Response Time | |||||
| Surprise | −1.15 ± 0.08 | −1.61 ± 0.09 | 161.5 | 0.067 | 0.03 * |
| Anger | 1.37 ± 0.05 | 0.24 ± 0.01 | 155.0 | 0.093 | 0.02 * |
| Happiness | −0.47 ± 0.03 | −0.49 ± 0.02 | 186.5 | 0.046 | 0.11 |
| Disgust | −0.86 ± 0.04 | −0.10 ± 0.01 | 248.0 | 0.005 | 0.69 |
| Sadness | 0.85 ± 0.04 | 1.24 ± 0.05 | 255.0 | 0.005 | 0.79 |
| Fear | 0.25 ± 0.02 | 0.73 ± 0.03 | 244.0 | 0.005 | 0.63 |
| Accuracy | |||||
| Surprise | 0.77 ± 0.03 | 1.01 ± 0.03 | 262.5 | 0.002 | 0.99 |
| Anger | −1.39 ± 0.05 | −0.30 ± 0.01 | 209.5 | 0.023 | 0.23 |
| Happiness | 1.32 ± 0.05 | 1.40 ± 0.03 | 251.5 | 0.001 | 0.72 |
| Disgust | 0.34 ± 0.02 | −0.96 ± 0.03 | 261.0 | 0.005 | 0.88 |
| Sadness | −0.41 ± 0.02 | −0.17 ± 0.01 | 257.0 | 0.004 | 0.79 |
| Fear | −0.63 ± 0.02 | −0.96 ± 0.03 | 261.5 | 0.001 | 0.85 |
| Correct Responses | |||||
| Surprise | −0.27 ± 0.04 | −0.29 ± 0.01 | 262.5 | 0.002 | 0.99 |
| Anger | −0.99 ± 0.10 | −0.62 ± 0.05 | 209.5 | 0.023 | 0.23 |
| Happiness | 1.57 ± 0.15 | 1.49 ± 0.15 | 251.5 | 0.001 | 0.72 |
| Disgust | −0.41 ± 0.05 | −0.79 ± 0.05 | 261.0 | 0.005 | 0.88 |
| Sadness | 0.85 ± 0.08 | 1.00 ± 0.07 | 257.0 | 0.004 | 0.79 |
| Fear | −0.74 ± 0.08 | −0.79 ± 0.05 | 261.5 | 0.001 | 0.85 |
| Errors | |||||
| Surprise | −1.62 ± 0.10 | −1.11 ± 0.05 | 266.0 | 0.001 | 0.93 |
| Anger | 1.03 ± 0.05 | 1.11 ± 0.04 | 258.5 | 0.001 | 1.00 |
| Happiness | −0.29 ± 0.02 | 1.11 ± 0.03 | 257.5 | 0.005 | 0.86 |
| Disgust | 0.14 ± 0.01 | −1.11 ± 0.03 | 239.0 | 0.013 | 0.45 |
| Sadness | −0.29 ± 0.02 | 0.18 ± 0.01 | 253.5 | 0.001 | 0.68 |
| Fear | 1.03 ± 0.04 | 0.18 ± 0.01 | 261.5 | 0.002 | 0.89 |
| Omissions | |||||
| Surprise | −0.63 ± 0.05 | −0.45 ± 0.02 | 266.5 | 0.001 | 1.00 |
| Anger | 1.07 ± 0.10 | −0.45 ± 0.02 | 209.0 | 0.034 | 0.20 |
| Happiness | −1.24 ± 0.10 | −1.54 ± 0.13 | 236.5 | 0.025 | 0.34 |
| Disgust | −0.63 ± 0.05 | 1.18 ± 0.07 | 220.5 | 0.027 | 0.19 |
| Sadness | 1.20 ± 0.10 | 0.63 ± 0.03 | 252.0 | 0.006 | 0.70 |
| Fear | 0.22 ± 0.02 | 0.63 ± 0.03 | 245.5 | 0.001 | 0.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Muñoz, M.; Fajardo-Araujo, M.E.; González-Pérez, E.G.; Aguirre-Arzola, V.E.; Solís-Ortiz, S. Facial Sadness Recognition is Modulated by Estrogen Receptor Gene Polymorphisms in Healthy Females. Brain Sci. 2018, 8, 219. https://doi.org/10.3390/brainsci8120219
Gutiérrez-Muñoz M, Fajardo-Araujo ME, González-Pérez EG, Aguirre-Arzola VE, Solís-Ortiz S. Facial Sadness Recognition is Modulated by Estrogen Receptor Gene Polymorphisms in Healthy Females. Brain Sciences. 2018; 8(12):219. https://doi.org/10.3390/brainsci8120219
Chicago/Turabian StyleGutiérrez-Muñoz, Mayra, Martha E. Fajardo-Araujo, Erika G. González-Pérez, Victor E. Aguirre-Arzola, and Silvia Solís-Ortiz. 2018. "Facial Sadness Recognition is Modulated by Estrogen Receptor Gene Polymorphisms in Healthy Females" Brain Sciences 8, no. 12: 219. https://doi.org/10.3390/brainsci8120219
APA StyleGutiérrez-Muñoz, M., Fajardo-Araujo, M. E., González-Pérez, E. G., Aguirre-Arzola, V. E., & Solís-Ortiz, S. (2018). Facial Sadness Recognition is Modulated by Estrogen Receptor Gene Polymorphisms in Healthy Females. Brain Sciences, 8(12), 219. https://doi.org/10.3390/brainsci8120219






