Peripheral Anti-Angiogenic Imbalance during Pregnancy Impairs Myogenic Tone and Increases Cerebral Edema in a Rodent Model of HELLP Syndrome
Abstract
1. Introduction
2. Methods
2.1. HELLP Rat Model
2.2. Measurement of Mean Arterial Pressure Conscious Rats and Determination of Hemolysis, Liver Enzymes, and Platelet Counts
2.3. Myogenic Response of Isolated Middle Cerebral Arteries (MCA)
2.4. Blood-Brain Barrier Permeability Studies
2.5. Evaluation of Occludin Protein Expression via Western Blot
2.6. Determination of Brain Water Content
2.7. Circulating and Brain Levels of sFlt-1 and sEng
2.8. Statistical Analysis
3. Results
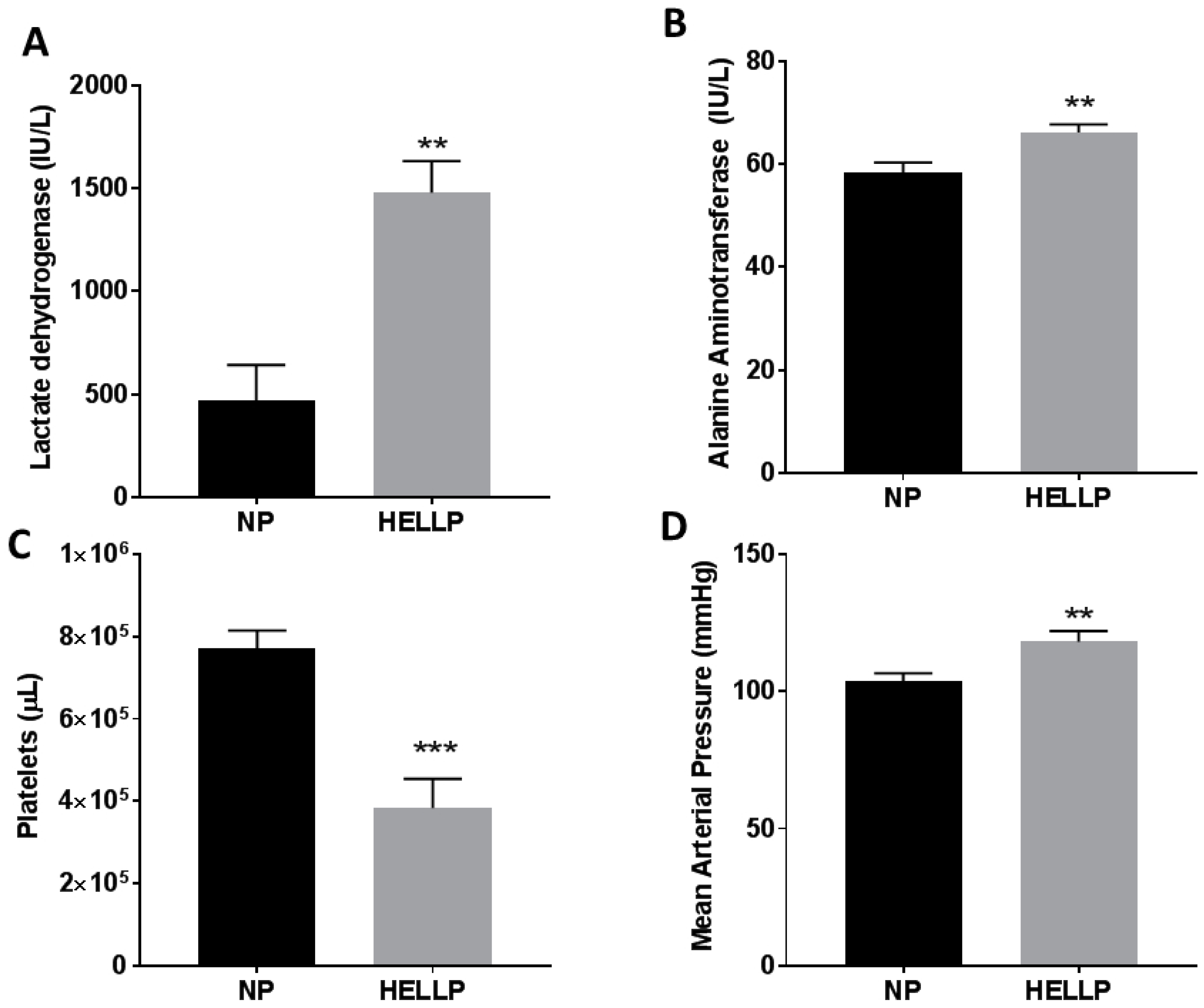
3.1. Mean Arterial Pressure and Symptomology of HELLP Syndrome Occur in Response to Infusion of sFlt-1 and sEng during Pregnancy
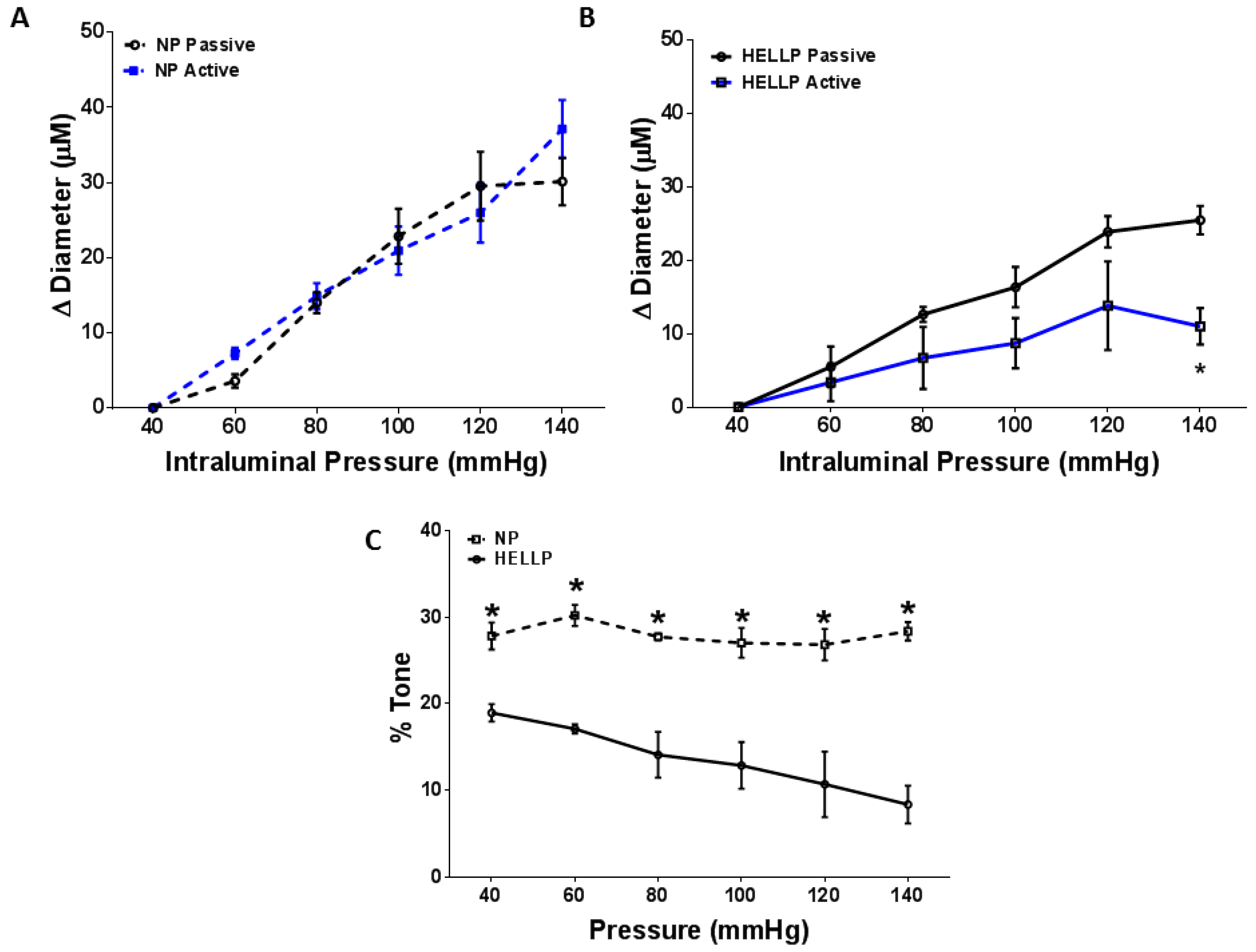
3.2. Myogenic Tone and BBB Are Impaired in Response to HELLP Syndrome
3.3. HELLP Syndrome Increases Brain Water Content in the Posterior Cortex
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abildgaard, U.; Heimdal, K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 166, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Haram, K.; Svendsen, E.; Abildgaard, U. The HELLP syndrome: Clinical issues and management. A Review. BMC Pregnancy Childbirth 2009, 9, 8. [Google Scholar] [CrossRef]
- Isler, C.M.; Rinehart, B.K.; Terrone, D.A.; Martin, R.W.; Magann, E.F.; Martin, J.N., Jr. Maternal mortality associated with HELLP syndrome. Am. J. Obstet. Gynecol. 1999, 181, 924–928. [Google Scholar] [CrossRef]
- Vigil-de Gracia, P. Maternal deaths due to eclampsia and HELLP syndrome. Int. J. Gynaecol. Obstet. 2009, 104, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Harscher, S.; Witte, O.W.; Möller, U.; Bloos, G.; Pfleiderer, S.O.; Terborg, C. Cerebral vasospasms with hemodynamic infarctions as a complication of HELLP syndrome. Nervenarzt 2003, 74, 1122–1126. [Google Scholar] [CrossRef]
- Schwartz, R.B.; Feske, S.K.; Polak, J.F.; DeGirolami, U.; Iaia, A.; Beckner, K.M.; Bravo, S.M.; Klufas, R.A.; Chai, R.Y.; Repke, J.T. Preeclampsia-eclampsia: Clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology 2000, 217, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.S.; Juneja, S.K.; Paul, G.; Gupta, S. Spectrum of neurological complications in HELLP syndrome. Neurol India 2013, 61, 467–471. [Google Scholar] [CrossRef]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.H.; Yuan, H.T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef]
- Aukes, A.M.; De Groot, J.C.; Wiegman, M.J.; Aarnoudse, J.G.; Sanwikarja, G.S.; Zeeman, G.G. Long-term cerebral imaging after pre-eclampsia. Br. J. Obstet. Gynaecol. 2012, 119, 1117–1122. [Google Scholar] [CrossRef]
- Wallace, K.; Tremble, S.M.; Owens, M.Y.; Morris, R.; Cipolla, M.J. Plasma from patients with HELLP Syndrome Increases Blood-brain barrier permeability. Reprod. Sci. 2015, 22, 278–284. [Google Scholar] [CrossRef]
- Wallace, K.; Cipolla, M. The Central Nervous System in HELLP Syndrome: Evidence for Impairment. In The 2015 Compendium for HELLP Syndrome; Martin, J.N., Ed.; Nova Biomedical: Hauppauge, NY, USA, 2015; Volume 1. [Google Scholar]
- Bean, C.; Spencer, S.K.; Bowles, T.; Kyle, P.B.; Williams, J.M.; Gibbens, J.; Wallace, K. Inhibition of T cell-activation attenuates hypertension, TNF-alpha, IL-17 and blood-brain barrier permeability in pregnant rats with angiogenic imbalance. Am. J. Reprod. Immunol. 2016, 76, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli, A.L.; Landi, B.; Corradetti, A.; Giannubilo, S.R.; Sartini, D.; Pozzi, V.; Emanuelli, M. Inflammatory cytokines patterns in the placenta of pregnancies complicated by HELLP (hemolysis, elevated liver enzyme, and low platelet) syndrome. Cytokine 2007, 40, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.; Martin, J.N., Jr.; Tam, K.T.; Wallukat, G.; Dechend, R.; Lamarca, B.; Owens, M.Y. Seeking the Mechanisms of Action for Corticosteroids in HELLP Syndrome: SMASH Study. Am. J. Obstet. Gynecol. 2013, 208, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.; Morris, R.; Kyle, P.B.; Cornelius, D.; Darby, M.; Scott, J.; Moseley, J.; Chatman, K.; Lamarca, B. Hypertension, inflammation and T lymphocytes are increased in a rat model of HELLP syndrome. Hypertens. Pregnancy 2014, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Spencer, S.K.; Barnes, A.; Bowles, T.; Kyle, P.B.; Wallace, K. Attenuation of oxidative stress and hypertension in an animal model of HELLP Syndrome. Eur. J. Pharmacol. 2018, 834, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Dhillion, P.; Wallace, K.; Herse, F.; Scott, J.; Wallukat, G.; Heath, J.; Moseley, J.; Martin, J.N., Jr.; Dechend, R.; LaMarca, B. IL-17 mediated oxidative stress is an important stimulator of AT1-AA and hypertension during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R353–R358. [Google Scholar] [CrossRef] [PubMed]
- Novotny, S.R.; Wallace, K.; Heath, J.; Moseley, J.; Dhillion, P.; Weimer, A.; Wallukat, G.; Herse, F.; Wenzel, K.; Martin, J.N., Jr.; et al. Activating autoantibodies to the angiotensin II type 1 receptor play an important role in mediating hypertension in response to adoptive transfer of CD4+ T lymphocytes from placental ischemic rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 301, R1197–R1201. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.; Cornelius, D.C.; Scott, J.; Heath, J.; Moseley, J.; Chatman, K.; LaMarca, B. CD4+ T cells are important mediators of oxidative stress that cause hypertension in response to placental ischemia. Hypertension 2014, 64, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.; Richards, S.; Dhillion, P.; Weimer, A.; Edholm, E.S.; Bengten, E.; Wilson, M.; Martin, J.N., Jr.; LaMarca, B. CD4+ T Helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 2011, 57, 949–955. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with Image J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Mackay, A.P.; Berg, C.J.; Liu, X.; Duran, C.; Hovert, D.L. Changes in pregnancy mortality ascertainment: United States, 1999–2005. Obstet. Gynecol. 2011, 118, 104–110. [Google Scholar] [CrossRef]
- Morris, R.; Spencer, S.K.; Kyle, P.B.; Williams, J.M.; Harris, A.; Owens, M.Y.; Wallace, K. Hypertension in an animal model of HELLP syndrome is associated with activation of endothelin-1. Reprod. Sci. 2016, 130, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Hammer, E.S.; Cipolla, M.J. Cerebrovascular Dysfunction in Preeclamptic Pregnancies. Curr. Hypertens. Rep. 2015, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Gilbert, E.L.; Glover, P.H.; George, E.M.; Masterson, C.W.; McLemore, G.R., Jr.; LaMarca, B.; Granger, J.P.; Drummond, H.A. Placental ischemia impairs middle cerebral artery myogenic responses in the pregnant rat. Hypertension 2011, 58, 1126–1131. [Google Scholar] [CrossRef]
- Amburgey, O.A.; Chapman, A.C.; May, V.; Bernstein, I.M.; Cipolla, M.J. Plasma from preeclamptic women increases blood-brain barrier permeability. Hypertension 2010, 56, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Van der Wijk, A.E.; Schreurs, M.P.; Cipolla, M.J. Pregnancy causes diminished myogenic tone and outward hypotrophic remodeling of the cerebral vein of Galen. J. Cereb. Blood Flow Metab. 2013, 33, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Cipolla, M.J. Impaired function of cerebral parenchymal arterioles in experimental preeclampsia. Microvasc. Res. 2018, 119, 64–72. [Google Scholar] [CrossRef]
- Warrington, J.P.; Fan, F.; Murphy, S.R.; Roman, R.J.; Drummond, H.A.; Granger, J.P.; Ryan, M.J. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiol. Rep. 2014, 2, e12134. [Google Scholar] [CrossRef]
- Porcello Marrone, L.C.; Gadonski, G.; de Oliveira Laguna, G.; Poli-de-Figueiredo, C.E.; Pinheiro da Costa, B.E.; Lopes, M.F.T.; Brunelli, J.P.F.; Diogo, L.P.; Marrone, A.C.; Da Costa, J.C. Blood-brain barrier breakdown in reduced uterine perfusion pressure: A possible model of posterior reversible encephalopathy syndrome. J. Stroke Cerebrovasc. Dis. 2014, 23, 2075–2079. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Bishop, N.; Chan, S.L. Effect of pregnancy on autoregulation of cerebral blood flow in anterior and posterior cerebrum. Hypertension 2012, 60, 705–711. [Google Scholar] [CrossRef]
- Euser, A.G.; Cipolla, M.J. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension 2007, 49, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Zunker, P.; Ley-Pozo, J.; Louwen, F.; Schuierer, G.; Holzgreve, W.; Ringelstein, E.B. Cerebral hemodynamics in pre-eclampsia/eclampsia syndrome. Ultrasound Obstet. Gynecol. 1995, 6, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Knopp, U.; Kehler, U.; Riskmann, H.; Arnold, H.; Gliemroth, J. Cerebral haemodynamic pathologies in HELLP syndrome. Clin. Neurol. Neurosurg. 2003, 105, 256–261. [Google Scholar] [CrossRef]
- Zunker, P.; Happe, S.; Georgiadis, A.L.; Louwen, F.; Georgiadis, D.; Ringelstein, E.B.; Holzgreve, W. Maternal cerebral hemodynamics in pregnancy-related hypertension. A prospective transcranial Doppler study. Ultrasound Obstet. Gynecol. 2000, 16, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.B. The blood-brain barrier and cerebral blood flow in acute hypertension. Acta Med. Scand. Suppl. 1983, 678, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, H.; Nara, S.; Kaneko, M.; Chiba, S.; Tamakawa, M. Magnetic resonance evaluation of brainstem dysfunction in eclampsia and the HELLP syndrome. J. Emerg. Med. 1995, 13, 191–194. [Google Scholar] [CrossRef]
- Johnson, A.C.; Tremble, S.M.; Chan, S.L.; Moseley, J.; Lamarca, B.; Nagle, K.J.; Cipolla, M.J. Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PLoS ONE 2014, 9, e113670. [Google Scholar] [CrossRef]
- Wallace, K.; Bean, C.; Bowles, T.; Spencer, S.K.; Randle, W.; Kyle, P.B.; Shaffery, J. Hypertension, Anxiety, and Blood-Brain Barrier Permeability Are Increased in Postpartum Severe Preeclampsia/Hemolysis, Elevated Liver Enzymes, and Low Platelet Count Syndrome Rats. Hypertension 2018, 72, 946–954. [Google Scholar] [CrossRef]
- Cipolla, M.J.; Sweet, J.G.; Chan, S.L. Cerebral vascular adaptation to pregnancy and its role in the neurological complications of eclampsia. J. Appl. Physiol. 2011, 110, 329–339. [Google Scholar] [CrossRef]
- Walshe, T.E.; Saint-Geniez, M.; Maharaj, A.S.; Sekiyama, E.; Maldonado, A.E.; D’Amore, P.A. TGF-B is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS ONE 2009, 4, e5149. [Google Scholar] [CrossRef]
- Betz, A.L.; Iannotti, F.; Hoff, J.T. Brain edema: A classification based on blood-brain barrier integrity. Cerebrovasc. Brain Metab. Rev. 1989, 1, 133–154. [Google Scholar] [PubMed]
- Easton, J.D. Severe preeclampsia/eclampsia: Hypertensive encephalopathy of pregnancy? Cerebrovasc. Dis. 1998, 8, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Hammers, E.S.; Sakkaki, S.; Tremble, S.M.; Holmes, G.L.; Cipolla, M.J. Inhibition of blood-brain barrier efflux transporters promotes seizures in pregnant rats: Role of circulating factors. Brain Behav. Immun. 2017, 67, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Warrington, J.P.; Drummond, H.A.; Granger, J.P.; Ryan, M.J. Placental ischemia-induced increases in brain water content and cerebrovascular permeability: Role of TNF-α. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1425–R1431. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, A.S.; Walshe, T.E.; Saint-Geniez, M.; Venkatesha, S.; Maldonado, A.E.; Himes, N.C.; Matharu, K.S.; Karumanchi, S.A.; D’Amore, P.A. VEGF and TGF-Beta are required for the maintenance of the choroid plexus and ependyma. J. Exp. Med. 2008, 205, 491–501. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bean, C.; Spencer, S.-K.; Pabbidi, M.R.; Szczepanski, J.; Araji, S.; Dixon, S.; Wallace, K. Peripheral Anti-Angiogenic Imbalance during Pregnancy Impairs Myogenic Tone and Increases Cerebral Edema in a Rodent Model of HELLP Syndrome. Brain Sci. 2018, 8, 216. https://doi.org/10.3390/brainsci8120216
Bean C, Spencer S-K, Pabbidi MR, Szczepanski J, Araji S, Dixon S, Wallace K. Peripheral Anti-Angiogenic Imbalance during Pregnancy Impairs Myogenic Tone and Increases Cerebral Edema in a Rodent Model of HELLP Syndrome. Brain Sciences. 2018; 8(12):216. https://doi.org/10.3390/brainsci8120216
Chicago/Turabian StyleBean, Cynthia, Shauna-Kay Spencer, Mallikarjuna R. Pabbidi, Jamie Szczepanski, Sarah Araji, Sellena Dixon, and Kedra Wallace. 2018. "Peripheral Anti-Angiogenic Imbalance during Pregnancy Impairs Myogenic Tone and Increases Cerebral Edema in a Rodent Model of HELLP Syndrome" Brain Sciences 8, no. 12: 216. https://doi.org/10.3390/brainsci8120216
APA StyleBean, C., Spencer, S.-K., Pabbidi, M. R., Szczepanski, J., Araji, S., Dixon, S., & Wallace, K. (2018). Peripheral Anti-Angiogenic Imbalance during Pregnancy Impairs Myogenic Tone and Increases Cerebral Edema in a Rodent Model of HELLP Syndrome. Brain Sciences, 8(12), 216. https://doi.org/10.3390/brainsci8120216





