Pedunculopontine Gamma Band Activity and Development
Abstract
:1. Introduction
2. Gamma Band Activity
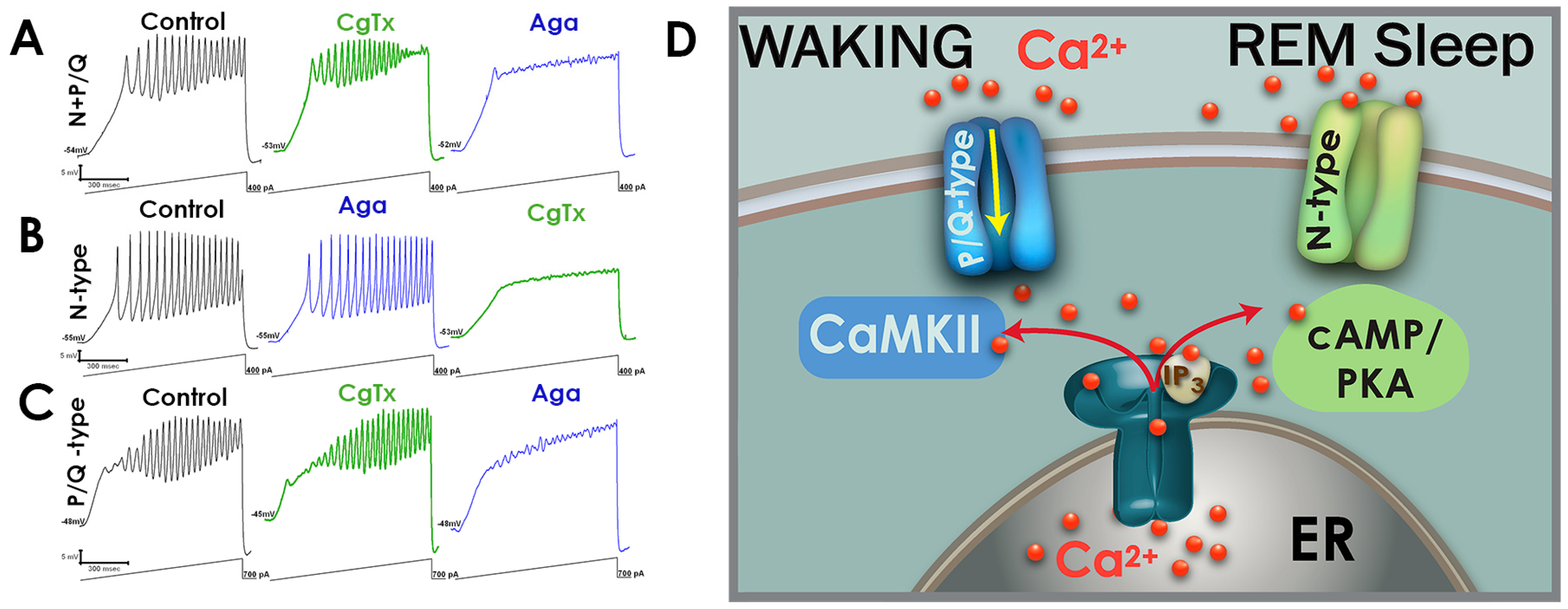
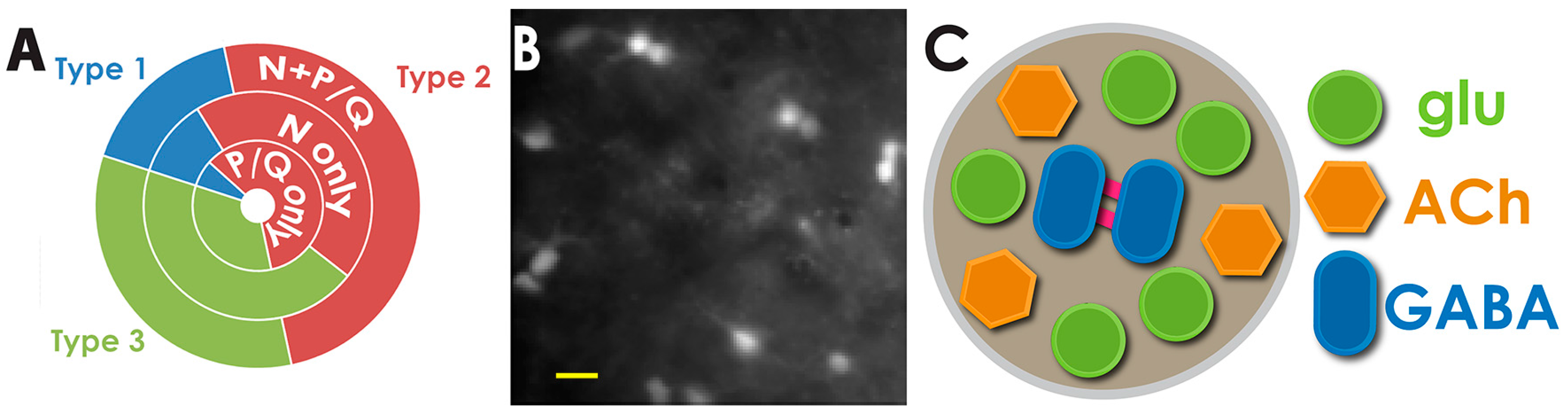
3. Electrical Coupling and Cell Ensembles
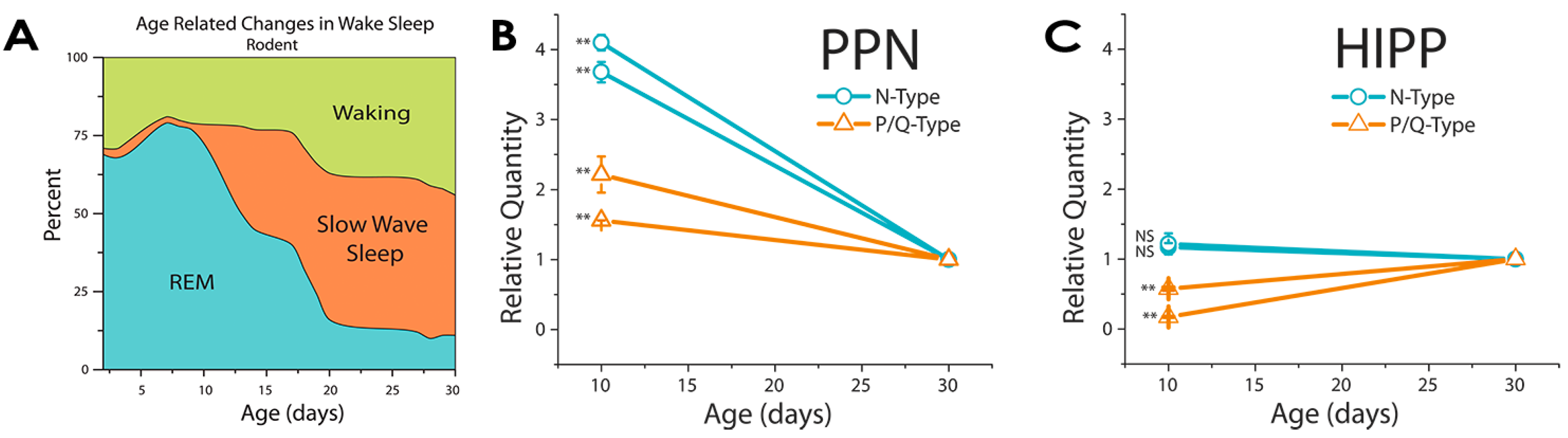
4. Development of REM Sleep
5. Basic Rest-Activity Cycle
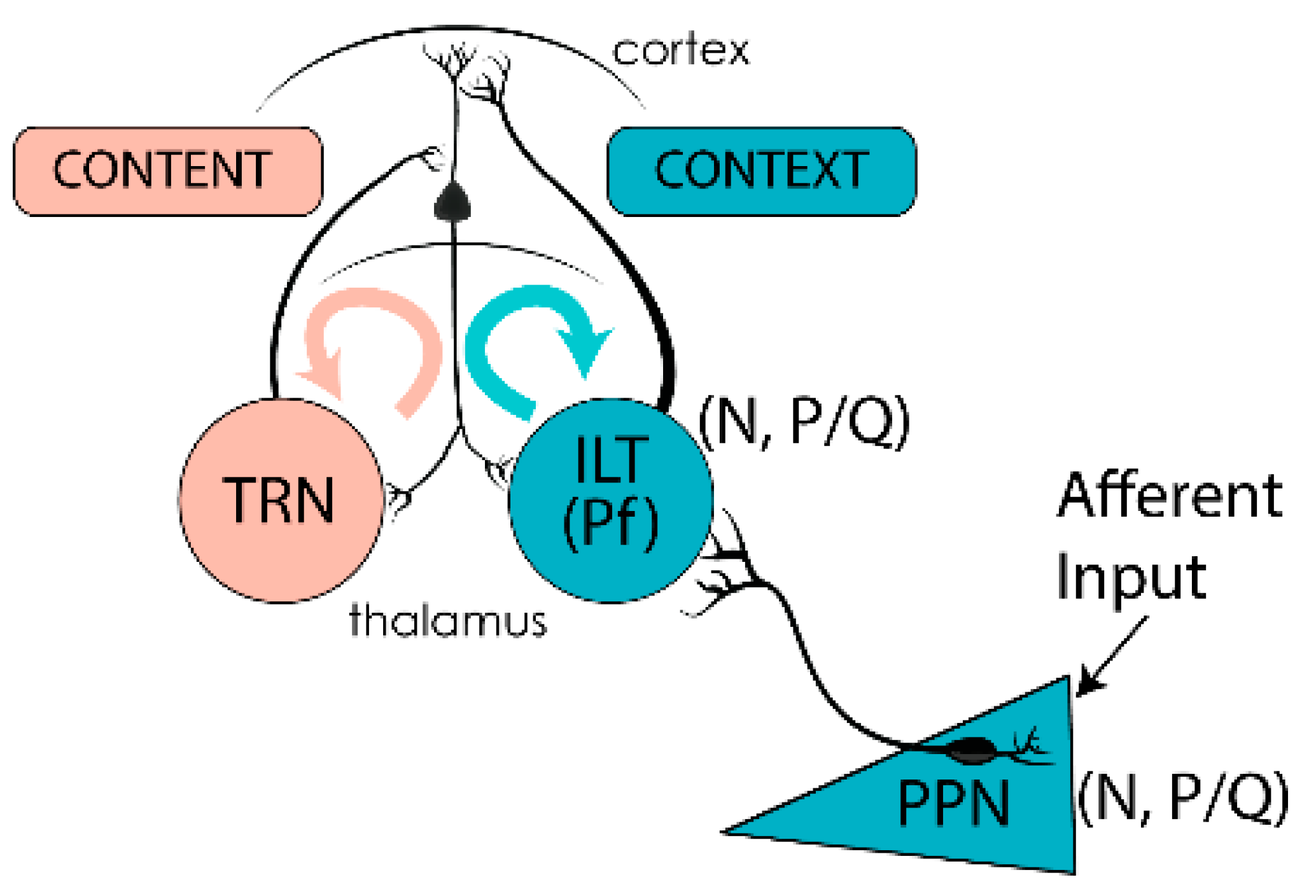
6. Preconscious Awareness
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Simon, C.; Kezunovic, N.; Ye, M.; Hyde, J.; Hayar, A.; Williams, D.K.; Garcia-Rill, E. Gamma band unit activity and population responses in the pedunculopontine nucleus. J. Neurophysiol. 2010, 104, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Kezunovic, N.; Urbano, F.J.; Simon, C.; Hyde, J.; Smith, K.; Garcia-Rill, E. Mechanism behind gamma band activity in the pedunculopontine nucleus. Eur. J. Neurosci. 2011, 34, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Bosman, C.A.; Lansink, C.S.; Pennartz, C.M. Functions of gamma-band synchronization in cognition: From single circuits to functional diversity across cortical and subcortical systems. Eur. J. Neurosci. 2014, 39, 1982–1999. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rill, E. Waking and the Reticular Activating System in Health and Disease; Garcia-Rill, E., Ed.; Springer: Boston, MA, USA, 2015. [Google Scholar]
- Garcia-Rill, E.; Kezunovic, N.; Hyde, J.; Simon, C.; Beck, P.; Urbano, F.J. Coherence and frequency in the reticular activating system (RAS). Sleep Med. Rev. 2013, 17, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Steriade, M.; Paré, D.; Datta, S.; Oakson, G.; Curro Dossi, R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J. Neurosci. 1990, 10, 2560–2579. [Google Scholar] [PubMed]
- Boucetta, S.; Cisse, Y.; Mainville, L.; Morales, M.; Jones, B.E. Discharge profiles across the sleep-waking cycle of identified cholinergic, gabaergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J. Neurosci. 2014, 34, 4708–4727. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Siwek, D.F. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J. Neurosci. Res. 2002, 70, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; el Mansari, M.; Jouvet, M. Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990, 527, 213–223. [Google Scholar] [CrossRef]
- Steriade, M.; Datta, S.; Paré, D.; Oakson, G.; Curro Dossi, R. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J. Neurosci. 1990, 10, 2541–2559. [Google Scholar] [PubMed]
- Hyde, J.; Kezunovic, N.; Urbano, F.J.; Garcia-Rill, E. Spatiotemporal properties of high-speed calcium oscillations in the pedunculopontine nucleus. J. Appl. Physiol. 2013, 115, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Kezunovic, N.; Hyde, J.; Goitia, B.; Bisagno, V.; Urbano, F.J.; Garcia-Rill, E. Muscarinic modulation of high frequency oscillations in pedunculopontine neurons. Front. Neurol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Kezunovic, N.; Hyde, J.; Simon, C.; Urbano, F.J.; Williams, D.K.; Garcia-Rill, E. Gamma band activity in the developing parafascicular nucleus. J. Neurophysiol. 2012, 107, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Kezunovic, N.; Williams, D.K.; Urbano, F.J.; Garcia-Rill, E. Cholinergic and glutamatergic agonists induce gamma frequency activity in dorsal subcoeruleus nucleus neurons. Am. J. Physiol. Cell Physiol. 2011, 301, C327–C335. [Google Scholar] [CrossRef] [PubMed]
- Hillman, D.; Chen, S.; Aung, T.T.; Cherksey, B.; Sugimori, M.; Llinas, R.R. Localization of P-type calcium channels in the central nervous system. Proc. Natl. Acad. Sci. USA 1991, 88, 7076–7080. [Google Scholar] [CrossRef] [PubMed]
- Uchitel, O.D.; Protti, D.A.; Sanchez, V.; Cherksey, B.D.; Sugimori, M.; Llinas, R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc. Natl. Acad. Sci. USA 1992, 89, 3330–3333. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Momiyama, A.; Uchitel, O.D.; Takahashi, T. Developmental changes in calcium channel types mediating central synaptic transmission. J. Neurosci. 2000, 20, 59–65. [Google Scholar] [PubMed]
- Santafe, N.M.; Urbano, F.J.; Lanuza, M.A.; Uchitel, O.D. Multiple types of calcium channels mediate transmitter release during functional recovery of botulinum toxin type A-poisoned mouse motor nerve terminals. Neurosci. 2000, 95, 227–234. [Google Scholar] [CrossRef]
- Iwasaki, S.; Takahashi, T. Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. J. Physiol. 1998, 509, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, D. Function and dysfunction of synaptic calcium channels: Insights from mouse models. Curr. Opin. Neurobiol. 2005, 15, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Jun, K.; Piedras-Renteria, E.S.; Smith, S.M.; Wheeler, D.B.; Lee, S.B.; Lee, T.G.; Chin, H.; Adams, M.E.; Scheller, R.H.; Tsien, R.W.; et al. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the α1A-subunit. Proc. Natl. Acad. Sci. USA 1999, 96, 15245–15250. [Google Scholar] [CrossRef] [PubMed]
- Llinas, R.R.; Choi, S.; Urbano, F.J.; Shin, H.S. Gamma-band deficiency and abnormal thalamocortical activity in P/Q-type channel mutant mice. Proc. Natl. Acad. Sci. USA 2007, 104, 17819–17824. [Google Scholar] [CrossRef] [PubMed]
- Luster, B.; D’Onofrio, S.; Urbano, F.; Garcia-Rill, E. High-threshold Ca2+ channels behind gamma band activity in the pedunculopontine nucleus (PPN). Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Siwek, D.F. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J. Neurophysiol. 1997, 77, 2975–2988. [Google Scholar] [PubMed]
- Datta, S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J. Neurophysiol. 2002, 87, 1790–1798. [Google Scholar] [PubMed]
- Datta, S.; Patterson, E.H.; Spoley, E.E. Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J. Neurosci. Res. 2001, 66, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; O’Malley, M.W.; Patterson, E.H. Calcium/calmodulin kinase II in the pedunculopontine tegmental nucleus modulates the initiation and maintenance of wakefulness. J. Neurosci. 2011, 31, 17007–17016. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rill, E.; Kezunovic, N.; D’Onofrio, S.; Luster, B.; Hyde, J.; Bisagno, V.; Urbano, F.J. Gamma band activity in the RAS-intracellular mechanisms. Exp. Brain Res. 2014, 232, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.; Mahaffey, S.; Urbano, F.J.; Garcia-Rill, E. Role of G-proteins in the effects of leptin on pedunculopontine nucleus neurons. J. Neurochem. 2013, 126, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Agler, H.L.; Evans, J.; Colecraft, H.M.; Yue, D.T. Custom distinctions in the interaction of G-protein beta subunits with N-type (CaV2.2) versus P/Q-type (CaV2.1) calcium channels. J. Gen. Physiol. 2003, 121, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Stea, A.; Soong, T.W.; Snutch, T.P. Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron 1995, 15, 929–940. [Google Scholar] [CrossRef]
- Jiang, X.; Lautermilch, N.J.; Watari, H.; Westenbroek, R.E.; Scheuer, T.; Catterall, W.A. Modulation of CaV2.1 channels by Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain. Proc. Natl. Acad. Sci. USA 2008, 105, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bucurenciu, I.; Bischofberger, J.; Jonas, P. A small number of open Ca2+ channels trigger transmitter release at a central GABAergic synapse. Nat. Neurosci. 2010, 13, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Glickfeld, L.L.; Scanziani, M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat. Neurosci. 2006, 9, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Hefft, S.; Jonas, P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat. Neurosci. 2005, 8, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Kerr, A.M.; Jonas, P. The two sides of hippocampal mossy fiber plasticity. Neuron 2008, 57, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.I.; Kunos, G.; Nicoll, R.A. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron 2001, 31, 453–462. [Google Scholar] [CrossRef]
- Isaacson, J.S.; Scanziani, M. How inhibition shapes cortical activity. Neuron 2011, 72, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.; Soltesz, I. Basket cell dichotomy in microcircuit function. J. Physiol. 2012, 590, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Colgin, L.L.; Denninger, T.; Fyhn, M.; Hafting, T.; Bonnevie, T.; Jensen, O.; Moser, M.B.; Moser, E.I. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 2009, 462, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Busey, T.A. Localization and identification tasks rely on different temporal frequencies. Vis. Res. 1999, 39, 513–532. [Google Scholar] [CrossRef]
- Colgin, L.L.; Moser, E.I. Gamma oscillations in the hippocampus. Physiology 2010, 25, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rill, E.; Charlesworth, A.; Heister, D.; Ye, M.; Hayar, A. The developmental decrease in REM sleep: The role of transmitters and electrical coupling. Sleep 2008, 31, 673–690. [Google Scholar] [CrossRef]
- Heister, D.S.; Hayar, A.; Charlesworth, A.; Yates, C.; Zhou, Y.H.; Garcia-Rill, E. Evidence for electrical coupling in the subcoeruleus (SubC) nucleus. J. Neurophysiol. 2007, 97, 3142–3147. [Google Scholar] [CrossRef] [PubMed]
- Urbano, F.J.; Leznik, E.; Llinas, R.R. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc. Natl. Acad. Sci. USA 2007, 104, 12554–12559. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Morales, M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur. J. Neurosci. 2009, 29, 340–358. [Google Scholar] [CrossRef] [PubMed]
- Feldt Muldoon, S.; Soltesz, I.; Cossart, R. Spatially clustered neuronal assemblies comprise the microstructure of synchrony in chronically epileptic networks. Proc. Natl. Acad. Sci. USA 2013, 110, 3567–3572. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G. Neural syntax: Cell assemblies, synapsembles, and readers. Neuron 2010, 68, 362–385. [Google Scholar] [CrossRef] [PubMed]
- Roffwarg, H.P.; Muzio, J.N.; Dement, W.C. Ontogenetic development of the human sleep-dream cycle. Science 1966, 152, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Marks, G.A.; Shaffery, J.P.; Oksenberg, A.; Speciale, S.G.; Roffwarg, H.P. A functional role for REM sleep in brain maturation. Behav. Brain Res. 1995, 69, 1–11. [Google Scholar] [CrossRef]
- Llinas, R.; Greenfield, S.A.; Jahnsen, H. Electrophysiology of pars compacta cells in the in vitro substantia nigra—A possible mechanism for dendritic release. Brain Res. 1984, 294, 127–132. [Google Scholar] [CrossRef]
- Jouvet-Mounier, D.; Astic, L.; Lacote, D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev. Psychobiol. 1970, 2, 216–239. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rill, E.; Ye, M.; Heister, D. Novel mechanism for sleep-wake control: Electrical coupling. SRS Bull. 2008, 14, 8–10. [Google Scholar] [PubMed]
- Garcia-Rill, E. Sleep and arousal states: Reticular activating system. In New Encyclopedia of Neuroscience; Squire, L.R., Bloom, F., Spitzer, N., Gage, F., Albright, T., Eds.; Elsevier: Oxford, UK, 2009; Volume 8, pp. 137–143. [Google Scholar]
- Garcia-Rill, E.; Mahaffey, S.; MacNicol, M. Correlation between the developmental decrease in REM sleep and N-type calcium channel expression. Neuroscience 2015, 39, 166. [Google Scholar]
- Kleitman, N. Sleep and Wakefulness; University of Chicago Press: Chicago, IL, USA, 1953. [Google Scholar]
- McPartland, R.J.; Kupfer, D.J. Rapid eye movement sleep cycle, clock time and sleep onset. Electroencephalogr. Clin. Neurophysiol. 1978, 45, 178–185. [Google Scholar] [CrossRef]
- Tsuji, Y.; Kobayashi, T. Short and long ultradian eeg components in daytime arousal. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 110–117. [Google Scholar] [CrossRef]
- Dantz, B.; Edgar, D.M.; Dement, W.C. Circadian rhythms in narcolepsy: Studies on a 90 minute day. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 24–35. [Google Scholar] [CrossRef]
- Sterman, M.B.; Hoppenbrouwers, T. The development of sleep-waking and rest-activity patters from fetus to adult in man. In Brain Development and Behavior; Sterman, M.B., McGinty, D.J., Adinolfi, A.M., Eds.; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Othmer, E.; Hayden, M.P.; Segelbaum, R. Encephalic cycles during sleep and wakefulness in humans: A 24-hour pattern. Science 1969, 164, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Kripke, D.F.; O’Donoghue, J.P. Perceptual deprivation, REM sleep, and an ultradian biological rhythm. Psychophysiology 1968, 5, 231–232. [Google Scholar]
- Globus, G.G.; Drury, R.L.; Phoebus, E.C.; Boyd, R. Ultradian rhythms in human performance. Percept. Mot. Skills 1971, 33, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Armitage, R. Rhythms in human performance: 1 1/2-hour oscillations in cognitive style. Science 1979, 204, 1326–1328. [Google Scholar] [CrossRef] [PubMed]
- Rechstaffen, A.; Wolpert, E.; Dement, W.; Mitchell, S.; Fisher, S. Nocturnal sleep in narcoleptics. Electroencephalogr. Clin. Neurophysiol. 1963, 15, 599–609. [Google Scholar]
- Friedman, S.; Fisher, C. On the presence of a rhythmic, diurnal, oral instinctual drive cycle in man: A preliminary report. J. Am. Psychoanal. Assoc. 1967, 15, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Oswald, I.; Merrington, J.; Lewis, H. Cyclical “on demand” oral intake by adults. Nature 1970, 225, 959–960. [Google Scholar] [CrossRef] [PubMed]
- Orr, W.C.; Hoffman, H.J. A 90-min cardiac biorhythm: Methodology and data analysis using modified periodograms and complex demodulation. IEEE Trans. Biomed. Eng. 1974, 21, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Lavie, P.; Kripke, D.F. Ultradian circa 11/2 hour rhythms: A multioscillatory system. Life Sci. 1981, 29, 2445–2450. [Google Scholar] [CrossRef]
- Monk, T.H.; Buysse, D.J.; Billy, B.D.; Kennedy, K.S.; Kupfer, D.J. The effects on human sleep and circadian rhythms of 17 days of continuous bedrest in the absence of daylight. Sleep 1997, 20, 858–864. [Google Scholar] [PubMed]
- Gronfier, C.; Simon, C.; Piquard, F.; Ehrhart, J.; Brandenberger, G. Neuroendocrine processes underlying ultradian sleep regulation in man. J. Clin. Endocrinol. Metab. 1999, 84, 2686–2690. [Google Scholar] [CrossRef] [PubMed]
- Kripke, D.F. Ultradian spectra in monkeys. Int. J. Chronobiol. 1976, 3, 193–204. [Google Scholar]
- Delorme, F.; Vimont, P.; Jouvet, D. Statistical study of the wakefulness-sleep cycle in the cat. C.R. Seances Soc. Biol. Fil. 1964, 158, 2128–2130. [Google Scholar] [PubMed]
- Sterman, M.B.; Knauss, T.; Lehmann, D.; Clemente, C.D. Circadian sleep and waking patterns in the laboratory cat. Electroencephalogr. Clin. Neurophysiol. 1965, 19, 509–517. [Google Scholar] [CrossRef]
- Ursin, R. The two stages of slow wave sleep in the cat and their relation to REM sleep. Brain Res. 1968, 11, 347–356. [Google Scholar] [CrossRef]
- Sterman, M.B.; Lucas, E.A.; MacDonald, L.R. Periodicity within sleep and operant performance in the cat. Brain Res. 1972, 38, 327–341. [Google Scholar] [CrossRef]
- Roldan, E.; Weiss, T. Neural mechanisms underlying sleep cycle in rodents. Bol. Inst. Estud. Med. Biol. Univ. Nac. Auton. Mex. 1963, 21, 467–483. [Google Scholar] [PubMed]
- Van Twyler, H. Sleep patterns of five rodent species. Physiol. Behav. 1969, 4, 901–905. [Google Scholar] [CrossRef]
- Timo-Iaria, C.; Negrao, N.; Schmidek, W.R.; Hoshino, K.; Lobato de Menezes, C.E.; Leme da Rocha, T. Phases and states of sleep in the rat. Physiol. Behav. 1970, 5, 1057–1062. [Google Scholar] [CrossRef]
- Garcia-Rill, E. Mechanisms of sleep and wakefulness. In Sleep Medicine; Lee-Chiong, T., Sateia, M.J., Carskadon, M.A., Eds.; Hanley & Belfus: Philadelphia, PA, USA, 2002; Volume 31–39. [Google Scholar]
- Vanhatalo, S.; Tallgren, P.; Becker, C.; Holmes, M.D.; Miller, J.W.; Kaila, K.; Voipio, J. Scalp-recorded slow EEG responses generated in response to hemodynamic changes in the human brain. Clin. Neurophysiol. 2003, 114, 1744–1754. [Google Scholar] [CrossRef]
- Vanhatalo, S.; Palva, J.M.; Holmes, M.D.; Miller, J.W.; Voipio, J.; Kaila, K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc. Natl. Acad. Sci. USA 2004, 101, 5053–5057. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, S.; Palva, J.M.; Andersson, S.; Rivera, C.; Voipio, J.; Kaila, K. Slow endogenous activity transients and developmental expression of K+-Cl− cotransporter 2 in the immature human cortex. Eur. J. Neurosci. 2005, 22, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.C.; Scheibel, A.B.; Murphy, G.M., Jr.; Harvey, T. On the brain of a scientist: Albert Einstein. Exp. Neurol. 1985, 88, 198–204. [Google Scholar] [CrossRef]
- Fellin, T.; D’Ascenzo, M.; Haydon, P.G. Astrocytes control neuronal excitability in the nucleus accumbens. Sci. World J. 2007, 7, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Parri, H.R.; Crunelli, V. Astrocytes, spontaneity, and the developing thalamus. J. Physiol. 2002, 96, 221–230. [Google Scholar]
- Cotrina, M.L.; Lin, J.H.; Alves-Rodrigues, A.; Liu, S.; Li, J.; Azmi-Ghadimi, H.; Kang, J.; Naus, C.C.; Nedergaard, M. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. USA 1998, 95, 15735–15740. [Google Scholar] [CrossRef] [PubMed]
- Pascual, O.; Casper, K.B.; Kubera, C.; Zhang, J.; Revilla-Sanchez, R.; Sul, J.Y.; Takano, H.; Moss, S.J.; McCarthy, K.; Haydon, P.G. Astrocytic purinergic signaling coordinates synaptic networks. Science 2005, 310, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Florian, C.; Fellin, T.; Munoz, J.R.; Lee, S.Y.; Abel, T.; Haydon, P.G.; Frank, M.G. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 2009, 61, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Dal Maschio, M.; Beltramo, R.; Haydon, P.G.; Benfenati, F.; Fellin, T. Integrated brain circuits: Neuron-astrocyte interaction in sleep-related rhythmogenesis. Sci. World J. 2010, 10, 1634–1645. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Manns, I.D.; Alonso, A.; Jones, B.E. Sleep-wake related discharge properties of basal forebrain neurons recorded with micropipettes in head-fixed rats. J. Neurophysiol. 2004, 92, 1182–1198. [Google Scholar] [CrossRef] [PubMed]
- Eckhorn, R.; Bauer, R.; Jordan, W.; Brosch, M.; Kruse, W.; Munk, M.; Reitboeck, H.J. Coherent oscillations: A mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol. Cybern. 1988, 60, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.; Takeda, Y. Greater frontal-parietal synchrony at low gamma-band frequencies for inefficient than efficient visual search in human EEG. Int. J. Psychophysiol. 2009, 73, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Palva, J.M.; Monto, S.; Kulashekhar, S.; Palva, S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc. Natl. Acad. Sci. USA 2010, 107, 7580–7585. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rill, E.; Skinner, R.D. The sleep state-dependent P50 midlatency auditory evoked potential. In Sleep Medicine; Lee-Chiong, T., Sateia, M.J., Carskadon, M.A., Eds.; Hanley & Belfus: Philadelphia, PA, USA, 2002. [Google Scholar]
- Garcia-Rill, E.; Miyazato, H.; Skinner, R.D.; Williams, K. Periodicity in the amplitudes of the sleep state-dependent, midlatency auditory evoked P1 potential in the human and P13 potential in the rat. Neuroscience 1999, 25, 627. [Google Scholar]
- Rasmussen, D.D. Physiological interactions of the basic rest—Activity cycle of the brain: Pulsatile luteinizing hormone secretion as a model. Psychoneuroendocrinology 1986, 11, 389–405. [Google Scholar] [CrossRef]
- Rasmussen, D.D.; Malven, P.V. Relationship between rhythmic motor activity and plasma luteinizing hormone in ovariectomized sheep. Neuroendocrinology 1981, 32, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Muse, K.N.; Cetel, N.S.; Futterman, L.A.; Yen, S.C. The premenstrual syndrome—Effects of medical ovariectomy. N. Engl. J. Med. 1984, 311, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Knobil, E.; Plant, T.M. The hypothalamic regulation of LH and FSH secretion in the rhesus monkey. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1978, 56, 359–372. [Google Scholar] [PubMed]
- Kripke, D.F.; Cook, B.; Lewis, O.F. Sleep of night workers: EEG recordings. Psychophysiology 1970, 7, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E. Endocrine rhythms. In Principles and Practice of Endocrinology and Metabolism; Becker, K.L., Ed.; Lippincott-Williams: Philadelphia, PA, USA, 2001; pp. 57–68. [Google Scholar]
- Del C. Arellanes-Licea, E.; Baez-Ruiz, A.; Carranza, M.E.; Aramburo, C.; Luna, M.; Diaz-Munoz, M. Daily patterns and adaptation of the ghrelin, growth hormone and insulin-like growth factor-1 system under daytime food synchronisation in rats. J. Neuroendocrinol. 2014, 26, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Kleitman, N. Basic rest-activity cycle—22 years later. Sleep 1982, 5, 311–317. [Google Scholar] [PubMed]
- De Castro, J.M.; Balagura, S. Ontogeny of meal patterning in rats and its recapitulation during recovery from lateral hypothalamic lesions. J. Comp. Physiol. Psychol. 1975, 89, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Strubbe, J.H. The psychobiology of meals. Psychon. Bull. Rev. 1994, 1, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Strubbe, J.H.; Woods, S.C. The timing of meals. Psychol. Rev. 2004, 111, 128–141. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Strubbe, J.H.; Wildering, W.C.; Gorter, J.A.; Prins, A.J. Patterns of body temperature during feeding in rats under varying ambient temperatures. Physiol. Behav. 1993, 53, 229–235. [Google Scholar] [CrossRef]
- Ootsuka, Y.; de Menezes, R.C.; Zaretsky, D.V.; Alimoradian, A.; Hunt, J.; Stefanidis, A.; Oldfield, B.J.; Blessing, W.W. Brown adipose tissue thermogenesis heats brain and body as part of the brain-coordinated ultradian basic rest-activity cycle. Neuroscience 2009, 164, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Blessing, W.; Mohammed, M.; Ootsuka, Y. Heating and eating: Brown adipose tissue thermogenesis precedes food ingestion as part of the ultradian basic rest-activity cycle in rats. Physiol. Behav. 2012, 105, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Blessing, W.; Mohammed, M.; Ootsuka, Y. Brown adipose tissue thermogenesis, the basic rest-activity cycle, meal initiation, and bodily homeostasis in rats. Physiol. Behav. 2013, 121, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Weigle, D.S.; Frayo, R.S.; Breen, P.A.; Ma, M.K.; Dellinger, E.P.; Purnell, J.Q. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 2002, 346, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Shiiya, T.; Nakazato, M.; Mizuta, M.; Date, Y.; Mondal, M.S.; Tanaka, M.; Nozoe, S.; Hosoda, H.; Kangawa, K.; Matsukura, S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J. Clin. Endocrinol. Metab. 2002, 87, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Tschop, M.; Wawarta, R.; Riepl, R.L.; Friedrich, S.; Bidlingmaier, M.; Landgraf, R.; Folwaczny, C. Post-prandial decrease of circulating human ghrelin levels. J. Endocrinol. Invest. 2001, 24, RC19–RC21. [Google Scholar] [CrossRef] [PubMed]
- Weigle, D.S.; Cummings, D.E.; Newby, P.D.; Breen, P.A.; Frayo, R.S.; Matthys, C.C.; Callahan, H.S.; Purnell, J.Q. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J. Clin. Endocrinol. Metab. 2003, 88, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Dornonville de la Cour, C.; Hakanson, R.; Lundquist, I. Effects of ghrelin on insulin and glucagon secretion: A study of isolated pancreatic islets and intact mice. Regul. Pept. 2004, 118, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, B.O.; Suchard, M.A.; Wong, M.L.; McCann, S.M.; Licinio, J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc. Natl. Acad. Sci. USA 2004, 101, 10434–10439. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Lotter, E.C.; McKay, L.D.; Porte, D., Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 1979, 282, 503–505. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Boyko, E.J.; Kahn, S.E.; Ravussin, E.; Bogardus, C. Reduced insulin secretion: An independent predictor of body weight gain. J. Clin. Endocrinol. Metab. 1995, 80, 1571–1576. [Google Scholar] [PubMed]
- McNay, E.C. Insulin and ghrelin: Peripheral hormones modulating memory and hippocampal function. Curr. Opin. Pharmacol. 2007, 7, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.; Urbano, F.J.; Williams, D.K.; Garcia-Rill, E. Effects of leptin on pedunculopontine nucleus (PPN) neurons. J. Neural Transm. (Vienna) 2013, 120, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Hayar, A.; Strotman, B.; Garcia-Rill, E. Cholinergic modulation of fast inhibitory and excitatory transmission to pedunculopontine thalamic projecting neurons. J. Neurophysiol. 2010, 103, 2417–2432. [Google Scholar] [CrossRef] [PubMed]
- Herlitze, S.; Garcia, D.E.; Mackie, K.; Hille, B.; Scheuer, T.; Catterall, W.A. Modulation of Ca2+ channels βγ G-protein py subunits. Nature 1996, 380, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.R. Voltage-dependent modulation of N-type calcium channels by G-protein β γsubunits. Nature 1996, 380, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994, 17, 531–536. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Bourinet, E.; Nelson, D.; Nargeot, J.; Snutch, T.P. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature 1997, 385, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Ellinor, P.T.; Aldrich, R.W.; Tsien, R.W. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G proteins. Neuron 1996, 17, 991–1003. [Google Scholar] [CrossRef]
- Beech, D.J.; Bernheim, L.; Hille, B. Pertussis toxin and voltage dependence distinguish multiple pathways modulating calcium channels of rat sympathetic neurons. Neuron 1992, 8, 97–106. [Google Scholar] [CrossRef]
- Elmslie, K.S. Calcium current modulation in frog sympathetic neurones: Multiple neurotransmitters and G proteins. J. Physiol. 1992, 451, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 1989, 340, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Carbone, E.; Lux, H.D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflug. Arch. 1986, 406, 104–111. [Google Scholar] [CrossRef]
- Bertram, R.; Behan, M. Implications of G-protein-mediated Ca2+ channel inhibition for neurotransmitter release and facilitation. J. Comput. Neurosci. 1999, 7, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Boland, L.M.; Bean, B.P. Modulation of N-type calcium channels in bullfrog sympathetic neurons by luteinizing hormone-releasing hormone: Kinetics and voltage dependence. J. Neurosci. 1993, 13, 516–533. [Google Scholar] [PubMed]
- Currie, K.P.; Fox, A.P. Comparison of N- and P/Q-type voltage-gated calcium channel current inhibition. J. Neurosci. 1997, 17, 4570–4579. [Google Scholar] [PubMed]
- Dunlap, K. Forskolin prolongs action potential duration and blocks potassium current in embryonic chick sensory neurons. Pflug. Archiv. 1985, 403, 170–174. [Google Scholar] [CrossRef]
- Strock, J.; Diverse-Pierluissi, M.A. Ca2+ channels as integrators of G protein-mediated signaling in neurons. Mol. Pharmacol. 2004, 66, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.D.; Wittemann, S.; Herlitze, S. G protein modulation of recombinant P/Q-type calcium channels by regulators of G protein signalling proteins. J. Physiol. 2000, 528, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Melliti, K.; Meza, U.; Fisher, R.; Adams, B. Regulators of G protein signaling attenuate the G protein-mediated inhibition of N-type Ca channels. J. Gen. Physiol. 1999, 113, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, S.; Hepler, J.R. Cellular regulation of RGS proteins: Modulators and integrators of G protein signaling. Pharmacol. Rev. 2002, 54, 527–559. [Google Scholar] [CrossRef] [PubMed]
- Diverse-Pierluissi, M.; Inglese, J.; Stoffel, R.H.; Lefkowitz, R.J.; Dunlap, K. G protein-coupled receptor kinase mediates desensitization of norepinephrine-induced Ca2+ channel inhibition. Neuron 1996, 16, 579–585. [Google Scholar] [CrossRef]
- Tedford, H.W.; Zamponi, G.W. Direct G protein modulation of Cav2 calcium channels. Pharmacol. Rev. 2006, 58, 837–862. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.S. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol. Rev. 2001, 53, 1–24. [Google Scholar] [PubMed]
- Ling, K.Y.; Maley, M.E.; Preston, R.R.; Saimi, Y.; Kung, C. New non-lethal calmodulin mutations in Paramecium. A structural and functional bipartition hypothesis. Eur. J. Biochem. 1994, 222, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci. 1994, 17, 406–412. [Google Scholar] [CrossRef]
- Schulman, H.; Heist, K.; Srinivasan, M. Decoding Ca2+ signals to the nucleus by multifunctional CaM kinase. Prog. Brain Res. 1995, 105, 95–104. [Google Scholar] [PubMed]
- Braun, A.P.; Schulman, H. The multifunctional calcium/calmodulin-dependent protein kinase: From form to function. Annu. Rev. Physiol. 1995, 57, 417–445. [Google Scholar] [CrossRef] [PubMed]
- Civin, M.; Lombardi, K.L. The preconscious and potential space. Psychoanal. Rev. 1990, 77, 573–585. [Google Scholar] [PubMed]
- Datta, S.; Li, G.; Auerbach, S. Activation of phasic pontine-wave generator in the rat: A mechanism for expression of plasticity-related genes and proteins in the dorsal hippocampus and amygdala. Eur. J. Neurosci. 2008, 27, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; O’Malley, M.W. Fear extinction memory consolidation requires potentiation of pontine-wave activity during REM sleep. J. Neurosci. 2013, 33, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Urbano, F.J.; D’Onofrio, S.M.; Luster, B.R.; Hyde, J.R.; Bosagno, V.; Garcia-Rill, E. Pedunculopontine nucleus gamma band activity-preconscious awareness, waking, and REM sleep. Front. Neurol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Llinas, R.R.; Leznik, E.; Urbano, F.J. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: A voltage-dependent dye-imaging study in mouse brain slices. Proc. Natl. Acad. Sci. USA 2002, 99, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rill, E.; Hyde, J.; Kezunovic, N.; Urbano, F.J.; Petersen, E. The physiology of the pedunculopontine nucleus: Implications for deep brain stimulation. J. Neural Transm. (Vienna) 2015, 122, 225–235. [Google Scholar] [CrossRef] [PubMed]
- James, W. The Principles of Psychology; Holt, H., Ed.; Dover Publications Inc.: New York, NY, USA, 1890; Volume 1, p. 225. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Rill, E.; Luster, B.; Mahaffey, S.; MacNicol, M.; Hyde, J.R.; D’Onofrio, S.M.; Phillips, C. Pedunculopontine Gamma Band Activity and Development. Brain Sci. 2015, 5, 546-567. https://doi.org/10.3390/brainsci5040546
Garcia-Rill E, Luster B, Mahaffey S, MacNicol M, Hyde JR, D’Onofrio SM, Phillips C. Pedunculopontine Gamma Band Activity and Development. Brain Sciences. 2015; 5(4):546-567. https://doi.org/10.3390/brainsci5040546
Chicago/Turabian StyleGarcia-Rill, Edgar, Brennon Luster, Susan Mahaffey, Melanie MacNicol, James R. Hyde, Stasia M. D’Onofrio, and Cristy Phillips. 2015. "Pedunculopontine Gamma Band Activity and Development" Brain Sciences 5, no. 4: 546-567. https://doi.org/10.3390/brainsci5040546
APA StyleGarcia-Rill, E., Luster, B., Mahaffey, S., MacNicol, M., Hyde, J. R., D’Onofrio, S. M., & Phillips, C. (2015). Pedunculopontine Gamma Band Activity and Development. Brain Sciences, 5(4), 546-567. https://doi.org/10.3390/brainsci5040546





