Forced Exercise Enhances Functional Recovery after Focal Cerebral Ischemia in Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Results
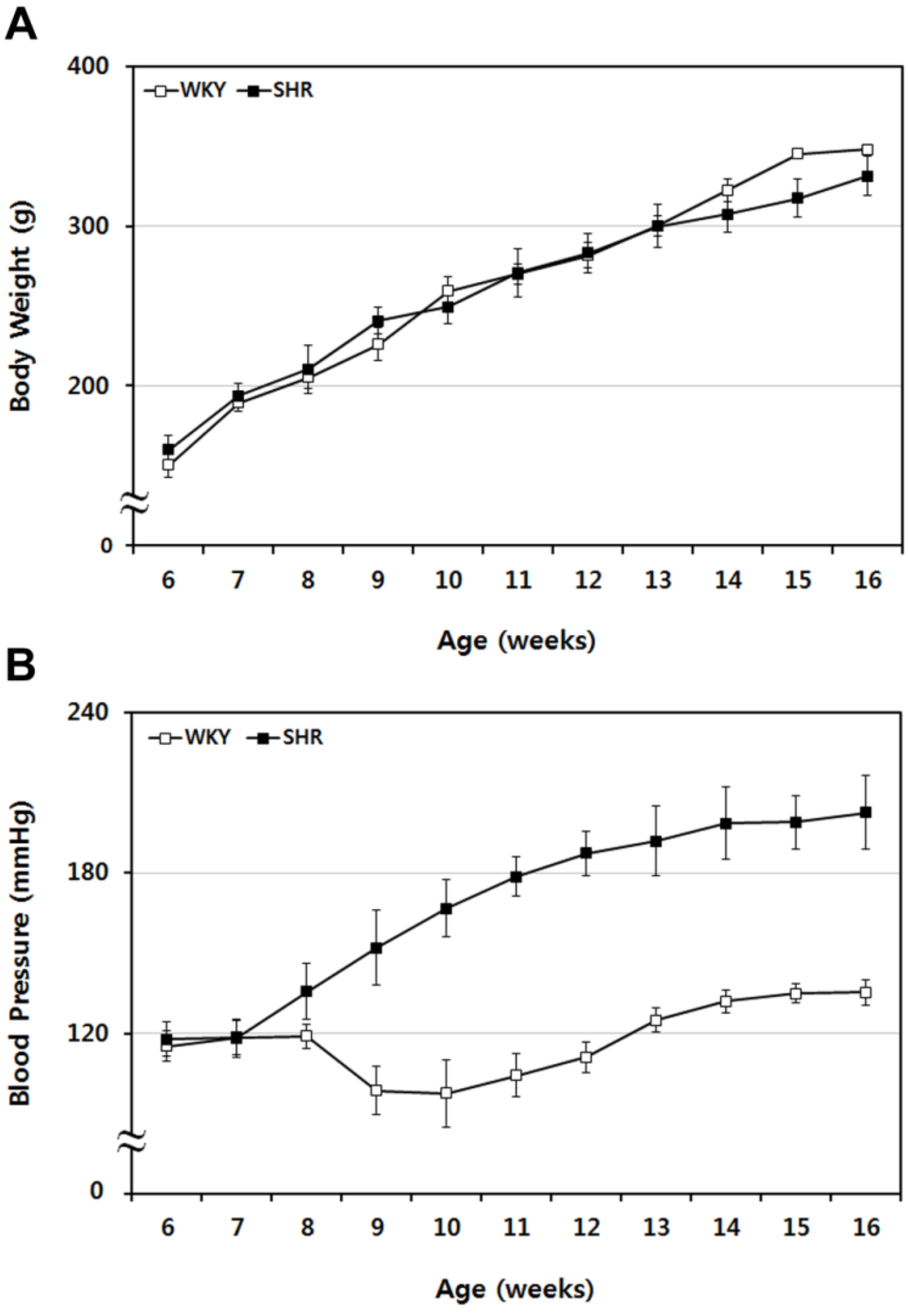
2.1. Sequential Tracing of Physiological Characteristics of WKY and SHR Rats
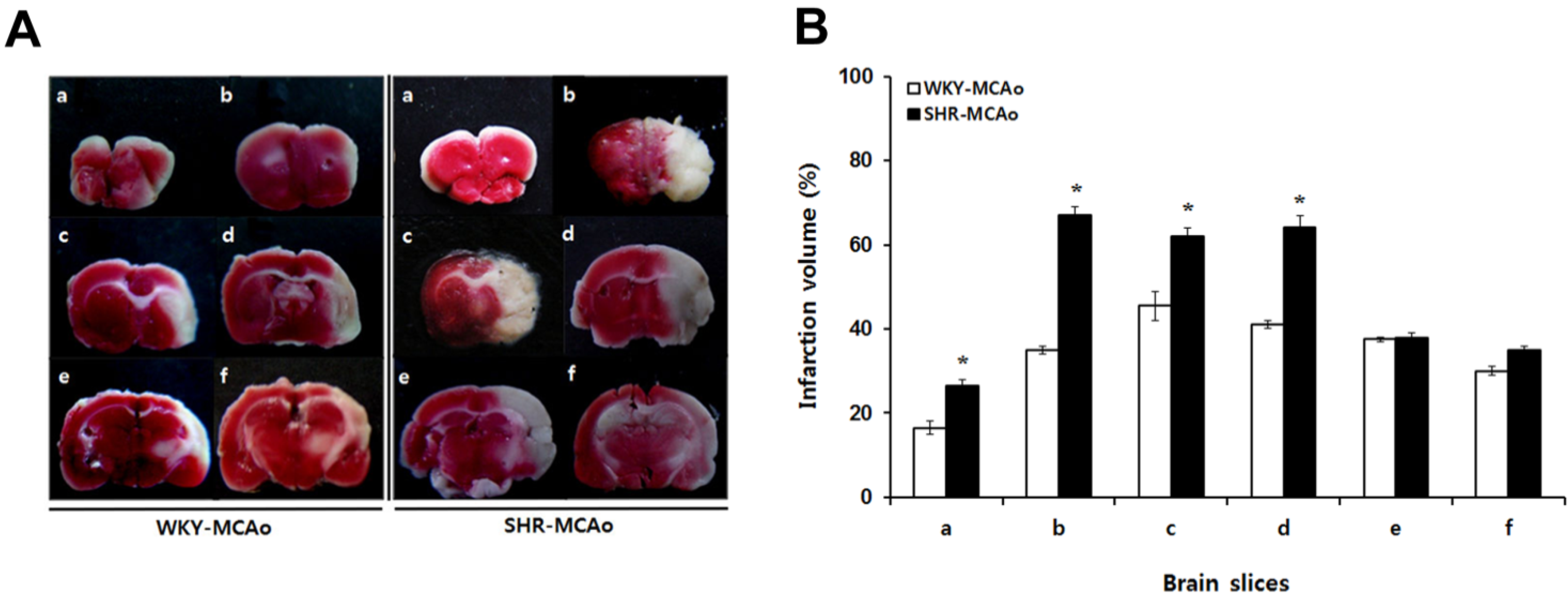
2.2. 2,3,5-Triphenyltetrazolium Chloride (TTC)-Defined Ischemic Lesion Volume in WKY and SHR Rats after MCAo Surgery
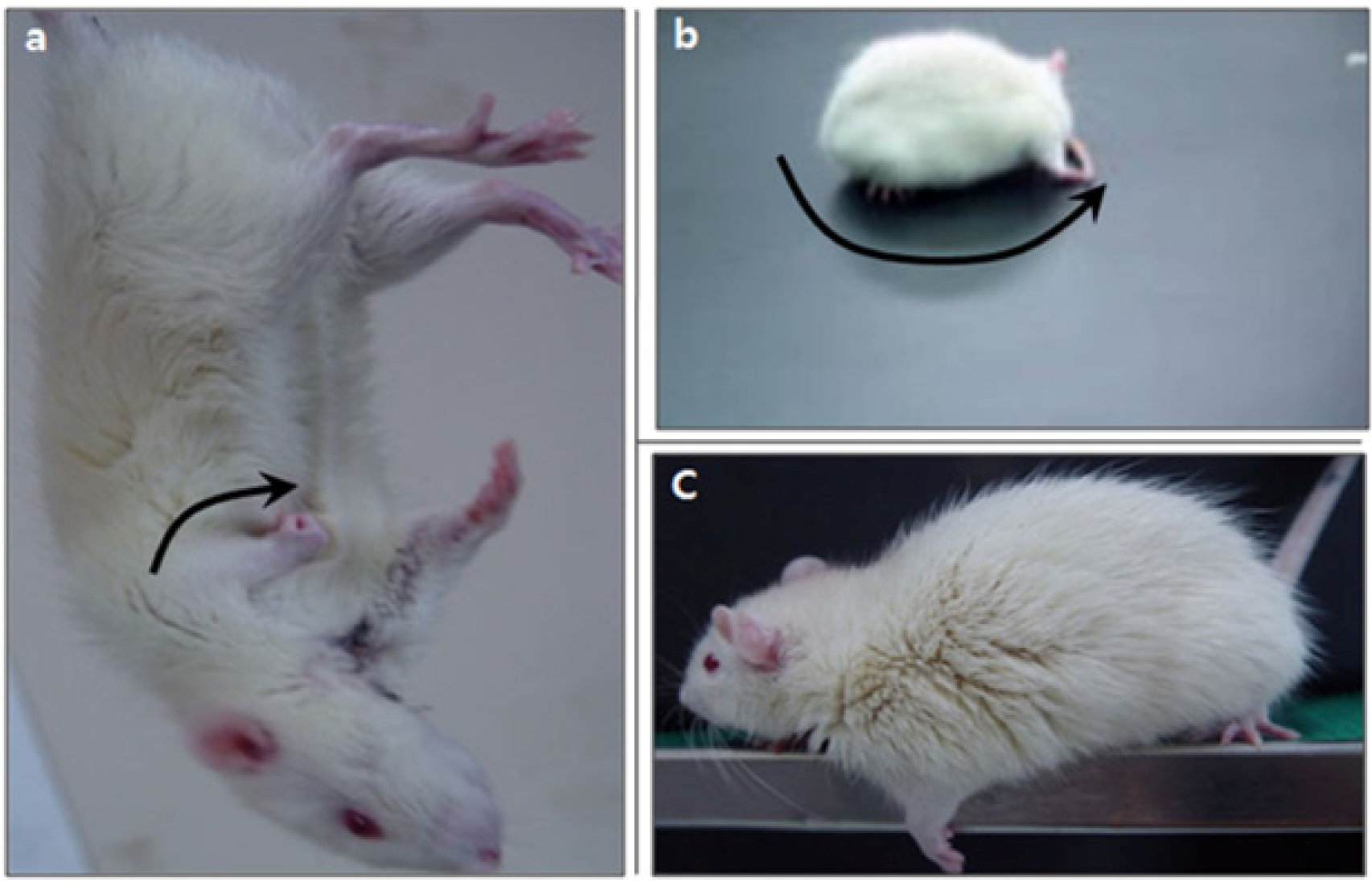
2.3. Neurological Deficiency and Motor Dysfunction Induced by Focal Cerebral Ischemia Were Reduced by Forced Exercise
| WKY | SHR | |||||||
|---|---|---|---|---|---|---|---|---|
| Sham | Sham + Ex | MCAo | MCAo + Ex | Sham | Sham + Ex | MCAo | MCAo + Ex | |
| mNSS (Score) | 1 (0–2) | 1 (0–2) | 11 (10–12) ** | 8 (8–10) # | 1 (1–2) | 1 (1–2) | 13 (12–14) **,a | 12 (9–12) b |
| Beam-walking test (Score) | 6 (5–6) | 6 (5–6) | 3 (2–4) ** | 4 (3–5) # | 6 (5–6) | 6 (5–6) | 3 (2–4) ** | 4 (2–5) |
| Hindlimb stride width (cm) | 5.2 (4.8–6.0) | 5.2 (5.1–5.7) | 7.1 (6.4–7.2) ** | 5.8 (5.6–6.1) ## | 5.1 (4.9–5.4) | 5.3 (4.7–5.8) | 7.4 (7.0–8.1) ** | 6.1 (5.7–7.1) ## |
2.4. Altered Expression of Caveolin and NOS Isoforms Due to Focal Cerebral Ischemia in WKY and SHR Rats
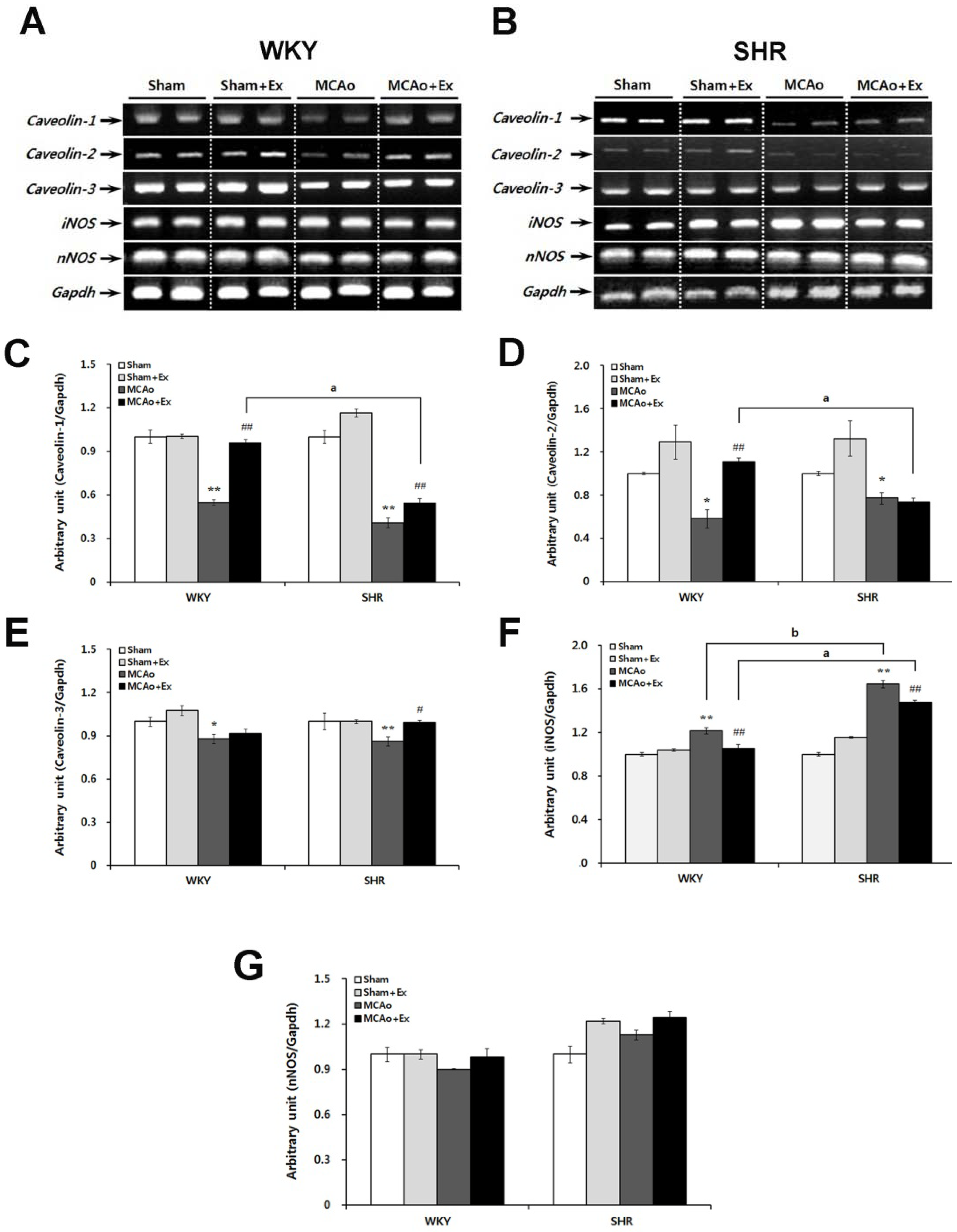
2.5. Effects of Forced Exercise on Caveolin-1 and iNOS Interaction and Autophagy Signaling after Focal Cerebral Ischemic Injury
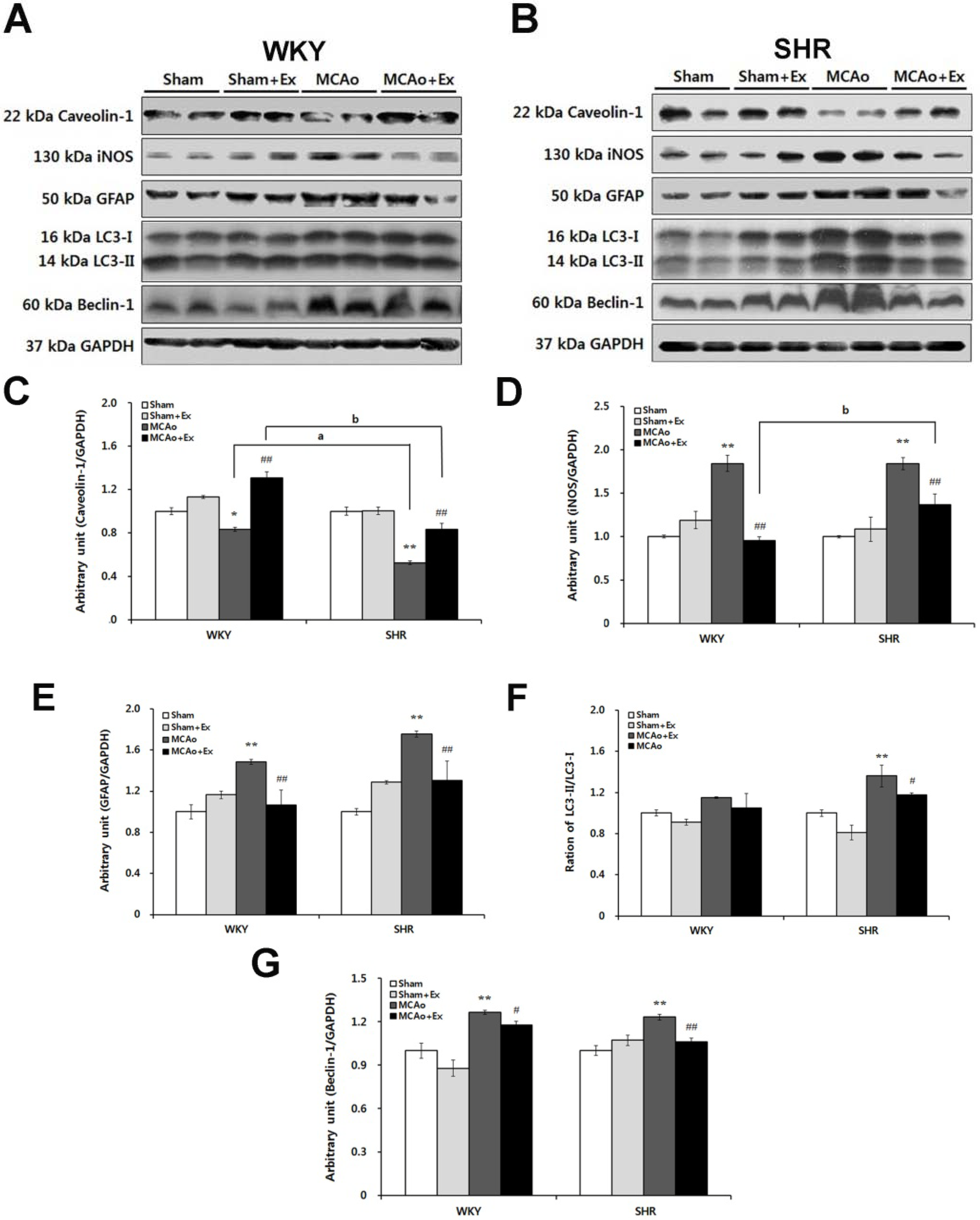
3. Discussion
4. Experimental Section
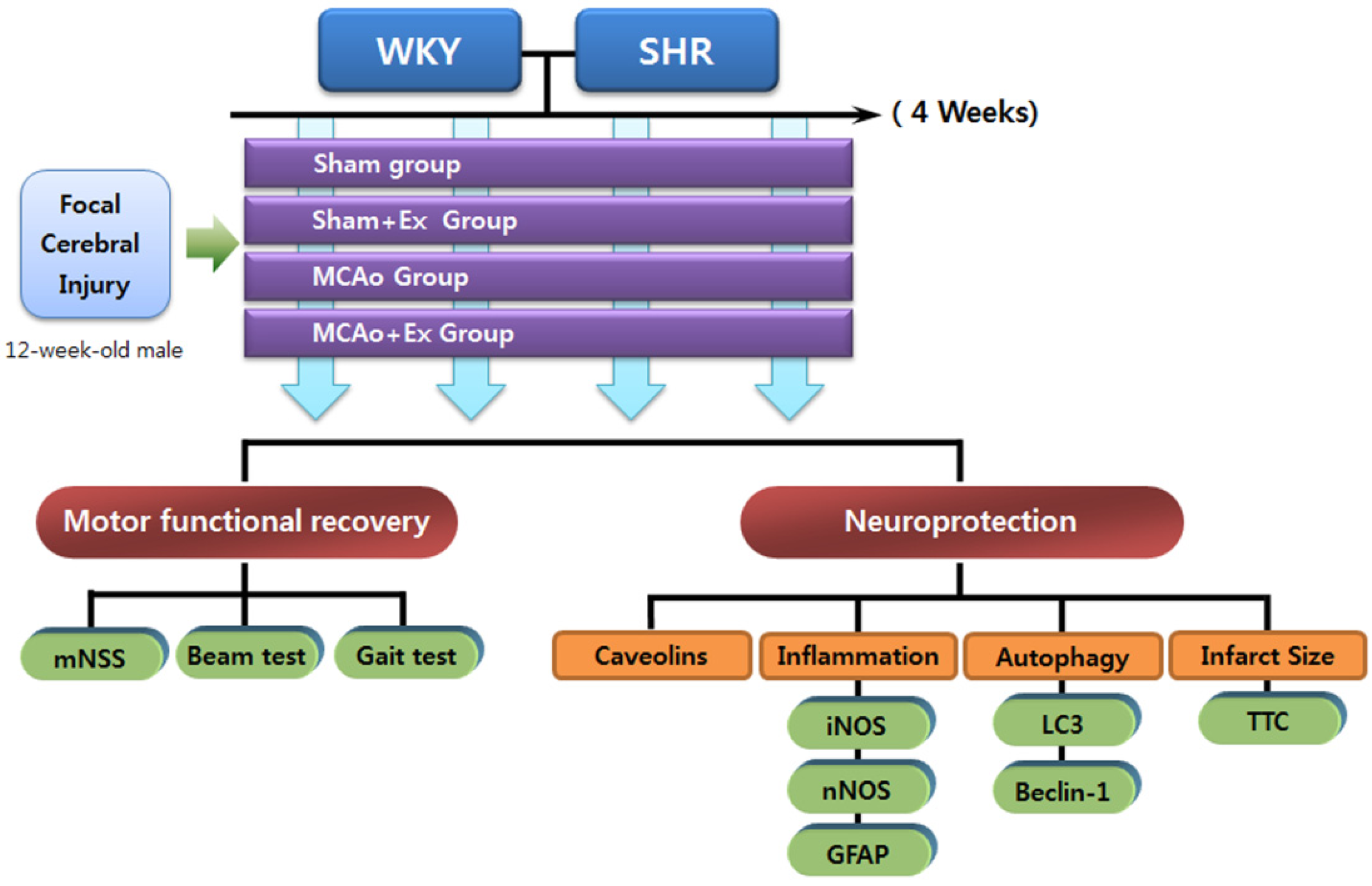
4.1. Experimental Animals
4.2. Experimental Groups
4.3. Measurement of Blood Pressure
4.4. Induction of Focal Cerebral Ischemia: MCAo
4.5. Protocol for Forced Treadmill Exercise
4.6. Modified Neurological Severity Score (mNSS)
4.7. Beam-Walking Test
4.8. Gait Analysis: Hindlimb Stride Width
4.9. Two Percent 2,3,5-Triphenyltetrazolium Chloride (TTC) Staining
4.10. Isolation of Brain Tissue
4.11. RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (PCR) Analysis
| Gene | Primer Sequence(5′–3′) | Tm (°C) | Product Length (bp) | GenBank Accession No. |
|---|---|---|---|---|
| Caveolin-1 | F: GATCAAGCTTATGTCTGGGGGCAAATAC | 55 | 537 | AY439333 |
| R: GATCGAATTCTCATATCTCTTTCTGC | ||||
| Caveolin-2 | F: GATCAAGCTTATGGGGCTGGAGACCGAG | 60 | 489 | NM_016900 |
| R: GATCGAATTCTCAGTCGTGGCTCAGTTG | ||||
| Caveolin-3 | F: GATCAAGCTTATGATGACCGAAGAGCAC | 60 | 456 | NM_007617 |
| R: GATCGAATTCTTAGCCTTCCCTTCGCAG | ||||
| iNOS | F: GTGTTCCACCAGGAGATGTTG | 60 | 272 | U03699 |
| R: GAAGGCGTAGCTGAACAAGG | ||||
| nNOS | F: TGGCAACAGCGACAATTTGA | 60 | 71 | NM_052799 |
| R: CACCCGAAGACCAGAACCAT | ||||
| GAPDH | F: GTATGACTCCACTCACGGCAAA | 60 | 100 | BC094037 |
| R: GGTCTCGCTCCTGGAAGATG |
4.12. Western Blot Analysis
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Feigin, V.L. Stroke epidemiology in the developing world. Lancet 2005, 365, 2160–2161. [Google Scholar] [CrossRef]
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; Straus, S.E.; Majumdar, S.R.; McAlister, F.A. Stroke: Strategies for primary prevention. Am. Fam. Physician 2003, 68, 2379–2386. [Google Scholar]
- Timsit, S.G.; Sacco, R.L.; Mohr, J.P.; Foulkes, M.A.; Tatemichi, T.K.; Wolf, P.A.; Price, T.R.; Hier, D.B. Brain infarction severity differs according to cardiac or arterial embolic source. Neurology 1993, 43, 728–733. [Google Scholar] [CrossRef]
- Hom, S.; Fleegal, M.A.; Egleton, R.D.; Campos, C.R.; Hawkins, B.T.; Davis, T.P. Comparative changes in the blood-brain barrier and cerebral infarction of SHR and WKY rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1881–R1892. [Google Scholar] [CrossRef]
- MacMahon, S. Blood pressure reduction and the prevention of stroke. J. Hypertens Suppl. 1991, 9, S7–S10. [Google Scholar] [CrossRef]
- Rahman, A.; Sward, K. The role of caveolin-1 in cardiovascular regulation. Acta Physiol. (Oxf.) 2009, 195, 231–245. [Google Scholar] [CrossRef]
- Rath, G.; Dessy, C.; Feron, O. Caveolae, caveolin and control of vascular tone: Nitric oxide (NO) and endothelium derived hyperpolarizing factor (EDHF) regulation. J. Physiol. Pharmacol. 2009, 60, 105–109. [Google Scholar]
- Sonveaux, P.; Martinive, P.; DeWever, J.; Batova, Z.; Daneau, G.; Pelat, M.; Ghisdal, P.; Gregoire, V.; Dessy, C.; Balligand, J.L.; Feron, O. Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis. Circ. Res. 2004, 95, 154–161. [Google Scholar] [CrossRef]
- Bauer, P.M.; Bauer, E.M.; Rogers, N.M.; Yao, M.; Feijoo-Cuaresma, M.; Pilewski, J.M.; Champion, H.C.; Zuckerbraun, B.S.; Calzada, M.J.; Isenberg, J.S. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc. Res. 2012, 93, 682–693. [Google Scholar] [CrossRef]
- Lisanti, M.P.; Scherer, P.E.; Tang, Z.; Sargiacomo, M. Caveolae, caveolin and caveolin-rich membrane domains: A signalling hypothesis. Trends Cell Biol. 1994, 4, 231–235. [Google Scholar] [CrossRef]
- Rothberg, K.G.; Heuser, J.E.; Donzell, W.C.; Ying, Y.S.; Glenney, J.R.; Anderson, R.G. Caveolin, a protein component of caveolae membrane coats. Cell 1992, 68, 673–682. [Google Scholar] [CrossRef]
- Das, K.; Lewis, R.Y.; Scherer, P.E.; Lisanti, M.P. The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers. Implications for the assembly of caveolae membranes in vivo. J. Biol. Chem. 1999, 274, 18721–18728. [Google Scholar]
- Couet, J.; Sargiacomo, M.; Lisanti, M.P. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J. Biol. Chem. 1997, 272, 30429–30438. [Google Scholar] [CrossRef]
- Mathew, R. Cell-specific dual role of caveolin-1 in pulmonary hypertension. Pulm. Med. 2011, 573432. [Google Scholar]
- Ikonen, E.; Parton, R.G. Caveolins and cellular cholesterol balance. Traffic 2000, 1, 212–217. [Google Scholar] [CrossRef]
- Van Helmond, Z.K.; Miners, J.S.; Bednall, E.; Chalmers, K.A.; Zhang, Y.; Wilcock, G.K.; Love, S.; Kehoe, P.G. Caveolin-1 and -2 and their relationship to cerebral amyloid angiopathy in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2007, 33, 317–327. [Google Scholar] [CrossRef]
- Gaudreault, S.B.; Blain, J.F.; Gratton, J.P.; Poirier, J. A role for caveolin-1 in post-injury reactive neuronal plasticity. J. Neurochem. 2005, 92, 831–839. [Google Scholar] [CrossRef]
- Lear, J.L.; Ackermann, R.; Kameyama, M.; Carson, R.; Phelps, M. Multiple-radionuclide autoradiography in evaluation of cerebral function. J. Cereb. Blood Flow Metab. 1984, 4, 264–269. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Benedek, A.; Moricz, K.; Juranyi, Z.; Gigler, G.; Levay, G.; Harsing, L.G., Jr.; Matyus, P.; Szenasi, G.; Albert, M. Use of TTC staining for the evaluation of tissue injury in the early phases of reperfusion after focal cerebral ischemia in rats. Brain Res. 2006, 1116, 159–165. [Google Scholar]
- Puurunen, K.; Jolkkonen, J.; Sirvio, J.; Haapalinna, A.; Sivenius, J. An alpha(2)-adrenergic antagonist, atipamezole, facilitates behavioral recovery after focal cerebral ischemia in rats. Neuropharmacology 2001, 40, 597–606. [Google Scholar] [CrossRef]
- Shen, J.; Ma, S.; Chan, P.; Lee, W.; Fung, P.C.; Cheung, R.T.; Tong, Y.; Liu, K.J. Nitric oxide down-regulates caveolin-1 expression in rat brains during focal cerebral ischemia and reperfusion injury. J. Neurochem. 2006, 96, 1078–1089. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, D.Y. Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 2009, 20, 223–230. [Google Scholar] [CrossRef]
- Samdani, A.F.; Dawson, T.M.; Dawson, V.L. Nitric oxide synthase in models of focal ischemia. Stroke 1997, 28, 1283–1288. [Google Scholar] [CrossRef]
- Malinski, T.; Bailey, F.; Zhang, Z.G.; Chopp, M. Nitric oxide measured by a porphyrinic microsensor in rat brain after transient middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 1993, 13, 355–358. [Google Scholar] [CrossRef]
- Matsui, T.; Nagafuji, T.; Kumanishi, T.; Asano, T. Role of nitric oxide in pathogenesis underlying ischemic cerebral damage. Cell. Mol. Neurobiol. 1999, 19, 177–189. [Google Scholar] [CrossRef]
- Northington, F.J.; Chavez-Valdez, R.; Martin, L.J. Neuronal cell death in neonatal hypoxia-ischemia. Ann. Neurol. 2011, 69, 743–758. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Liu, J.X.; Li, X.Z.; Xu, L.; Xu, Y.G. RNA interference-mediated downregulation of Beclin1 attenuates cerebral ischemic injury in rats. Acta Pharmacol. Sin. 2009, 30, 919–927. [Google Scholar] [CrossRef]
- Rami, A.; Langhagen, A.; Steiger, S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol. Dis. 2008, 29, 132–141. [Google Scholar] [CrossRef]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Hinescu, M.E.; Popescu, L.M. Myocardial interstitial Cajal-like cells (ICLC) in caveolin-1 KO mice. J. Cell. Mol. Med. 2009, 13, 202–206. [Google Scholar] [CrossRef]
- Anderson, R.G. The caveolae membrane system. Annu. Rev. Biochem. 1998, 67, 199–225. [Google Scholar] [CrossRef]
- Jasmin, J.F.; Malhotra, S.; Singh Dhallu, M.; Mercier, I.; Rosenbaum, D.M.; Lisanti, M.P. Caveolin-1 deficiency increases cerebral ischemic injury. Circ. Res. 2007, 100, 721–729. [Google Scholar] [CrossRef]
- Barone, F.C.; Price, W.J.; White, R.F.; Willette, R.N.; Feuerstein, G.Z. Genetic hypertension and increased susceptibility to cerebral ischemia. Neurosci. Biobehav. Rev. 1992, 16, 219–233. [Google Scholar] [CrossRef]
- Liu, Y.; Schlumberger, A.; Wirth, K.; Schmidtbleicher, D.; Steinacker, J.M. Different effects on human skeletal myosin heavy chain isoform expression: Strength vs. combination training. J. Appl. Physiol. 2003, 94, 2282–2288. [Google Scholar]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Chen, M.J.; Russo-Neustadt, A.A. Exercise activates the phosphatidylinositol 3-kinase pathway. Brain Res. Mol. Brain Res. 2005, 135, 181–193. [Google Scholar]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef]
- Arvanitis, D.N.; Wang, H.; Bagshaw, R.D.; Callahan, J.W.; Boggs, J.M. Membrane-associated estrogen receptor and caveolin-1 are present in central nervous system myelin and oligodendrocyte plasma membranes. J. Neurosci. Res. 2004, 75, 603–613. [Google Scholar] [CrossRef]
- Gaudreault, S.B.; Dea, D.; Poirier, J. Increased caveolin-1 expression in Alzheimer’s disease brain. Neurobiol. Aging 2004, 25, 753–759. [Google Scholar] [CrossRef]
- Trushina, E.; Du Charme, J.; Parisi, J.; McMurray, C.T. Neurological abnormalities in caveolin-1 knock out mice. Behav. Brain. Res. 2006, 172, 24–32. [Google Scholar] [CrossRef]
- Park, K.; Lee, Y.; Park, S.; Lee, S.; Hong, Y.; Kil Lee, S. Synergistic effect of melatonin on exercise-induced neuronal reconstruction and functional recovery in a spinal cord injury animal model. J. Pineal Res. 2010, 48, 270–281. [Google Scholar] [CrossRef]
- Messing, A.; Brenner, M. GFAP: Functional implications gleaned from studies of genetically engineered mice. Glia 2003, 43, 87–90. [Google Scholar] [CrossRef]
- Yang, Y.R.; Wang, R.Y.; Wang, P.S.; Yu, S.M. Treadmill training effects on neurological outcome after middle cerebral artery occlusion in rats. Can. J. Neurol. Sci. 2003, 30, 252–258. [Google Scholar]
- Park, S.; Lee, S.K.; Park, K.; Lee, Y.; Hong, Y.; Lee, S.; Jeon, J.C.; Kim, J.H.; Lee, S.R.; Chang, K.T. Beneficial effects of endogenous and exogenous melatonin on neural reconstruction and functional recovery in an animal model of spinal cord injury. J. Pineal Res. 2012, 52, 107–119. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Park, S.; Shin, J.; Hong, Y.; Kim, S.; Lee, S.; Park, K.; Lkhagvasuren, T.; Lee, S.-R.; Chang, K.-T.; Hong, Y. Forced Exercise Enhances Functional Recovery after Focal Cerebral Ischemia in Spontaneously Hypertensive Rats. Brain Sci. 2012, 2, 483-503. https://doi.org/10.3390/brainsci2040483
Park S, Shin J, Hong Y, Kim S, Lee S, Park K, Lkhagvasuren T, Lee S-R, Chang K-T, Hong Y. Forced Exercise Enhances Functional Recovery after Focal Cerebral Ischemia in Spontaneously Hypertensive Rats. Brain Sciences. 2012; 2(4):483-503. https://doi.org/10.3390/brainsci2040483
Chicago/Turabian StylePark, Sookyoung, Jinhee Shin, Yunkyung Hong, Sunmi Kim, Seunghoon Lee, Kanghui Park, Tserentogtokh Lkhagvasuren, Sang-Rae Lee, Kyu-Tae Chang, and Yonggeun Hong. 2012. "Forced Exercise Enhances Functional Recovery after Focal Cerebral Ischemia in Spontaneously Hypertensive Rats" Brain Sciences 2, no. 4: 483-503. https://doi.org/10.3390/brainsci2040483
APA StylePark, S., Shin, J., Hong, Y., Kim, S., Lee, S., Park, K., Lkhagvasuren, T., Lee, S.-R., Chang, K.-T., & Hong, Y. (2012). Forced Exercise Enhances Functional Recovery after Focal Cerebral Ischemia in Spontaneously Hypertensive Rats. Brain Sciences, 2(4), 483-503. https://doi.org/10.3390/brainsci2040483






