From Bacillus Criminalis to the Legalome: Will Neuromicrobiology Impact 21st Century Criminal Justice?
Abstract
1. Introduction
“Of course, if it is the bad bacilli that make men murderers and robbers, they should not be punished. A man is not responsible for what his bacilli force him to do. He is acting under physiological distress and might be a good man if he had a better set of microbes in him.”Editorial, “The Bacillus of Crime”—The Springfield Daily Leader, 1910 [1]
2. Auto-Brewery Syndrome
3. Legalomics and Involuntary Intoxication
4. Neuromicrobiology—Potential Mechanisms
5. Microbial Signatures and Multi-Omics Support
6. Are Gut Microbes a Causal Factor?
7. Match to Existing Biopsychosocial Research
8. Response to Glucose Testing and Brain Fog
9. New Bottles for Old Wine
10. Legalome—Future Directions
- Expansion of preclinical mechanistic research, including a greater linkage between specific biological markers and justice-related behavior. Further identify microbiota-mediated pathways between dietary components and EtOH production [278]. While EtOH is easily understood from a forensics perspective, the courts will need reliable causal links between specific microbes and microbe-manufactured chemicals (e.g., p-cresol, propionic acid [279,280]) and microbe-related chemicals (e.g., plasma pro-inflammatory cytokines [281]). Relationships between elevated uric acid [282] and low bilirubin [283] have been noted in impulsive aggression, and these associations are likely mediated, at least in part, by gut microbiota.
- Approach criminology with the exposome in mind. That is, consider the biological responses of the “total organism to the total environment” throughout the life course [284]. With advances in exposome science, aided by omics and inclusive of microbiome endpoints, the capacity to examine total lived experiences (both positive and negative “exposures”) interacting with genes (over time) is within reach [285,286]. It is important to consider the potential of microbiomes as contributors to the neurobiology of love and other positive emotions [287].
- Expansion of high-quality randomized controlled studies, including designs that target the microbiome. With appropriate informed consent and ethical guardrails in place, greater inclusion of at-risk or justice-involved persons in microbiome research. The emergence of microbial signatures related to specific disorders and behaviors requires consideration of vulnerable populations, often excluded from research endeavors. Meaningful inclusion, with consideration of life course experiences and the social exposome, will better inform researchers as they attempt to expand microbiome knowledge [288]. Ethical inclusions of justice-involved persons can help researchers pursue meaningful endeavors, especially those that might improve individualized outcomes.
- It is important to examine non-violent criminal behavior. Approximately half of the adults in the US jail/prison population are incarcerated for non-violent reasons [289]. So-called white-collar crime is associated with significantly higher rates of recidivism than violent crime [290]. White-collar crime has been linked to genetics and personality traits [291,292]. How might microbial signatures interact with multi-omics and polygenic markers in non-violent populations? How might microbial signatures influence risk-taking in healthy populations [293], and people involved with the justice system?
- Determine whether or not microbiome or omics-based markers change in response to therapeutic interventions in carceral settings. Does the gut microbiome play a critical role in explaining observed associations between inflammatory diets and behavioral disinhibition [294]? Some US prisons are making efforts to transform their food systems for the better, which represents an opportunity for microbiome and behavior research [295]. Given emerging evidence indicating that baseline microbiome profiles predict antidepressant treatment outcomes [296,297], it might be worth querying whether microbial profiles predict responsiveness to various carceral programs. Meta-analyses of human studies demonstrate the value of various strains and species of probiotics in neuropsychiatric conditions [298,299], yet carceral populations have thus far not been included in this research.
- Incorporation of a ‘justice lens’ in microbiome research. For example, the phenomenon of ‘brain fog’ is witnessing increased research attention, especially because it is a central complaint associated with rising rates of post-COVID conditions (also known as, ‘long COVID’) [300]. When researchers examine phenomena such as ‘brain fog’ and mental fatigue using multi-omics and machine learning approaches [301], there is an opportunity to consider the risks of justice involvement. Recent population studies have linked adherence to ADHD medications with lower subsequent risk of criminality [302,303]. Given that ADHD medications may (at least in part) operate via the gut microbiome [304], this is an area worthy of scrutiny.
- Scrutinize the role of the microbiome in the links between trauma exposure and subsequent justice involvement. Military veterans with posttraumatic stress disorder (PTSD) are 61% more likely (vs. veterans without PTSD) to be justice-involved, and the odds of arrest for violent offenses are 59% higher [305]. Research shows that the gut microbiome is disturbed in PTSD, with one recent study linking dysbiosis to executive function in veterans with PTSD [306]. Given the long-standing research connecting deficits in executive function to significantly higher risks of justice involvement [307], this is an area worthy of pursuit. Since gut microbes appear to mediate the startle response [308], deficits of which have been found in PTSD and violent/antisocial behavior [309,310,311], this is an area ripe for research.
- Revisit existing research with legalomics in mind. For example, multiple studies have connected the quantity and accessibility of residential greenspace with positive mental health, and even reduced violence and crime [312,313]. The mechanisms are poorly understood, but are often assumed to operate through sensory pathways leading to cognitive restoration and stress reduction. However, emerging research is connecting environmental exposures, including greenspace, to differences in gut microbes [314] and temperament [315]. Moreover, emerging research has linked skin microbiota to psychological wellbeing [316].
- Expand the use of neuroimaging and electroencephalogram research in concert with omics, microbial signatures, and interventions targeting the microbiome. For now, only a small number of studies have incorporated neuroimaging and EEG into intervention trials [317] or research examining microbiome signatures with brain areas involved in with memory, language, and emotion processing [318].
- Explore how neuromicrobiology and metabolomic markers can enhance the predictive value of currently used “paper and pencil” instruments used in parole and probation risk assessments. Viewed through a lens of diagnostic accuracy in health and medicine, commonly used criminogenic risk assessments would be rated as “poor” or “fair”—they may be enhanced significantly by supplementing with biological markers [319].
- Enhance education, scientific literacy, and develop ethical frameworks. Legalome research—linking microbiology to legal responsibility—raises fundamental questions about punishment. Studies show that many lawyers and judges lack formal scientific training and rely heavily on personal beliefs [320]. Research has found that potential jurors with higher levels of scientific knowledge are less likely to support harsh sentencing [321]. Continuing education for the legal community, encompassing advances in microbiology and gut microbiome, should be explored. Education and science literacy will be essential to science-based reforms. Building bridges between microbiology and justice will require new frameworks, deeper collaborations, and cultural shifts in how responsibility, punishment, and public safety are understood. Matters of privacy and the risks of “dangerousness” labeling will need to be considered [322]. How might potential biases or misinterpretations in the analysis of gut–brain axis findings lead to potential harms to an individual and society?
- Establish an epidemiological understanding of auto-brewery syndrome and explore connections to mental health and suicidality [323]. While there are multiple reasons to suspect that the condition is more common than currently appreciated, the frequency of the condition remains a matter of speculation. Cases are typically uncovered and reported only when the BAC is exceedingly high [324].
- Explore short-term changes in cognition and behavior after single or several meals. There is evidence to suggest that meals rich in ultra-processed or fast foods can lead to changes in post-prandial physiology [325], cognition [326], and the microbiome transcriptome [327]. How might this influence the “irresistible impulse” in criminal law or the rapid decisions made by criminal justice professionals?
- Begin to examine relationships between the lifestyles of criminal justice professionals, occupational performance, and objective microbiome-related markers. For many in law enforcement, an inflammatory diet may be the norm [328,329]. Recent evidence suggests that many correction officers may be underpaid and living with food insecurity [330]. Given links between food insecurity and higher consumption of ultra-processed foods [331], and alterations in the microbiome [332], there may be microbiome-mediated behavioral changes in the workplace.
- Cross-jurisdictional data integration. Synchronize microbiome, behavioral and legal outcome datasets across countries and legal systems to understand how cultural, dietary, environmental, and policy differences modulate microbiome–justice relationships. Bioinformatics pipelines, including microbiota analysis for personalized care, at a nascent stage. Per the recent Position statement of the Microbiota International Clinical Society, “explicit reporting of software and database releases, key parameters and their rationale, and, where feasible, simple sensitivity checks to confirm the robustness of main findings” are recommended [333].
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABS | Auto-brewery syndrome |
| AhR | Aryl Hydrocarbon Receptor |
| BBB | Blood–brain barrier |
| MAMPs | Microbial-associated molecular patterns |
References
- Anonymous. The Bacillus of Crime. Springfield Leader and Press, 31 July 1910; 6. [Google Scholar]
- Anonymous. Cops and Bacilli. The Minneapolis Journal, 1 May 1901; 13. [Google Scholar]
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part I—Autointoxication revisited. Gut Patho. 2013, 5, 5. [Google Scholar] [CrossRef]
- Bested, A.C.; Logan, A.C.; Selhub, E.M. Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part II–contemporary contextual research. Gut Pathog. 2013, 5, 3. [Google Scholar] [CrossRef]
- Lydston, G.F. The Diseases of Society: The Vice and Crime Problem; J.B. Lippincott: Philadelphia, PA, USA, 1904. [Google Scholar]
- Adami, J.G. An Address on chronic intestinal stasis: Autointoxication and sub-infection. Br. Med. J. 1914, 1, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Autointoxication. In The Encyclopedia Britannica; Encyclopædia Britannica, Inc.: Chicago, IL, USA, 1922; Volume 31, p. 547. [Google Scholar]
- Anonymous. Doctor Who Claims That Thieves and Even Murderers Can Be Cured. Waco News Tribune, 14 November 1889; 4. [Google Scholar]
- Mearkle, A.L. The civilization bacillus. Mind 1897, 1, 358–365. [Google Scholar]
- Prescott, S.L. History of medicine: Origin of the term microbiome and why it matters. Hum. Microbiome J. 2017, 4, 24–25. [Google Scholar] [CrossRef]
- Eugene, E.M. Handbook on Diet; FA Davis Publishers: Philadelphia, PA, USA, 1928. [Google Scholar]
- Charles, C.F. Human Distillery. The St. Louis Star and Times, 29 December 1924; 5. [Google Scholar]
- Reuters. Doctors Close Up Distillery—In a Patient’s Stomach. The Buffalo News, 9 July 1959; 4. [Google Scholar]
- Anonymous. Medicine: The Secret Still. Time, 20 July 1959; 69. [Google Scholar]
- Iwata, K. A Review of the Literature on Drunken Symptoms due to Yeasts in the Gastro Intestinal Tract. In Yeasts and Yeast-Like Microorganisms in Medical Science: Proceedings of the Second International Specialized Symposium on Yeasts, Science Council of Japan, Tokyo, Japan, August 7-10, 1972; University of Tokyo Press: Tokyo, Japan, 1972; pp. 260–268. [Google Scholar]
- Swaart, C. The Secret Still is Not Still Secret. The Arizona Republic Sunday Magazine, 7 August 1977; 6–14. [Google Scholar]
- Swaart, C. My Bout with the Secret Still. Star: Kansas City Star Sunday Magazine, 16 October 1977; 16–21. [Google Scholar]
- Logan, A.C. Dysbiotic drift: Mental health, environmental grey space, and microbiota. J. Physiol. Anthropol. 2015, 34, 1–6. [Google Scholar] [CrossRef]
- Prescott, S.L.; Wegienka, G.; Logan, A.C.; Katz, D.L. Dysbiotic drift and biopsychosocial medicine: How the microbiome links personal, public and planetary health. Biopsychosoc. Med. 2018, 12, 1–2. [Google Scholar] [CrossRef]
- Cordell, B. My Gut Makes Alcohol: The Science and Stories of Auto-Brewery Syndrome; BookBaby: Pennsauken, NJ, USA, 2019. [Google Scholar]
- Yates, S.R.; Saito, A. Auto-Brewery syndrome after COVID-19 infection. ACG Case Rep. J. 2024, 11, e01248. [Google Scholar] [CrossRef]
- Mulholland, J.H.; Townsend, F.J. Bladder beer-a new clinical observation. Trans. Am. Clin. Climatol. Assoc. 1984, 95, 34–39. [Google Scholar]
- Gillies, M.; Ranakusuma, A.; Hoffmann, T.; Thorning, S.; McGuire, T.; Glasziou, P.; Mar, C. Common harms from amoxicillin: A systematic review and meta-analysis of randomized placebo-controlled trials for any indication. Can. Med. Assoc. J. 2015, 187, E21–E31. [Google Scholar] [CrossRef]
- Mbaye, B.; Wasfy, R.M.; Alou, M.T.; Borentain, P.; Gerolami, R.; Dufour, J.C.; Million, M. A catalog of ethanol-producing microbes in humans. Future Microbiol. 2024, 19, 697–714. [Google Scholar] [CrossRef]
- Xue, G.; Feng, J.; Zhang, R.; Du, B.; Sun, Y.; Liu, S.; Yan, C.; Liu, X.; Du, S.; Feng, Y.; et al. Three Klebsiella species as potential pathobionts generating endogenous ethanol in a clinical cohort of patients with auto-brewery syndrome: A case control study. EBioMedicine 2023, 91, 104560. [Google Scholar] [CrossRef] [PubMed]
- Hecht, A.L.; Harling, L.C.; Friedman, E.S.; Tanes, C.; Lee, J.; Firrman, J.; Hao, F.; Tu, V.; Liu, L.S.; Patterson, A.D.; et al. Dietary carbohydrates regulate intestinal colonization and dissemination of Klebsiella pneumoniae. J. Clin. Investig. 2024, 134, e174726. [Google Scholar] [CrossRef]
- Lin, Y.T.; Liu, C.J.; Yeh, Y.C.; Chen, T.J.; Fung, C.P. Ampicillin and amoxicillin use and the risk of Klebsiella pneumoniae liver abscess in Taiwan. J. Infect. Dis. 2013, 208, 211–217. [Google Scholar] [CrossRef]
- Associated Press. Disease Makes Rep. Vargas a Human Brewery; Carlsbad Current-Argus: Carlsbad, NM, USA, 1990; p. A–3. [Google Scholar]
- Glen, R. Rare disease afflicts Vargas, lawyer says. Albuq. J. 1990, A–1, A–10. [Google Scholar]
- The Editors. A new DWI defense. Albuq. J. 1990, A–8. [Google Scholar]
- Ray, T. Vargas case breaks new ground in DWI case. Albuq. J. 1991, A–15. [Google Scholar]
- Esch, M. Woman Beats DWI Rap with Claim Her Body Brews Alcohol. The Post-Standard, 31 December 2015; A12. [Google Scholar]
- Logan, A.C.; Prescott, S.L.; LaFata, E.M.; Nicholson, J.J.; Lowry, C.A. Beyond Auto-Brewery: Why Dysbiosis and the Legalome Matter to Forensic and Legal Psychology. Laws 2024, 13, 46. [Google Scholar] [CrossRef]
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Katsafanas, E.; Schweinfurth, L.A.; Savage, C.L.; Adamos, M.B.; Sweeney, K.M.; Origoni, A.E.; Khushalani, S.; et al. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophr. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Yuan, X.; Li, X.; Hei, G.; Zhang, X.; Song, X. Intestinal mycobiota dysbiosis associated inflammation activation in chronic schizophrenia. Behav. Brain Res. 2024, 472, 115149. [Google Scholar] [CrossRef] [PubMed]
- Shinar, D. Alcohol and Driving. In Traffic Safety and Human Behavior, 2nd ed.; Shinar, D., Ed.; Emerald Publishing: Bingley, UK, 2017; pp. 563–636. [Google Scholar]
- Frank, A.H. Low-Dose Alcohol Effects on Human Behavior and Performance: A Review of Post 1984 Research; Office of Aviation Medicine, Federal Aviation Administration: Washington, DC, USA, 1994. Available online: https://rosap.ntl.bts.gov/view/dot/21422/dot_21422_DS1.pdf (accessed on 2 May 2025).
- Marcelline, M.H.; Burns, M.; Williams, A.F. Skills performance at low blood alcohol levels. J. Stud. Alcohol 1985, 46, 482–485. [Google Scholar] [CrossRef]
- Dyke, N.A.; Fillmore, M.T. Laboratory analysis of risky driving at 0.05% and 0.08% blood alcohol concentration. Drug Alcohol Depend. 2017, 175, 127–132. [Google Scholar] [CrossRef]
- Wenger, L.P.; Hamm, O.; Mühle, C.; Hoffmann, S.; Reinhard, I.; Bach, P.; Kornhuber, J.; Alpers, G.W.; Kiefer, F.; Leménager, T.; et al. Alcohol does not influence trust in others or oxytocin, but increases positive affect and risk-taking: A randomized, controlled, within-subject trial. Eur. Arch. Psychiatry Clin. Neurosci. 2024, 274, 311–320. [Google Scholar] [CrossRef]
- Giancola, P.R.; Zeichner, A. Alcohol-related aggression in males and females: Effects of blood alcohol concentration, subjective intoxication, personality, and provocation. Alcohol. Clin. Exp. Res. 1995, 19, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Virkkunen, M. Alcohol as a factor precipitating aggression and conflict behaviour leading to homicide. Br. J. Addict. Alcohol Other Drugs 1974, 69, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Markey, L.; Hooper, A.; Melon, L.C.; Baglot, S.; Hill, M.N.; Maguire, J.; Kumamoto, C.A. Colonization with the commensal fungus Candida albicans perturbs the gut-brain axis through dysregulation of endocannabinoid signaling. Psychoneuroendocrinology 2020, 121, 104808. [Google Scholar] [CrossRef] [PubMed]
- Sontate, K.V.; Rahim Kamaluddin, M.; Naina Mohamed, I.; Mohamed, R.M.; Shaikh, M.F.; Kamal, H.; Kumar, J. Alcohol, aggression, and violence: From public health to neuroscience. Front. Psychol. 2021, 12, 699726. [Google Scholar] [CrossRef] [PubMed]
- Kuhns, J.B.; Wilson, D.B.; Clodfelter, T.A.; Maguire, E.R.; Ainsworth, S.A. A meta-analysis of alcohol toxicology study findings among homicide victims. Addiction 2011, 106, 62–72. [Google Scholar] [CrossRef]
- Naimi, T.S.; Xuan, Z.; Cooper, S.E.; Coleman, S.M.; Hadland, S.E.; Swahn, M.H.; Heeren, T.C. Alcohol involvement in homicide victimization in the United States. Alcohol. Clin. Exp. Res. 2016, 40, 2614–2621. [Google Scholar] [CrossRef]
- Lira, M.C.; Sarda, V.; Heeren, T.C.; Miller, M.; Naimi, T.S. Alcohol policies and motor vehicle crash deaths involving blood alcohol concentrations below 0.08%. Am. J. Prev. Med. 2020, 58, 622–629. [Google Scholar] [CrossRef]
- Dan, H. Prosecutors Won’t Appeal Bizarre DWI Dismissal. The Buffalo News, 1 May 2016; D1–D2. [Google Scholar]
- Logan, A.C.; Katzman, M. Major depressive disorder: Probiotics may be an adjuvant therapy. Med. Hypotheses 2005, 64, 533–538. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Gawey, B.J.; Mars, R.A.; Kashyap, P.C. The role of the gut microbiome in disorders of gut–brain interaction. FEBS J. 2025, 292, 1357–1377. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The gut microbiota-immune-brain axis: Therapeutic implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, G.; Dudhabhate, B.B.; Kokare, D.M.; Sakharkar, A.J. Gut microbiota regulates epigenetic remodelling in the amygdala: A role in Repeated Mild Traumatic Brain Injury (rMTBI)-induced anxiety. Mol. Neurobiol. 2024, 61, 9892–9914. [Google Scholar] [CrossRef] [PubMed]
- Erny, D.; Dokalis, N.; Mezö, C.; Castoldi, A.; Mossad, O.; Staszewski, O.; Frosch, M.; Villa, M.; Fuchs, V.; Mayer, A.; et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021, 33, 2260–2276. [Google Scholar] [CrossRef] [PubMed]
- Potel, C.M.; Burtscher, M.L.; Garrido-Rodriguez, M.; Brauer-Nikonow, A.; Becher, I.; Le Sueur, C.; Typas, A.; Zimmermann, M.; Savitski, M.M. Uncovering protein glycosylation dynamics and heterogeneity using deep quantitative glycoprofiling (DQGlyco). Nat. Struct. Mol. Biol. 2025, 32, 1111–1126. [Google Scholar] [CrossRef]
- Dardani, C.; Robinson, J.W.; Jones, H.J.; Rai, D.; Stergiakouli, E.; Grove, J.; Gardner, R.; McIntosh, A.M.; Havdahl, A.; Hemani, G.; et al. Immunological drivers and potential novel drug targets for major psychiatric, neurodevelopmental, and neurodegenerative conditions. Mol. Psychiatry 2025. [Google Scholar] [CrossRef]
- Sırlıer Emir, B.; Yıldız, S.; Kazğan Kılıçaslan, A.; Kurt, O.; Uğur, K.; Tabara, M.F.; Aydın, S. Inflammation Markers in Patients with Bipolar Disorder Who Have Committed Offenses and Their Relationship with Criminal Behavior. Medicina 2023, 59, 1725. [Google Scholar] [CrossRef]
- Kaya, S.; Tasci, G.; Kilic, N.; Karadayi, H.; Ozsoy, F.; Atmaca, M. Examination of the Relationship between Peripheral Inflammation Markers and Impulsivity and Aggression in Schizophrenia Patients Involved and Not Involved in Crime. J. Pers. Med. 2023, 13, 475. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Lee, R.; Coussons-Read, M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. J. Am. Med. Assoc. Psychiatry 2014, 71, 158–165. [Google Scholar] [CrossRef]
- Warren, A.; Nyavor, Y.; Zarabian, N.; Mahoney, A.; Frame, L.A. The microbiota-gut-brain-immune interface in the pathogenesis of neuroinflammatory diseases: A narrative review of the emerging literature. Front. Immunol. 2024, 15, 1365673. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Kumar, V.; Rohilla, A.; Ahire, J.J. Omega-3 fatty acids and the gut microbiome: A new frontier in cardiovascular disease prevention. Discov. Med. 2025, 2, 53. [Google Scholar] [CrossRef]
- Hyży, A.; Rozenek, H.; Gondek, E.; Jaworski, M. Effect of Antioxidants on the Gut Microbiome Profile and Brain Functions: A Review of Randomized Controlled Trial Studies. Foods 2025, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Conly, J.M.; Stein, K. The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog. Food Nutr. Sci. 1992, 16, 307–343. [Google Scholar] [PubMed]
- Selma, M.V.; Tomas-Barberan, F.A.; Beltran, D.; García-Villalba, R.; Espín, J.C. Gordonibacter urolithinfaciens sp. nov., a urolithin-producing bacterium isolated from the human gut. Int. J. Syst. Evol. Microbiol. 2014, 64, 2346–2352. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Mariappan, T.; Jeyaraman, N.; Muthu, S.; Ramasubramanian, S.; Santos, G.S.; da Fonseca, L.F.; Lana, J.F. Gut microbiome: A revolution in type II diabetes mellitus. World J. Diabetes 2024, 15, 1874. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Grundeken, E.; Aidy, S.E. Enteroendocrine cells: The gatekeepers of microbiome-gut-brain communication. NPJ Biofilms Microbiomes 2025, 11, 179. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Bhattacharyya, S.; Brydges, C.R.; Jia, W.; Fiehn, O.; Rush, A.J.; Dunlop, B.W.; Kaddurah-Daouk, R. Gut microbiome-linked metabolites in the pathobiology of major depression with or without anxiety—A role for bile acids. Front. Neurosci. 2022, 16, 937906. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzmanr, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Sanz, Y.; Cryan, J.F.; Deschasaux-Tanguy, M.; Elinav, E.; Lambrecht, R.; Veiga, P. The gut microbiome connects nutrition and human health. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 534–555. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Lathe, R.; Schultek, N.M.; Balin, B.J.; Ehrlich, G.D.; Auber, L.A.; Perry, G.; Breitschwerdt, E.B.; Corry, D.B.; Doty, R.L.; Rissman, R.A.; et al. Establishment of a consensus protocol to explore the brain pathobiome in patients with mild cognitive impairment and Alzheimer’s disease: Research outline and call for collaboration. Alzheimer’s Dement. 2023, 19, 5209–5231. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G.; et al. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflamm. 2015, 12, 223. [Google Scholar] [CrossRef]
- Shanmugam, N.K.; Eimer, W.A.; Kumar, D.K.; Tanzi, R.E. The brain pathobiome in Alzheimer’s disease. Neurotherapeutics 2024, 21, e00475. [Google Scholar] [CrossRef]
- Harrison, N.A.; Brydon, L.; Walker, C.; Gray, M.A.; Steptoe, A.; Critchley, H.D. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry 2009, 66, 407–414. [Google Scholar] [CrossRef]
- Favril, L.; Rich, J.D.; Hard, J.; Fazel, S. Mental and physical health morbidity among people in prisons: An umbrella review. Lancet Public Health 2024, 9, e250–e260. [Google Scholar] [CrossRef]
- Al-Rousan, T.; Rubenstein, L.; Sieleni, B.; Deol, H.; Wallace, R.B. Inside the nation’s largest mental health institution: A prevalence study in a state prison system. BMC Public Health 2017, 17, 342. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Logan, A.C. The Legalome: Nutritional Psychology and Microbiome Sciences at the Intersection of Criminal Justice, Mens Rea, and Mitigation. Crim. Justice Behav. 2024, 52, 990–1004. [Google Scholar] [CrossRef]
- Gkougka, D.; Mitropoulos, K.; Tzanakaki, G.; Panagouli, E.; Psaltopoulou, T.; Thomaidis, L.; Tsolia, M.; Sergentanis, T.N.; Tsitsika, A. Gut microbiome and attention deficit/hyperactivity disorder: A systematic review. Pediatr. Res. 2022, 92, 1507–1519. [Google Scholar] [CrossRef]
- He, Q.; Wang, W.; Xu, D.; Xiong, Y.; Tao, C.; You, C.; Ma, L.; Ma, J. Potential causal association between gut microbiome and posttraumatic stress disorder. Transl. Psychiatry 2024, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Baranova, A.; Cao, H.; Sun, J.; Zhang, F. Gut microbiome and schizophrenia: Insights from two-sample Mendelian randomization. Schizophrenia 2024, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Neurodevelopmental disorders associated with gut microbiome dysbiosis in children. Children. 2024, 11, 796. [Google Scholar] [CrossRef]
- Young, S.; Cocallis, K. ADHD and offending. J. Neural Transm. 2021, 128, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Fairchild, G.; Hawes, D.J.; Frick, P.J.; Copeland, W.E.; Odgers, C.L.; Franke, B.; Freitag, C.M.; De Brito, S.A. Conduct disorder. Nat. Rev. Dis. Primers 2019, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Kelton, K.; Blonigen, D.M.; Keith Mcinnes, D.; Clark, S.; Blue-Howells, J.; Hooshyar, D. A research agenda for criminal justice involvement among US veterans. Mil. Med. 2024, 189, e481–e485. [Google Scholar] [CrossRef]
- Dean, K.; Singh, S.; Soon, Y.L. Decriminalizing severe mental illness by reducing risk of contact with the criminal justice system, including for forensic patients. CNS Spectr. 2020, 25, 687–700. [Google Scholar] [CrossRef]
- Orme, W.; Grimm, S.L.; Vella, D.S.; Fowler, J.C.; Frueh, B.C.; Weinstein, B.L.; Petrosino, J.; Coarfa, C.; Madan, A. Relationships of Personality Traits With the Taxonomic Composition of the Gut Microbiome Among Psychiatric Inpatients. J. Neuropsychiatry Clin. Neurosci. 2025; ahead of print. [Google Scholar]
- Ke, S.; Guimond, A.J.; Tworoger, S.S.; Huang, T.; Chan, A.T.; Liu, Y.Y.; Kubzansky, L.D. Gut feelings: Associations of emotions and emotion regulation with the gut microbiome in women. Psychol. Med. 2023, 53, 7151–7160. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, P.; Costabile, A.; Imakulata, F.; Pandey, N.; Hepsomali, P. A preliminary examination of gut microbiota and emotion regulation in 2-to 6-year-old children. Front. Dev. Psychol. 2024, 2, 1445642. [Google Scholar] [CrossRef]
- An, E.; Delgadillo, D.R.; Yang, J.; Agarwal, R.; Labus, J.S.; Pawar, S.; Leitman, M.; Kilpatrick, L.A.; Bhatt, R.R.; Vora, P.; et al. Stress-resilience impacts psychological wellbeing as evidenced by brain–gut microbiome interactions. Nat. Ment. Health 2024, 2, 935–950. [Google Scholar] [CrossRef]
- Ueda, E.; Matsunaga, M.; Fujihara, H.; Kajiwara, T.; Takeda, A.K.; Watanabe, S.; Hagihara, K.; Myowa, M. Temperament in early childhood is associated with gut microbiota composition and diversity. Dev. Psychobiol. 2024, 66, e22542. [Google Scholar] [CrossRef]
- Sumich, A.; Heym, N.; Lenzoni, S.; Hunter, K. Gut microbiome-brain axis and inflammation in temperament, personality and psychopathology. Curr. Opin. Behav. Sci. 2022, 44, 101101. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, X.; Wu, T.C.; Liu, J.; Le, C.; Tu, X.M.; Knight, R.; Jeste, D.V. Association of loneliness and wisdom with gut microbial diversity and composition: An exploratory study. Front. Psychiatry 2021, 12, 648475. [Google Scholar] [CrossRef]
- Konstanti, P.; Gómez-Martínez, C.; Muralidharan, J.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Tinahones, F.J.; Torres-Collado, L.; Coltell, O.; et al. Faecal microbiota composition and impulsivity in a cohort of older adults with metabolic syndrome. Sci. Rep. 2024, 14, 28075. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, L.; Deng, Z.; Liu, R.; Ma, T.; Xin, Y.; Xie, Y.; Zhou, Y.; Tang, Y. Associations between impulsivity and fecal microbiota in individuals abstaining from methamphetamine. CNS Neurosci. Ther. 2024, 30, e14580. [Google Scholar] [CrossRef]
- Jakobi, B.; Cimetti, C.; Mulder, D.; Vlaming, P.; Franke, B.; Hoogman, M.; Arias-Vasquez, A. The role of diet and the gut microbiota in reactive aggression and adult ADHD—An exploratory analysis. Nutrients 2024, 16, 2174. [Google Scholar] [CrossRef]
- Costanza, A.; Alexander, J.; Amerio, A.; Aguglia, A.; Magnani, L.; Parise, A.; Saverino, D.; Serafini, G.; Amore, M.; Nguyen, K.D. Microbial Dysbiosis as an Emerging Pathology of Suicidal Behaviour? A Critical Review, Passing Through Depression to Chronic Pain. Ann. Neurosci. 2025; ahead of print. [Google Scholar]
- Chen, X.; Xu, J.; Wang, H.; Luo, J.; Wang, Z.; Chen, G.; Jiang, D.; Cao, R.; Huang, H.; Luo, D.; et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int. J. Leg. Med. 2021, 135, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; He, L.; Wang, C.; Zhang, T.; Guo, H.; Zhang, H.; Song, Y.; Chen, B. Altered gut microbiota and its metabolites correlate with plasma cytokines in schizophrenia inpatients with aggression. BMC Psychiatry 2022, 22, 629. [Google Scholar] [CrossRef]
- Konstanti, P.; Ahrens, K.F.; Neumann, R.J.; Plichta, M.M.; Schiweck, C.; Ruf, A.; Fiebach, C.J.; Kalisch, R.; Basten, U.; Wessa, M.; et al. Impulsivity among healthy adults is associated with diet and fecal microbiota composition. Transl. Psychiatry 2025, 15, 263. [Google Scholar] [CrossRef]
- Ioannou, M.; Borkent, J.; Andreu-Sánchez, S.; Wu, J.; Fu, J.; Sommer, I.E.; Haarman, B.C. Reproducible gut microbial signatures in bipolar and schizophrenia spectrum disorders: A metagenome-wide study. Brain Behav. Immun. 2024, 121, 165–175. [Google Scholar] [CrossRef]
- Peralta-Marzal, L.N.; Rojas-Velazquez, D.; Rigters, D.; Prince, N.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P.; Lopez-Rincon, A. A robust microbiome signature for autism spectrum disorder across different studies using machine learning. Sci. Rep. 2024, 14, 814. [Google Scholar] [CrossRef]
- Richarte, V.; Sánchez-Mora, C.; Corrales, M.; Fadeuilhe, C.; Vilar-Ribó, L.; Arribas, L.; Garcia, E.; Rosales-Ortiz, S.K.; Arias-Vasquez, A.; Soler-Artigas, M.; et al. Gut microbiota signature in treatment-naïve attention-deficit/hyperactivity disorder. Transl. Psychiatry 2021, 11, 382. [Google Scholar] [CrossRef]
- Cherro, M.; Itani, H.; Ghossoub, E.; Maalouf, F. Predictors of suicide attempts among adolescents with suicidal ideations and a plan: Results from the National Survey on Drug use and Health (NSDUH). PLoS ONE 2025, 20, e0331261. [Google Scholar] [CrossRef] [PubMed]
- Prandovszky, E.; Liu, H.; Severance, E.G.; Splan, V.W.; Dickerson, F.B.; Yolken, R.H. Altered gut microbial diversity, composition, and metabolomic potential in patients with major depressive disorder and recent suicide attempt. Brain Behav. Immun.-Health 2025, 48, 101081. [Google Scholar] [CrossRef]
- Joos, R.; Boucher, K.; Lavelle, A.; Arumugam, M.; Blaser, M.J.; Claesson, M.J.; Clarke, G.; Cotter, P.D.; De Sordi, L.; Dominguez-Bello, M.G.; et al. Examining the healthy human microbiome concept. Nat. Rev. Microbiol. 2025, 23, 192–205. [Google Scholar] [CrossRef]
- Mulder, D.; Jakobi, B.; Shi, Y.; Mulders, P.; Kist, J.D.; Collard, R.M.; Vrijsen, J.N.; van Eijndhoven, P.; Tendolkar, I.; Bloemendaal, M.; et al. Gut microbiota composition links to variation in functional domains across psychiatric disorders. Brain Behav. Immun. 2024, 120, 275–287. [Google Scholar] [CrossRef]
- Hagenbeek, F.A.; Kluft, C.; Hankemeier, T.; Bartels, M.; Draisma, H.H.; Middeldorp, C.M.; Berger, R.; Noto, A.; Lussu, M.; Pool, R.; et al. Discovery of biochemical biomarkers for aggression: A role for metabolomics in psychiatry. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171, 719–732. [Google Scholar] [CrossRef]
- Hagenbeek, F.A.; van Dongen, J.; Pool, R.; Boomsma, D.I. Twins and Omics: The Role of Twin Studies in Multi-Omics. In Twin Research for Everyone; Tarnocki, A., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 547–584. [Google Scholar]
- Hernández-Cacho, A.; García-Gavilán, J.F.; Atzeni, A.; Konstanti, P.; Belzer, C.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Mela, V.; et al. Multi-omics approach identifies gut microbiota variations associated with depression. NPJ Biofilms Microbiomes 2025, 11, 68. [Google Scholar] [CrossRef]
- Hagenbeek, F.A.; Dongen, J.; Pool, R.; Roetman, P.J.; Harms, A.C.; Hottenga, J.J.; Kluft, C.; Colins, O.F.; van Beijsterveldt, C.E.; Fanos, V.; et al. Integrative multi-omics analysis of childhood aggressive behavior. Behav. Genet. 2023, 53, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Laudani, S.; Torrisi, S.A.; Alboni, S.; Bastiaanssen, T.F.; Benatti, C.; Rivi, V.; Moloney, R.D.; Fuochi, V.; Furneri, P.M.; Drago, F.; et al. Gut microbiota alterations promote traumatic stress susceptibility associated with p-cresol-induced dopaminergic dysfunctions. Brain Behav. Immun. 2023, 107, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Killingsworth, J.; Sawmiller, D.; Shytle, R.D. Propionate and Alzheimer’s disease. Front. Aging Neurosci. 2021, 12, 580001. [Google Scholar] [CrossRef]
- Al Suhaibani, A.; Ben Bacha, A.; Alonazi, M.; Bhat, R.S.; El-Ansary, A. Testing the combined effects of probiotics and prebiotics against neurotoxic effects of propionic acid orally administered to rat pups. Food Sci. Nutr. 2021, 9, 4440–4451. [Google Scholar] [CrossRef]
- Zhvania, M.G.; Lobzhanidze, G.; Pochkhidze, N.; Japaridze, N.; Tchelidze, P.; Rzayev, F.; Gasimov, E. Propionic acid affects the synaptic architecture of rat hippocampus and prefrontal cortex. Micron 2024, 181, 103624. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C.J. Genetic contributions to antisocial personality and behavior: A meta-analytic review from an evolutionary perspective. J. Soc. Psychol. 2010, 150, 160–180. [Google Scholar] [CrossRef]
- Rhee, S.H.; Waldman, I.D. Genetic and environmental influences on aggression. In Human Aggression and Violence: Causes, Manifestations, and Consequences; Shaver, P.R., Mikulincer, M., Eds.; American Psychological Association: Washington, DC, USA, 2011; pp. 143–163. [Google Scholar]
- Bruins, S.; van der Laan, C.M.; Bartels, M.; Dolan, C.V.; Boomsma, D. Genetic and Environmental Influences on Aggression Across the Lifespan: A Longitudinal Twin Study. PsyArXiv. 2024. preprints. Available online: https://osf.io/preprints/psyarxiv/jxmzs_v1 (accessed on 2 July 2025).
- Farahany, N.A.; Robinson, G.E. The rise and fall of the “warrior gene” defense. Science 2021, 371, 1320. [Google Scholar] [CrossRef]
- Tanksley, P.T.; Brislin, S.J.; Wertz, J.; de Vlaming, R.; Courchesne-Krak, N.S.; Mallard, T.T.; Raffington, L.L.; Karlsson Linnér, R.; Koellinger, P.; Palmer, A.A.; et al. Do polygenic indices capture “direct” effects on child externalizing behavior problems? Within-family analyses in two longitudinal birth cohorts. Clin. Psychol. Sci. 2025, 13, 316–331. [Google Scholar] [CrossRef]
- Farsetti, A.; Illi, B.; Gaetano, C. How epigenetics impacts on human diseases. Eur. J. Intern. Med. 2023, 114, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Nohesara, S.; Abdolmaleky, H.M.; Dickerson, F.; Pinto-Tomás, A.A.; Jeste, D.V.; Thiagalingam, S. Maternal Gut Microbiome-Mediated Epigenetic Modifications in Cognitive Development and Impairments: A New Frontier for Therapeutic Innovation. Nutrients 2024, 16, 4355. [Google Scholar] [CrossRef]
- Sahebi, K.; Arianejad, M.; Azadi, S.; Hosseinpour-Soleimani, F.; Kazemi, R.; Tajbakhsh, A.; Negahdaripour, M. The Interplay between Gut Microbiome, Epigenetics, and Substance Use Disorders: From Molecular to Clinical Perspectives. Eur. J. Pharmacol. 2025, 998, 177630. [Google Scholar] [CrossRef]
- Odintsova, V.V.; Hagenbeek, F.A.; Van der Laan, C.M.; Van de Weijer, S.; Boomsma, D.I. Genetics and epigenetics of human aggression. Handb. Clin. Neurol. 2023, 197, 13–44. [Google Scholar] [PubMed]
- Bohare, N. Exploring the Role of Epigenetic Modifications in Criminal Behavior: Implications for Prevention and Intervention. Int. J. Innov. Res. Technol. Sci. 2024, 12, 434–441. [Google Scholar]
- Yuan, S.; Zhu, T.; Gu, J.; Hua, L.; Sun, J.; Deng, X.; Ran, J. Associations of Ultra-Processed Food Intake and Its Circulating Metabolomic Signature with Mental Disorders in Middle-Aged and Older Adults. Nutrients 2025, 17, 1582. [Google Scholar] [CrossRef]
- Abar, L.; Steele, E.M.; Lee, S.K.; Kahle, L.; Moore, S.C.; Watts, E.; O’Connell, C.P.; Matthews, C.E.; Herrick, K.A.; Hall, K.D.; et al. Identification and validation of poly-metabolite scores for diets high in ultra-processed food: An observational study and post-hoc randomized controlled crossover-feeding trial. PLoS Med. 2025, 22, e1004560. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Shemirani, P.; Sood, T.; Paré, G. From omics to multi-omics technologies: The discovery of novel causal mediators. Curr. Atheroscler. Rep. 2023, 25, 55–65. [Google Scholar] [CrossRef]
- Men, J.; Cui, C.; Li, H.; Li, Z.; Zhang, Y.; Liu, Z.; Wang, Q.; Liu, P.; Zou, S.; Yu, Z.; et al. Cold water swimming reshapes gut microbiome to improve high-fat diet-induced obesity. Front. Microbiol. 2025, 16, 1589902. [Google Scholar] [CrossRef]
- Wei, N.; Ju, M.; Su, X.; Zhang, Y.; Huang, Y.; Rao, X.; Cui, L.; Lin, Z.; Dong, Y. Transplantation of gut microbiota derived from patients with schizophrenia induces schizophrenia-like behaviors and dysregulated brain transcript response in mice. Schizophrenia 2024, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yan, J.; Yang, T.; Zhu, J.; Li, T.; Wei, H.; Chen, J. Fecal Microbiome Transplantation from Children with Autism Spectrum Disorder Modulates Tryptophan and Serotonergic Synapse Metabolism and Induces Altered Behaviors in Germ-Free Mice. mSystems 2021, 6, e01343-20. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Wolstenholme, J.T.; Saunders, J.M.; Smith, M.; Kang, J.D.; Hylemon, P.B.; Gonzalez-Maeso, J.; Fagan, A.; Zhao, D.; Sikaroodi, M.; Herzog, J.; et al. Reduced alcohol preference and intake after fecal transplant in patients with alcohol use disorder is transmissible to germ-free mice. Nat. Commun. 2022, 13, 6198. [Google Scholar] [CrossRef]
- Wang, T.; Hao, L.; Yang, K.; Feng, W.; Guo, Z.; Liu, M.; Xiao, R. Fecal microbiota transplantation derived from mild cognitive impairment individuals impairs cerebral glucose uptake and cognitive function in wild-type mice: Bacteroidetes and TXNIP-GLUT signaling pathway. Gut Microbes 2024, 16, 2395907. [Google Scholar] [CrossRef]
- Cai, M.; Xue, S.S.; Zhou, C.H.; Feng, Y.C.; Liu, J.Z.; Liu, R.; Wang, P.; Wang, H.N.; Peng, Z.W. Effects of fecal microbiota transplantation from patients with generalized anxiety on anxiety-like behaviors: The role of the gut-microbiota-endocannabinoid-brain Axis. J. Affect. Disord. 2025, 381, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, P.; Tong, J.; Yan, S.; Gao, G.; Tao, F.; Huang, K. Effects of Early-life Antibiotic Use on Emotional and Behavioral Development Trajectories in Preschool Children. J. Psychiatr. Res. 2025, 186, 244–251. [Google Scholar] [CrossRef]
- Uzan-Yulzari, A.; Turjeman, S.; Moadi, L.; Getselter, D.; Sharon, E.; Rautava, S.; Isolauri, E.; Khatib, S.; Elliott, E.; Koren, O. A gut reaction? The role of the microbiome in aggression. Brain Behav. Immun. 2024, 122, 301–312. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, C.; Zhang, Q.; Lv, Q.; Liu, H.; Yuan, H.; Wang, C.; Meng, F.; Guo, Y.; Pei, J.; et al. Gut microbiota modulates depressive-like behaviors induced by chronic ethanol exposure through short-chain fatty acids. J. Neuroinflamm. 2024, 21, 290. [Google Scholar] [CrossRef]
- Saeedi, N.; Pourabdolhossein, F.; Dadashi, M.; Suha, A.J.; Janahmadi, M.; Behzadi, G.; Hosseinmardi, N. Faecal Microbiota Transplantation Modulates Morphine Addictive—Like Behaviours Through Hippocampal Metaplasticity. Addict. Biol. 2025, 30, e70034. [Google Scholar] [CrossRef]
- Dong, X.; Su, Y.; Luo, Z.; Li, C.; Gao, J.; Han, X.; Yao, S.; Wu, W.; Tian, L.; Bai, Y.; et al. Fecal microbiota transplantation alleviates cognitive impairment by improving gut microbiome composition and barrier function in male rats of traumatic brain injury following gas explosion. Front. Microbiol. 2024, 15, 1485936. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C.; Mishra, P.; Prescott, S.L. The Legalome: Microbiology, Omics and Criminal Justice. Microb. Biotechnol. 2025, 18, e70129. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chang, K.T.; Chang, F. Just a gut feeling: Faecal microbiota transplant for treatment of depression–A mini-review. J. Psychopharmacol. 2024, 38, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Castells-Nobau, A.; Puig, I.; Motger-Albertí, A.; de la Vega-Correa, L.; Rosell-Díaz, M.; Arnoriaga-Rodríguez, M.; Escrichs, A.; Garre-Olmo, J.; Puig, J.; Ramos, R.; et al. Microviridae bacteriophages influence behavioural hallmarks of food addiction via tryptophan and tyrosine signalling pathways. Nat. Metab. 2024, 6, 2157–2186. [Google Scholar] [CrossRef]
- Matsuo, K.; Haku, A.; Bi, B.; Takahashi, H.; Kamada, N.; Yaguchi, T.; Saijo, S.; Yoneyama, M.; Goto, Y. Fecal microbiota transplantation prevents Candida albicans from colonizing the gastrointestinal tract. Microbiol. Immunol. 2019, 63, 155–163. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, H.; Qin, X.; Song, H.; Yang, M.; Zhang, L.; Liu, R. Is anxiety and depression transmissible? Depressed mother rats transmit anxiety-and depression-like phenotypes to cohabited rat pups through gut microbiota assimilation. J. Affect. Disord. 2024, 366, 124–135. [Google Scholar] [CrossRef]
- Yirmiya, K.; Turjeman, S.; Shtossel, O.; Zagoory-Sharon, O.; Moadi, L.; Rubin, E.; Sharon, E.; Louzoun, Y.; Koren, O.; Feldman, R. Microbiome signature of posttraumatic stress disorder and resilience in youth. Psychol. Trauma Theory Res. Pract. Policy, 2024; ahead of print. [Google Scholar]
- Ritz, N.L.; Brocka, M.; Butler, M.I.; Cowan, C.S.; Barrera-Bugueño, C.; Turkington, C.J.; Draper, L.A.; Bastiaanssen, T.F.; Turpin, V.; Morales, L.; et al. Social anxiety disorder-associated gut microbiota increases social fear. Proc. Natl. Acad. Sci. USA 2024, 121, e2308706120. [Google Scholar] [CrossRef]
- Pillai, S. Are Microbes Responsible for Criminal Behavior? TEDx Talks. Available online: https://www.youtube.com/watch?v=Y1ldMa9vpqM (accessed on 2 July 2025).
- Borrego-Ruiz, A.; Borrego, J.J. Fecal Microbiota Transplantation as a Tool for Therapeutic Modulation of Neurological and Mental Disorders. SciBase Neurol. 2024, 2, 1018. [Google Scholar] [CrossRef]
- He, H.; Li, M.; Qiu, Y.; Wu, Z.; Wu, L. Washed microbiota transplantation improves sleep quality in patients with sleep disorder by the gut-brain axis. Front. Neurosci. 2024, 18, 1415167. [Google Scholar] [CrossRef]
- Hu, D.X.; Lu, C.M.; Si, X.Y.; Wu, Q.T.; Wu, L.H.; Zhong, H.J.; He, X.X. Effects of gastrointestinal symptoms on the efficacy of washed microbiota transplantation in patients with autism. Front. Pediatr. 2025, 13, 1528167. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Sun, J.; Zhang, G. Pick fecal microbiota transplantation to enhance therapy for major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2024, 128, 110860. [Google Scholar] [CrossRef]
- Hazan, S.; Haroon, J.; Jordan, S.; Walker, S.J. Improvements in gut microbiome composition and clinical symptoms following familial fecal microbiota transplantation in a nineteen-year-old adolescent with severe autism. J. Med. Cases 2024, 15, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yao, T.; Li, W.; Pan, N.; Xu, H.; Zhao, Q.; Wang, J. Efficacy and safety of fecal microbiota transplantation for chronic insomnia in adults: A real world study. Front. Microbiol. 2022, 14, 1299816. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Gavis, E.A.; Fagan, A.; Wade, J.B.; Thacker, L.R.; Fuchs, M.; Patel, S.; Davis, B.; Meador, J.; Puri, P.; et al. A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology 2021, 73, 1688–1700. [Google Scholar] [CrossRef]
- Casañas-Martínez, M.; Barbero-Herranz, R.; Alegre-González, D.; Mosquera-Lozano, J.D.; Del Campo, R.; Llorente-Artero, M.; Ponce-Alonso, M.; Baeza-Trinidad, R. Fecal microbiota transplantation in a long-standing auto-brewery syndrome with complex symptomatology. J. Hepatol. 2025, 82, e186–e188. [Google Scholar] [CrossRef]
- Vandekerckhove, E.; Janssens, F.; Tate, D.; De Looze, D. Treatment of gut fermentation syndrome with fecal microbiota transplantation. Ann. Intern. Med. 2020, 173, 855. [Google Scholar] [CrossRef]
- Wilson, B.C.; Zuppi, M.; Derraik, J.G.B.; Albert, B.B.; Tweedie-Cullen, R.Y.; Leong, K.S.W.; Beck, K.L.; Vatanen, T.; O’Sullivan, J.M.; Cutfield, W.S. Gut Bugs Study Group. Long-term health outcomes in adolescents with obesity treated with faecal microbiota transplantation: 4-year follow-up. Nat. Commun. 2025, 16, 7786. [Google Scholar] [CrossRef]
- Zhang, N.; Dong, X. Causal relationship between gut microbiota, lipids, and neuropsychiatric disorders: A Mendelian randomization mediation study. J. Affect. Disord. 2025, 379, 19–35. [Google Scholar] [CrossRef]
- Xu, W.M.; Zhang, H.F.; Feng, Y.H.; Li, S.J.; Xie, B.Y. Genetically predicted fatty liver disease and risk of psychiatric disorders: A Mendelian randomization study. World J. Clin. Cases 2024, 12, 2359. [Google Scholar] [CrossRef]
- Fatoba, A.; Simpson, C. Exploring the potential causal association of gut microbiota on panic and conduct disorder: A two-sample Mendelian randomization approach. J. Affect. Disord. 2025, 385, 119312. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, M.; Wang, R. Causal link between gut microbiome and schizophrenia: A Mendelian randomization study. Psychiatr. Genet. 2024, 34, 43–53. [Google Scholar] [CrossRef]
- Zhao, Q.; Cao, H.; Baranova, A.; Zhang, F. Evaluating Causal Effects of Gut Microbiome on Bipolar Disorder. Bipolar Disord. 2025; ahead of print. [Google Scholar]
- Wang, X.; Pan, L.; Gu, J.; Gu, L.; Lou, M.; Liu, Y. Associations Between Gut Microbiota and Alcohol Abuse: A Mendelian Randomisation and Bioinformatics Study. J. Mol. Neurosci. 2024, 74, 80. [Google Scholar] [CrossRef]
- Ma, Z.; Zhao, H.; Zhao, M.; Zhang, J.; Qu, N. Gut microbiotas, inflammatory factors, and mental-behavioral disorders: A mendelian randomization study. J. Affect. Disord. 2025, 371, 113–123. [Google Scholar] [CrossRef]
- Montazeri, R.S.; Shidfar, F.; Hosseini-Baharanchi, F.S.; Kholasezadeh, G. The Effect of Synbiotic Supplementation on Self-Reported Aggression in Healthy Adult Men: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Nutr. Food Secur. 2025, 10, 124–133. [Google Scholar] [CrossRef]
- Eastwood, J.; van Hemert, S.; Stolaki, M.; Williams, C.; Walton, G.; Lamport, D. Exploring the acute and chronic effects of a multistrain probiotic supplement on cognitive function and mood in healthy older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2025, 121, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Marticella, M.; Arija, V.; Canals-Sans, J. Effect of Probiotics on the Symptomatology of Autism Spectrum Disorder and/or Attention Deficit/Hyperactivity Disorder in Children and Adolescents: Pilot Study. Res. Child Adolesc. Psychopathol. 2025, 53, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Roman, P.; Estévez, A.F.; Miras, A.; Sánchez-Labraca, N.; Cañadas, F.; Vivas, A.B.; Cardona, D. A pilot randomized controlled trial to explore cognitive and emotional effects of probiotics in fibromyalgia. Sci. Rep. 2018, 8, 10965. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gonzalez, C.; Cardona, D.; Rueda-Ruzafa, L.; Rodriguez-Arrastia, M.; Ropero-Padilla, C.; Roman, P. Cognitive and Emotional Effect of a Multi-species Probiotic Containing Lactobacillus rhamnosus and Bifidobacterium lactis in Healthy Older Adults: A Double-Blind Randomized Placebo-Controlled Crossover Trial. Probiotics Antimicrob. Proteins, 2024; ahead of print. [Google Scholar]
- Marotta, A.; Sarno, E.; Del Casale, A.; Pane, M.; Mogna, L.; Amoruso, A.; Felis, G.E.; Fiorio, M. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Front. Psychiatry 2019, 10, 164. [Google Scholar] [CrossRef]
- Huang, P.W.; Chia-Min, C.; Sun, C.K.; Cheng, Y.S.; Tang, Y.H.; Liu, C.; Hung, K.C. Therapeutic effects of probiotics on symptoms of irritability/emotional lability associated with neurodevelopmental conditions: A systematic review and meta-analysis of placebo-controlled trials. Complement. Ther. Med. 2025, 89, 103132. [Google Scholar] [CrossRef]
- Johnson, K.V.; Steenbergen, L. Probiotics reduce negative mood over time: The value of daily self-reports in detecting effects. NPJ Ment. Health Res. 2025, 4, 10. [Google Scholar] [CrossRef]
- Falkenstein, M.; Simon, M.C.; Mantri, A.; Weber, B.; Koban, L.; Plassmann, H. Impact of the gut microbiome composition on social decision-making. PNAS Nexus 2024, 3, pgae166. [Google Scholar] [CrossRef]
- Dantas, A.M.; Sack, A.T.; Bruggen, E.; Jiao, P.; Schuhmann, T. The effects of probiotics on risk and time preferences. Sci. Rep. 2022, 12, 12152. [Google Scholar] [CrossRef]
- Niu, Q.; Wang, W.; Liang, Y.; Fang, S.; Wan, L.; Yang, G. Efficacy and Safety of a Probiotic Mixture Containing Bifidobacterium animalis subsp. lactis BLa80 and Lacticaseibacillus rhamnosus LRa05 in Children With Attention-Deficit/Hyperactivity Disorder. Mol. Nutr. Food Res. 2025; ahead of print. [Google Scholar]
- Baião, R.; Capitão, L.P.; Higgins, C.; Browning, M.; Harmer, C.J.; Burnet, P.W. Multispecies probiotic administration reduces emotional salience and improves mood in subjects with moderate depression: A randomised, double-blind, placebo-controlled study. Psychol. Med. 2023, 53, 3437–3447. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef]
- Robinson, J.M.; Cameron, R. The Holobiont blindspot: Relating host-microbiome interactions to cognitive biases and the concept of the “umwelt”. Front. Psychol. 2020, 11, 591071. [Google Scholar] [CrossRef] [PubMed]
- Van Draanen, J.; Tsang, C.; Mitra, S.; Karamouzian, M.; Richardson, L. Socioeconomic marginalization and opioid-related overdose: A systematic review. Drug Alcohol Depend. 2020, 214, 108127. [Google Scholar] [CrossRef] [PubMed]
- De Courson, B.; Nettle, D. Why do inequality and deprivation produce high crime and low trust? Sci. Rep. 2021, 11, 1937. [Google Scholar] [CrossRef]
- Barnert, E.S.; Schlichte, L.M.; Tolliver, D.G.; La Charite, J.; Biely, C.; Dudovitz, R.; Leifheit, K.; Russ, S.; Sastry, N.; Yama, C.; et al. Parents’ adverse and positive childhood experiences and offspring involvement with the criminal legal system. J. Am. Med. Assoc. Netw. Open 2023, 6, e2339648. [Google Scholar] [CrossRef]
- Hawks, L.C.; Walker, R.J.; Egede, L.E. Association Between Social Adaptability Index Score and Lifetime Criminal Legal Involvement in US Adults. Health Equity 2022, 6, 240–247. [Google Scholar] [CrossRef]
- DeMarco, L.M.; Dwyer, R.E.; Haynie, D.L. The accumulation of disadvantage: Criminal justice contact, credit, and debt in the transition to adulthood. Criminology 2021, 59, 545–580. [Google Scholar] [CrossRef]
- López-Moreno, A.; Torres-Sánchez, A.; Suárez, A.; Ruiz-Rodríguez, A.; Aguilera, M. Perinatal bisphenol A exposure impairs gut microbial colonization: Implications for offspring obesity and neurodevelopment. Ecotoxicol. Environ. Saf. 2025, 298, 118295. [Google Scholar] [CrossRef] [PubMed]
- Zuniga-Chaves, I.; Eggers, S.; Kates, A.E.; Safdar, N.; Suen, G.; Malecki, K.M. Neighborhood socioeconomic status is associated with low diversity gut microbiomes and multi-drug resistant microorganism colonization. NPJ Biofilms Microbiomes 2023, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Usyk, M.; Beggs, D.; Choi, H.; Ahdoot, D.; Wu, F.; Maceda, L.; Li, H.; Im, E.O.; Han, H.R.; et al. Sociobiome-Individual and neighborhood socioeconomic status influence the gut microbiome in a multi-ethnic population in the US. NPI Biofilms Microbiomes 2024, 10, 19. [Google Scholar] [CrossRef]
- Batalha, M.A.; LeCroy, M.N.; Lin, J.; Peters, B.A.; Qi, Q.; Wang, Z.; Wang, T.; Gallo, L.C.; Talavera, G.A.; McClain, A.C.; et al. Life-course socioeconomic position and the gut microbiome in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Gut Microbes 2025, 17, 2479772. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, C.C. From Mouse to Man: How the Human Post Mortem Microbiome Relates to Local Area Crime Level. Ph.D. Dissertation, Michigan State University, East Lansing, MI, USA, 2022. [Google Scholar]
- Bai, Y.; Shu, C.; Zhang, S.; Gui, G.; Zhang, R.; Yu, S.; Dong, C.; Duan, R.; Ai, X.; Liu, R.; et al. The role of gut microbiota in adolescent depression: Insights from adverse childhood experiences. J. Affect. Disord. 2025, 392, 120141. [Google Scholar] [CrossRef]
- Langmajerová, M.; Ježková, J.; Kreisinger, J.; Semerád, J.; Titov, I.; Procházková, P.; Cajthaml, T.; Jiřička, V.; Vevera, J.; Roubalová, R. Gut microbiome in impulsively violent female convicts. Neuropsychobiology 2025, 84, 1–14. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, X.; Yang, Y.; Gu, L.; Liu, L.; Yang, Y.; Zhou, J.; Wu, C.; Jin, F. Marked shifts in gut microbial structure and neurotransmitter metabolism in fresh inmates revealed a close link between gut microbiota and mental health: A case-controlled study. Int. J. Clin. Health Psychol. 2022, 22, 100323. [Google Scholar] [CrossRef]
- Mikhael-Moussa, H.; Desprez, C.; Gillibert, A.; Leroi, A.M.; Mion, F.; Gourcerol, G.; Melchior, C. Is Carbohydrate Intolerance associated with carbohydrate malabsorption in Disorders of Gut-Brain Interaction (DGBI)? Am. J. Gastroenterol. ACG, 2022; ahead of print. [Google Scholar]
- El Halabi, M.; Arwani, R.; Rao, S.C.; Parkman, H.P. Brain Fog in Gastrointestinal Disorders: Small Intestinal Bacterial Overgrowth, Gastroparesis, Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2024, 59, 842–848. [Google Scholar] [CrossRef]
- Alim-Marvasti, A.; Ciocca, M.; Kuleindiren, N.; Lin, A.; Selim, H.; Mahmud, M. Subjective brain fog: A four-dimensional characterization in 25,796 participants. Front. Hum. Neurosci. 2024, 18, 1409250. [Google Scholar] [CrossRef]
- Hunnisett, A.; Howard, J.; Davies, S. Gut fermentation (or the ‘auto-brewery’) syndrome: A new clinical test with initial observations and discussion of clinical and biochemical implications. J. Nutr. Med. 1990, 1, 33–38. [Google Scholar] [CrossRef]
- Alastair, M. Sweet Tooth Could Lead to Driving Ban; The Daily Telegraph: London, UK, 1990; Volume 9. [Google Scholar]
- Eaton, K.K.; Chan, R.; Howard, M.A.; McLaren-Howard, J.M. A Comparison of Lactulose Breath Hydrogen Measurements with Gut Fermentation Profiles in Patients with Fungal-type Dysbiosis. J. Nutr. Environ. Med. 2004, 14, 171–180. [Google Scholar] [CrossRef]
- Panculescu, F.G.; Catrinoiu, D.; Mihai, C.M.; Chisnoiu, T. The predictive utility of one-hour plasma glucose in oral glucose tolerance test: A study of 231 nondiabetic patients. Rom. J. Diabetes Nutr. Metab. Dis. 2024, 31, 404–410. [Google Scholar]
- Yuan, J.; Chen, C.; Cui, J.; Lu, J.; Yan, C.; Wei, X.; Zhao, X.; Li, N.; Li, S.; Xue, G.; et al. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab. 2019, 30, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Callas, P.W.; Alexander, K.S.; Wadley, V.; Zakai, N.A.; Lidofsky, S.D.; Unverzagt, F.W.; Judd, S.E. Nonalcoholic fatty liver disease and cognitive impairment: A prospective cohort study. PLoS ONE 2023, 18, e0282633. [Google Scholar] [CrossRef]
- Quesada-Vázquez, S.; Bone, C.; Saha, S.; Triguero, I.; Colom-Pellicer, M.; Aragonès, G.; Hildebrand, F.; Del Bas, J.M.; Caimari, A.; Beraza, N.; et al. Microbiota dysbiosis and gut barrier dysfunction associated with non-alcoholic fatty liver disease are modulated by a specific metabolic cofactors’ combination. Int. J. Mol. Sci. 2022, 23, 13675. [Google Scholar] [CrossRef]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-Producing Bacteria in Gut Microbiome of Human NAFLD as a Putative Link to Systemic T-Cell Activation and Advanced Disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef]
- Schwiertz, A.; Taras, D.; Schäfer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in Lean and Overweight Healthy Subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Y.N.; Zhang, J.; Liu, J. Elevated serum levels of diamine oxidase, D-lactate and lipopolysaccharides are associated with metabolic-associated fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2023, 35, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Rehman, A.; Yu, S.; De Andino, N.M. Brain fogginess, gas and bloating: A link between SIBO, probiotics and metabolic acidosis. Clin. Transl. Gastroenterol. 2018, 9, e162. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, J.R.; Wettenhall, R.E.; Scanlon, D.; Gooley, P.R.; Lewis, D.P.; Mcgregor, N.; Stapleton, D.I.; Butt, H.L.; Meirleir, K.L. Increased d-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. Vivo 2009, 23, 621–628. [Google Scholar]
- Liu, S.; Zhang, L.; Fan, X.; Wang, G.; Liu, Q.; Yang, Y.; Shao, M.; Song, M.; Li, W.; Lv, L.; et al. Lactate levels in the brain and blood of schizophrenia patients: A systematic review and meta-analysis. Schizophr. Res. 2024, 264, 29–38. [Google Scholar] [CrossRef]
- Shan, B.; Ai, Z.; Zeng, S.; Song, Y.; Song, J.; Zeng, Q.; Liao, Z.; Wang, T.; Huang, C.; Su, D. Gut microbiome-derived lactate promotes to anxiety-like behaviors through GPR81 receptor-mediated lipid metabolism pathway. Psychoneuroendocrinology 2020, 117, 104699. [Google Scholar] [CrossRef]
- Hanstock, T.L.; Clayton, E.H.; Li, K.M.; Mallet, P.E. Anxiety and aggression associated with the fermentation of carbohydrates in the hindgut of rats. Physiol. Behav. 2004, 82, 357–368. [Google Scholar] [CrossRef]
- Henneman, D.H.; Altschule, M.D.; Goncz, R.M. Carbohydrate metabolism in brain disease: II. Glucose metabolism in schizophrenic, manic-depressive, and involutional psychoses. AMA Arch. Intern. Med. 1954, 94, 402–416. [Google Scholar] [CrossRef]
- Henneman, D.H.; Altschule, M.D.; Goncz, R.M. Carbohydrate Metabolism in Brain Disease: III. Fructose Metabolism in Schizophrenic, Manic-Depressive, and Involutional Psychoses. AMA Arch. Neurol. Psychiatry 1954, 72, 696–704. [Google Scholar] [CrossRef]
- Altschule, M.D.; Henneman, D.H.; Goncz, R.M. Carbohydrate Metabolism in Brain Disease: VI. Lactate Metabolism after Infusion of Sodium d-Lactate in Manic-Depressive and Schizophrenic Psychoses. AMA Arch. Intern. Med. 1956, 98, 35–38. [Google Scholar] [CrossRef]
- Zada, D.K.; Fang, H.; Gagnon, C.; Tchernof, A.; Marette, A.; Schertzer, J. 9171 Microbial D-lactate Contributes To Hyperglycemia During Obesity And Is A Potential Therapeutic Target. J. Endocr. Soc. 2024, 8, bvae163–bvae849. [Google Scholar] [CrossRef]
- Fang, H.; Anhê, F.F.; Zada, D.K.; Barra, N.G.; E-Lacerda, R.R.; McAlpin, B.T.; Wylie, R.; Berthiaume, L.; Audet-Walsh, É.; O’Dwyer, C.; et al. Gut substrate trap of D-lactate from microbiota improves blood glucose and fatty liver disease in obese mice. Cell Metab. 2025, 37, 1806–1819.e7. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.S.; Sperber, A.D.; Bangdiwala, S.; Whitehead, W.E. Prevalence and associated factors of disorders of gut-brain interaction in the United States: Comparison of two nationwide internet surveys. Neurogastroenterol. Motil. 2023, 35, e14564. [Google Scholar] [CrossRef]
- Kalligeros, M.; Vassilopoulos, A.; Vassilopoulos, S.; Victor, D.W.; Mylonakis, E.; Noureddin, M. Prevalence of steatotic liver disease (MASLD, MetALD, and ALD) in the United States: NHANES 2017–2020. Clin. Gastroenterol. Hepatol. 2024, 22, 1330–1332. [Google Scholar] [CrossRef]
- Fava, M.; Anderson, K.; Rosenbaum, J.F. “ Anger attacks”: Possible variants of panic and major depressive disorders. Am. J. Psychiatry 1990, 147, 867–870. [Google Scholar]
- Fava, M.; Rosenbaum, J.F.; McCarthy, M.; Pava, J.; Steingard, R.; Bless, E. Anger attacks in depressed outpatients and their response to fluoxetine. Psychopharmacol. Bull. 1991, 27, 275–279. [Google Scholar] [CrossRef]
- Cox, B.J.; Swinson, R.P.; Endler, N.S.; Norton, G.R. The symptom structure of panic attacks. Compr. Psychiatry 1994, 35, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Fava, M.; Minhajuddin, A.; Chin Fatt, C.; Mischoulon, D.; Cusin, C.; Trivedi, M.H. Association of anger attacks with suicidal ideation in adults with major depressive disorder: Findings from the EMBARC study. Depress. Anxiety 2021, 38, 57–66. [Google Scholar] [CrossRef]
- Jha, M.K.; Fava, M.; Minhajuddin, A.; Fatt, C.C.; Mischoulon, D.; Wakhlu, N.; Trombello, J.M.; Cusin, C.; Trivedi, M.H. Anger attacks are associated with persistently elevated irritability in MDD: Findings from the EMBARC study. Psychol. Med. 2021, 51, 1355–1363. [Google Scholar] [CrossRef]
- Hoşgören Alici, Y.; Ceran, S.; Hasanli, J.; Asut, G.; Özel, B.; Ucar Hasanli, Z.; Saygi, G.; Bağcaz, A.; Misir, E. Why Do Some Depressive Patients Have Suicidal Ideation but Others Not? Suicidal Ideation From the Perspective of Affective Neuroscience Personality Traits. Brain Behav. 2024, 14, e70077. [Google Scholar] [CrossRef]
- Punzi, G.; Ursini, G.; Chen, Q.; Radulescu, E.; Tao, R.; Huuki, L.A.; Di Carlo, P.; Collado-Torres, L.; Shin, J.H.; Catanesi, R.; et al. Genetics and brain transcriptomics of completed suicide. Am. J. Psychiatry 2022, 179, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Dumais, A.; Lesage, A.D.; Lalovic, A.; Séguin, M.; Tousignant, M.; Chawky, N.; Turecki, G. Is violent method of suicide a behavioral marker of lifetime aggression? Am. J. Psychiatry 2005, 162, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Kozhakhmetov, S.; Kossumov, A.; Zhakupova, T.; Polyakova, T.; Imambayeva, N.; Syzdykova, B.; Rakhmankulova, A.; Dalibayeva, G.; Kovenskiy, A.; Jarmukhanov, Z.; et al. Characterization of Gut Microbiome Composition in Depression and Completed Suicide. Int. J. Mol. Sci. 2025, 26, 4880. [Google Scholar] [CrossRef]
- Pedersen, M.G.; Mortensen, P.B.; Norgaard-Pedersen, B.; Postolache, T.T. Toxoplasma gondii infection and self-directed violence in mothers. Arch. Gen. Psychiatry 2012, 69, 1123–1130. [Google Scholar] [CrossRef]
- Cook, T.B.; Brenner, L.A.; Cloninger, C.R.; Langenberg, P.; Igbide, A.; Giegling, I.; Hartmann, A.M.; Konte, B.; Friedl, M.; Brundin, L.; et al. “Latent” infection with Toxoplasma gondii: Association with trait aggression and impulsivity in healthy adults. J. Psychiatr. Res. 2015, 60, 87–94. [Google Scholar] [CrossRef]
- Martinez, V.O.; de Mendonça Lima, F.W.; De Carvalho, C.F.; Menezes-Filho, J.A. Toxoplasma gondii infection and behavioral outcomes in humans: A systematic review. Parasitol. Res. 2018, 117, 3059–3065. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, X.; Chen, M.; Hu, Y.; Chang, Y.; Wu, X. Research Progress on the Association between Schizophrenia and Toxoplasma gondii Infection. Biomed. Environ. Sci. 2024, 37, 647–660. [Google Scholar]
- Postolache, T.T.; Duncan, E.; Yen, P.; Potocki, E.; Barnhart, M.; Federline, A.; Massa, N.; Dagdag, A.; Joseph, J.; Wadhawan, A.; et al. Toxoplasma gondii, suicidal behaviour and suicide risk factors in US Veterans enrolled in mental health treatment. Folia Parasitol. 2025, 72, 1–24. [Google Scholar] [CrossRef]
- Zerekidze, A.; Li, M.; Refisch, A.; Shameya, J.; Sobanski, T.; Walter, M.; Wagner, G. Impact of Toxoplasma gondii and human microbiome on suicidal behavior: A systematic review. J. Clin. Med. 2024, 13, 593. [Google Scholar] [CrossRef]
- Akaltun, İ.; Kara, T.; Ayaydın, H.; Alyanak, B.; Beka, H.; Ağaçfidan, A. The relation between serum Toxoplasma gondii IgG antibody in children and ADHD and its severity. Psychiatry Clin. Psychopharmacol. 2019, 29, 326–331. [Google Scholar] [CrossRef]
- Yereli, K.; Balcioğlu, I.C.; Özbilgin, A. Is Toxoplasma gondii a potential risk for traffic accidents in Turkey? Forensic Sci. Int. 2006, 163, 34–37. [Google Scholar] [CrossRef]
- Kocazeybek, B.; Oner, Y.A.; Turksoy, R.; Babur, C.; Cakan, H.; Sahip, N.; Unal, A.; Ozaslan, A.; Kılıc, S.; Saribas, S.; et al. Higher prevalence of toxoplasmosis in victims of traffic accidents suggest increased risk of traffic accident in Toxoplasma-infected inhabitants of Istanbul and its suburbs. Forensic Sci. Int. 2009, 18, 103–108. [Google Scholar] [CrossRef]
- Stepanova, E.V.; Kondrashin, A.V.; Sergiev, V.P.; Morozova, L.F.; Turbabina, N.A.; Maksimova, M.S.; Brazhnikov, A.I.; Shevchenko, S.B.; Morozov, E.N. Significance of chronic toxoplasmosis in epidemiology of road traffic accidents in Russian Federation. PLoS ONE 2017, 12, e0184930. [Google Scholar] [CrossRef]
- Flegr, J.; Havlícek, J.; Kodym, P.; Malý, M.; Smahel, Z. Increased risk of traffic accidents in subjects with latent toxoplasmosis: A retrospective case-control study. BMC Infect. Dis. 2002, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Abdelati Abdelsalam, A.A.; Woods, S.; Henriquez, S.; Curran, L.; Westrop, G.; Roberts, C.W. Toxoplasma gondii infection of BALB/c mice perturbs host neurochemistry. Parasite Immunol. 2024, 46, e13073. [Google Scholar] [CrossRef]
- Everett, A.; Elsheikha, H.M. Neuroinflammation and schizophrenia: The role of Toxoplasma gondii infection and astrocytic dysfunction. J. Neuroimmunol. 2025, 403, 578588. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, Y.; Cui, B.; Yang, J.; Yuan, B.; Cao, Y.; Zhao, Z.; Sun, Z.; Wang, Q.; Yang, X.; et al. Gut microbiota-derived butyrate alleviates the impairment of mice intestinal integrity caused by toxoplasma gondii infection. Life Sci. 2025, 374, 123709. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, C.; Yang, Z.; Wu, W.; Zou, W.; Xin, Z.; Zheng, S.; Liu, R.; Yang, L.; Peng, H. Intestinal microbiota imbalance resulted by anti-Toxoplasma gondii immune responses aggravate gut and brain injury. Parasites Vectors 2024, 17, 284. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Tan, S.; Tian, X.; Meng, X.; Li, Y.; Zhou, B.; Zhao, G.; Ge, X.; He, C.; et al. Alterations in gut microbiota contribute to cognitive deficits induced by chronic infection of Toxoplasma gondii. Brain Behav. Immun. 2024, 119, 394–407. [Google Scholar] [CrossRef]
- Luo, X.; Yang, X.; Tan, S.; Zhang, Y.; Liu, Y.; Tian, X.; Huang, Y.; Zhou, Y.; He, C.; Yin, K.; et al. Gut microbiota mediates anxiety-like behaviors induced by chronic infection of Toxoplasma gondii in mice. Gut Microbes 2024, 16, 2391535. [Google Scholar] [CrossRef]
- Board of Directors. Pediatric acute-onset neuropsychiatric syndrome (PANS): Clinical report. Pediatrics 2025, 155, e2024070334. [Google Scholar] [CrossRef]
- Gupta, S.; Aggarwal, S. Obsesssive-compulsive disorder: Juvenile crime and teenage suicide on the rise due to adolescents being made vulnerable by OCD. BIOKEMI 2023, 5, 45–54. [Google Scholar]
- Tagi, V.M.; Tosi, M.; Greco, I.P.; Stucchi, E.; Verduci, E.; Zuccotti, G. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections and gut microbiota composition: What do we know? Front. Nutr. 2025, 11, 1477893. [Google Scholar] [CrossRef]
- Wong, Y.X.; Ubhi, B. 5975 Enuresis as a presentation of paediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS). Arch. Dis. Child. 2024, 109, A92. [Google Scholar]
- Gurvits, T.V.; Lasko, N.B.; Schachter, S.C.; Kuhne, A.A.; Orr, S.P.; Pitman, R.K. Neurological status of Vietnam veterans with chronic posttraumatic stress disorder. J. Neuropsychiatry Clin. Neurosci. 1993, 5, 183–188. [Google Scholar]
- Michaels, J.J.; Steinberg, A. Persistent enuresis and juvenile delinquency. Br. J. Delinq. 1952, 3, 114–123. [Google Scholar]
- Koposov, R.A.; Stickley, A.; Isaksson, J.; Ruchkin, V. Enuresis in young offenders–a study on prevalence and mental health comorbidity. Front. Psychiatry 2024, 15, 1328767. [Google Scholar] [CrossRef] [PubMed]
- Maternik, M. Understanding of and misconceptions around monosymptomatic nocturnal enuresis: Findings from patient and physician surveys. J. Pediatr. Urol. 2019, 15, 37.e1–37.e8. [Google Scholar] [CrossRef]
- Nishizaki, N.; Oshiro, S.; Tohya, M.; Watanabe, S.; Okazaki, T.; Takahashi, K.; Kirikae, T.; Shimizu, T. Propionimicrobium lymphophilum in urine of children with monosymptomatic nocturnal enuresis. Front. Cell. Infect. Microbiol. 2024, 14, 1377992. [Google Scholar] [CrossRef]
- Akagawa, S.; Tsuji, S.; Urakami, C.; Kaneko, K. Dysbiosis of urinary microbiome (urobiome) in male children with nocturnal enuresis. JU Open Plus 2024, 2, e00074. [Google Scholar] [CrossRef]
- Prescott, S.L.; Logan, A.C. Commentary: Propionimicrobium lymphophilum in urine of children with monosymptomatic nocturnal enuresis. Front. Cell. Infect. Microbiol. 2025, 15, 1553911. [Google Scholar] [CrossRef] [PubMed]
- Bransfield, R.C. Aggressiveness, violence, homicidality, homicide, and Lyme disease. Neuropsychiatr. Dis. Treat. 2018, 14, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Madsen, T.; Erlangsen, A.; Benros, M.E. Lyme borreliosis and associations with mental disorders and suicidal behavior: A nationwide Danish cohort study. Am. J. Psychiatry 2021, 178, 921–931. [Google Scholar] [CrossRef]
- Bransfield, R.C. The Association between Infectious Diseases, Mental Impairments, Violent Behavior, and Global Instability. Med. Res. Arch. 2025, 13. [Google Scholar] [CrossRef]
- Bransfield, R.C.; Gadila, S.K.; Kursawe, L.J.; Dwork, A.J.; Rosoklija, G.; Horn, E.J.; Cook, M.J.; Embers, M.E. Late-stage borreliosis and substance abuse. Heliyon 2024, 10, e31159. [Google Scholar] [CrossRef]
- Morrissette, M.; Pitt, N.; González, A.; Strandwitz, P.; Caboni, M.; Rebman, A.W.; Knight, R.; D’onofrio, A.; Aucott, J.N.; Soloski, M.J.; et al. A distinct microbiome signature in posttreatment Lyme disease patients. MBio 2020, 11, e02310-20. [Google Scholar] [CrossRef]
- Hazan, S.; Dave, S.; Goudzwaard, A.; Barrows, B.; Borody, T.J. S551 Loss of Bifidobacteria in Lyme Disease: Cause or Effect. Am. J. Gastroenterol. 2022, 117, e389–e390. [Google Scholar] [CrossRef]
- Moitas, B.; Caldas, I.M.; Sampaio-Maia, B. Forensic microbiology and geographical location: A systematic review. Aust. J. Forensic Sci. 2024, 56, 416–431. [Google Scholar] [CrossRef]
- Tripathi, P.; Render, R.; Nidhi, S.; Tripathi, V. Microbial genomics: A potential toolkit for forensic investigations. Forensic Sci. Med. Pathol. 2025, 21, 417–429. [Google Scholar] [CrossRef]
- Robinson, J.M.; Pasternak, Z.; Mason, C.E.; Elhaik, E. Forensic applications of microbiomics: A review. Front. Microbiol. 2021, 11, 608101. [Google Scholar] [CrossRef]
- Logan, A.C.; Berryessa, C.M.; Mishra, P.; Prescott, S.L. On Gastronomic Jurisprudence and Judicial Wellness as a Matter of Competence. Laws 2025, 14, 39. [Google Scholar] [CrossRef]
- Logan, A.C.; Prescott, S.L. The Big Minority View: Do Prescientific Beliefs Underpin Criminal Justice Cruelty, and Is the Public Health Quarantine Model a Remedy? Int. J. Environ. Res. Public Health 2025, 22, 1170. [Google Scholar] [CrossRef]
- Gaudet, L.M.; Marchant, G.E. Under the radar: Neuroimaging evidence in the criminal courtroom. Drake Law Rev. 2016, 64, 577. [Google Scholar]
- Brewer, J. Genes as a defense to homicide: Trends in neurocriminology. In Psychological Applications and Trends; Pracana, C., Wang, M., Eds.; inScience Press: Lisboa, Portugal, 2022; pp. 55–459. [Google Scholar] [CrossRef]
- Berryessa, C.M. The potential influence of criminological rationales in considering childhood abuse as mitigating to sentencing. Child Abus. Negl. 2021, 111, 104818. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C.; Mishra, P. The Promise of Neurolaw in Global Justice: An Interview with Dr. Pragya Mishra. Challenges 2025, 16, 15. [Google Scholar] [CrossRef]
- Sissoko, S.; Armstrong, N.; Alou, M.T.; Fadlane, A.; Thera, M.A.; Gerolami, R.; Raoult, D.; Ranque, S. Transgenic corn syrup enhances in vitro yeast ethanol production: Hidden cause of the US MASH epidemic? Microbe 2025, 7, 100416. [Google Scholar] [CrossRef]
- Siracusano, M.; Arturi, L.; Riccioni, A.; Noto, A.; Mussap, M.; Mazzone, L. Metabolomics: Perspectives on clinical employment in autism spectrum disorder. Int. J. Mol. Sci. 2023, 24, 13404. [Google Scholar] [CrossRef]
- Benitah, K.C.; Kavaliers, M.; Ossenkopp, K.P. The enteric metabolite, propionic acid, impairs social behavior and increases anxiety in a rodent ASD model: Examining sex differences and the influence of the estrous cycle. Pharmacol. Biochem. Behav. 2023, 231, 173630. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Lee, R.; Breen, E.C.; Irwin, M.R. Plasma and cerebrospinal fluid inflammatory markers and human aggression. Neuropsychopharmacology 2023, 48, 1060–1066. [Google Scholar] [CrossRef]
- Logan, A.C.; Mishra, P. Aggression and Justice Involvement: Does Uric Acid Play a Role? Brain Sci. 2025, 15, 268. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Stein, M.B.; Coccaro, E.F.; Meruelo, A.D. Exploring the metabolic signature of intermittent explosive disorder: Preliminary evidence and potential mechanisms for altered bilirubin metabolism. Compr. Psychoneuroendocr. 2025, 22, 100294. [Google Scholar] [CrossRef]
- Logan, A.C.; Nicholson, J.J.; Schoenthaler, S.J.; Prescott, S.L. Neurolaw: Revisiting Huberty v. McDonald’s through the lens of nutritional criminology and food crime. Laws 2024, 13, 17. [Google Scholar] [CrossRef]
- Anderer, S. Beyond Genes: Human Exposome Project to Tackle External Drivers of Disease. J. Am. Med. Assoc. 2025, 334, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Barouki, R. The exposome as the science of social-to-biological transitions. Environ. Int. 2022, 165, 107312. [Google Scholar] [CrossRef]
- Robinson, J.M.; Crino, O.L.; Camargo, A.; Breed, M.F. Does a microbial-endocrine interplay shape love-associated emotions in humans? A hypothesis. mSystems 2025, 10, e0041525. [Google Scholar] [CrossRef] [PubMed]
- Ahalt, C.; Haney, C.; Kinner, S.; Williams, B. Balancing the rights to protection and participation: A call for expanded access to ethically conducted correctional health research. J. Gen. Intern. Med. 2018, 33, 764–768. [Google Scholar] [CrossRef]
- Irwin, J.; Schiraldi, V.; Ziedenberg, J. America’s one million nonviolent prisoners. Soc. Justice 2000, 27, 135–147. [Google Scholar]
- Fredericks, K.A.; McComas, R.E.; Weatherby, G.A. White collar crime: Recidivism, deterrence, and social impact. Forensic Res. Criminol. Int. J. 2016, 2, 00039. [Google Scholar]
- Kendler, K.S.; Maes, H.H.; Lönn, S.L.; Morris, N.A.; Lichtenstein, P.; Sundquist, J.; Sundquist, K. A Swedish national twin study of criminal behavior and its violent, white-collar and property subtypes. Psychol. Med. 2015, 45, 2253–2262. [Google Scholar] [CrossRef]
- Peled-Laskov, R. When personal rational decision-making fails: Examining the psychological limits of criminal punishment as a successful deterrent for white-collar offenders. J. Forensic Psychiatry Psychol. 2024, 35, 331–355. [Google Scholar] [CrossRef]
- Musculus, L.; Broeker, L.; Tolentino-Castro, J.W.; Hoffmann, S.; Pedraza-Ramirez, I.; Laborde, S.; Lobinger, B.; Borges, U.; Liepelt, R.; Pizzera, A.; et al. Reassessing the gut–cognition link: Exploring psychophysiological mechanisms of risky decision-making. Decision 2025, 12, 216. [Google Scholar] [CrossRef]
- Shi, H.; Schweren, L.J.; Ter Horst, R.; Bloemendaal, M.; van Rooij, D.; Vasquez, A.A.; Hartman, C.A.; Buitelaar, J.K. Low-grade inflammation as mediator between diet and behavioral disinhibition: A UK Biobank study. Brain Behav. Immun. 2022, 106, 100–110. [Google Scholar] [CrossRef]
- Prescott, S.L.; Logan, A.C.; LaFata, E.M.; Naik, A.; Nelson, D.H.; Robinson, M.B.; Soble, L. Crime and nourishment: A narrative review examining ultra-processed foods, brain, and behavior. Dietetics 2024, 3, 318–345. [Google Scholar] [CrossRef]
- Dong, Z.; Shen, X.; Hao, Y.; Li, J.; Xu, H.; Yin, L.; Kuang, W. Gut microbiome: A potential indicator for predicting treatment outcomes in major depressive disorder. Front. Neurosci. 2022, 16, 813075. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Yuan, J.J.; Xu, Y.Q.; Gou, Y.H.; Zhu, Y.Y.; Chen, C.; Huang, X.X.; Ma, X.M.; Pi, M.; Yang, Z.X. Fecal microbiota as a predictor of acupuncture responses in patients with postpartum depressive disorder. Front. Cell. Infect. Microbiol. 2023, 13, 1228940. [Google Scholar] [CrossRef] [PubMed]
- Zandifar, A.; Badrfam, R.; Mohammaditabar, M.; Kargar, B.; Goodarzi, S.; Hajialigol, A.; Ketabforoush, S.; Heidari, A.; Fathi, H.; Shafiee, A.; et al. The Effect of Prebiotics and Probiotics on Levels of Depression, Anxiety, and Cognitive Function: A Meta—Analysis of Randomized Clinical Trials. Brain Behav. 2025, 15, e70401. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, B.; Qu, Z.; Liu, S.; Li, S. Efficacy of probiotics in patients with cognitive impairment: A systematic review and meta-analysis. PLoS ONE 2025, 20, e0321567. [Google Scholar] [CrossRef]
- Lawrence, M.R.; Arnetz, J.E.; Counts, S.E.; Ahmed, A.; Arnetz, B.B. Self-reported health, neuropsychological tests and biomarkers in fully recovered COVID-19 patients vs patients with post-COVID cognitive symptoms: A pilot study. PLoS ONE 2025, 20, e0315486. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fujiwara, N.; Murakami, Y.; Ishida, S.; Kinguchi, S.; Haze, T.; Azushima, K.; Fujiwara, A.; Wakui, H.; Sakakura, M.; et al. Visualizing fatigue mechanisms in non-communicable diseases: An integrative approach with multi-omics and machine learning. BMC Med. Inform. Decis. Mak. 2025, 25, 204. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Coghill, D.; Sjölander, A.; Yao, H.; Zhang, L.; Kuja-Halkola, R.; Brikell, I.; Lichtenstein, P.; D’Onofrio, B.M.; Larsson, H.; et al. Increased Prescribing of Attention-Deficit/Hyperactivity Disorder Medication and Real-World Outcomes Over Time. J. Am. Med. Assoc. Psychiatry 2025, 82, 830–837. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, N.; Sjölander, A.; Nourredine, M.; Li, L.; Garcia-Argibay, M.; Kuja-Halkola, R.; Brikell, I.; Lichtenstein, P.; D’Onofrio, B.M.; et al. ADHD drug treatment and risk of suicidal behaviours, substance misuse, accidental injuries, transport accidents, and criminality: Emulation of target trials. BMJ 2025, 390, e083658. [Google Scholar] [CrossRef]
- Stiernborg, M.; Debelius, J.W.; Yang, L.L.; Skott, E.; Millischer, V.; Giacobini, M.; Melas, P.A.; Boulund, F.; Lavebratt, C. Bacterial gut microbiome differences in adults with ADHD and in children with ADHD on psychostimulant medication. Brain Behav. Immun. 2023, 110, 310–321. [Google Scholar] [CrossRef]
- Taylor, E.N.; Timko, C.; Nash, A.; Owens, M.D.; Harris, A.H.; Finlay, A.K. Posttraumatic stress disorder and justice involvement among military veterans: A systematic review and meta-analysis. J. Trauma. Stress 2020, 33, 804–812. [Google Scholar] [CrossRef]
- Li, Y.I.; Pagulayan, K.; Rau, H.; Hendrickson, R.; Schindler, A.G. Gut Microbial Composition Is Associated with Symptom Self-Report in Trauma-Exposed Iraq and Afghanistan Veterans. Neurotrauma Rep. 2025, 6, 1–12. [Google Scholar] [CrossRef]
- Griffith, R.L.; Nowalis, S.; Monroe-Gulick, A. Executive functioning and offending behavior: An updated meta-analysis. Crim. Justice Behav. 2024, 51, 528–551. [Google Scholar] [CrossRef]
- Gattuso, J.J.; Kong, G.; Bezcioglu, B.; Lu, D.; Ekwudo, M.N.; Wilson, C.; Gubert, C.; Hannan, A.J.; Renoir, T. Chronic psilocybin administration increases sociability and alters the gut microbiome in male wild-type mice but not in a preclinical model of obsessive-compulsive disorder. Neuropharmacology 2025, 279, 110648. [Google Scholar] [CrossRef]
- Kumari, V.; Das, M.; Hodgins, S.; Zachariah, E.; Barkataki, I.; Howlett, M.; Sharma, T. Association between violent behaviour and impaired prepulse inhibition of the startle response in antisocial personality disorder and schizophrenia. Behav. Brain Res. 2005, 158, 159–166. [Google Scholar] [CrossRef]
- Sedgwick, O.; Young, S.; Greer, B.; Arnold, J.; Parsons, A.; Puzzo, I.; Terracciano, M.; Das, M.; Kumari, V. Sensorimotor gating characteristics of violent men with comorbid psychosis and dissocial personality disorder: Relationship with antisocial traits and psychosocial deprivation. Schizophr. Res. 2018, 198, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Santos-Carrasco, D.; De la Casa, L.G. Prepulse inhibition deficit as a transdiagnostic process in neuropsychiatric disorders: A systematic review. BMC Psychol. 2023, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, B.; Chan, C.S. Green space is associated with lower violent assault rates: A longitudinal remote sensing study. Landsc. Urban Plan. 2025, 253, 105200. [Google Scholar] [CrossRef]
- Wang, R.; Cleland, C.L.; Weir, R.; McManus, S.; Martire, A.; Grekousis, G.; Bryan, D.; Hunter, R.F. Rethinking the association between green space and crime using spatial quantile regression modelling: Do vegetation type, crime type, and crime rates matter? Urban For. Urban Green. 2024, 101, 128523. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.Y.; Zhu, X.Q.; Jiang, J.C.; Li, C.; Zheng, Z.H.; Wang, Z.; Zhao, T.Y.; Markevych, I.; Heinrich, J.; et al. Intervention effects of greenspace exposure on human microbiota: A randomized controlled trial in Chinese young adults. Ecotoxicol. Environ. Saf. 2025, 296, 118183. [Google Scholar] [CrossRef]
- Fang, Q.; Feng, Z.; Qiu, T.; Tian, X.; Cao, Y.; Bai, J.; Zhao, I.Y.; Sali, A.; Liu, Y. Mediating Role of Gut Microbiota in the Relationship Between Residential Greenness Exposure and Young Children’s Temperament: A Pilot Study. Infancy 2025, 30, e70025. [Google Scholar] [CrossRef]
- Tyson-Carr, J.; Leng, J.; Scott, M.; Adams, S.; Hoptroff, M.; Murphy, B.; Fallon, N.; Paterson, S.; Thomas, A.; Giesbrecht, T.; et al. Body-site specific associations between human skin microbiome composition and psychological wellbeing. Br. J. Dermatol. 2025; ahead of print. [Google Scholar]
- Crocetta, A.; Liloia, D.; Costa, T.; Duca, S.; Cauda, F.; Manuello, J. From gut to brain: Unveiling probiotic effects through a neuroimaging perspective—A systematic review of randomized controlled trials. Front. Nutr. 2024, 11, 1446854. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.I.; Cruz-Gil, S.; Fernández, C.M.; Fernández, L.P.; Sobrino-Santos, A.; Espinosa-Salinas, I.; Maestre, C.; Latasa, M.J.; Cebrián, M.; Ramos-Ruiz, R.; et al. Gut microbiota profiles are associated with different spontaneous cortical activity in healthy older people. Sci. Rep. 2025, 15, 31590. [Google Scholar] [CrossRef] [PubMed]
- Dongen, J.D.; Haveman, Y.; Sergiou, C.S.; Choy, O. Neuroprediction of violence and criminal behavior using neuro-imaging data: From innovation to considerations for future directions. Aggress. Violent Behav. 2025, 80, 102008. [Google Scholar] [CrossRef]
- Costanzo, M.; Krauss, D. Forensic Legal Psychology; Macmillan: New York, NY, USA, 2021. [Google Scholar]
- Thomaidou, M.A.; Berryessa, C.M. A jury of scientists: Formal education in biobehavioral sciences reduces the odds of punitive criminal sentencing. Behav. Sci. Law 2022, 40, 787–817. [Google Scholar] [CrossRef]
- Jones, G.; Cohen, B. Sentencing the ‘Psychopath’: How Labelling Affects Judges’ Decision Making in Aotearoa/New Zealand. N. Z. Sociol. 2025, 40, 1–15. [Google Scholar]
- Stamation, R. Endogenous ethanol production in the human alimentary tract: A literature review. J. Gastroenterol. Hepatol. 2025, 40, 783–790. [Google Scholar] [CrossRef]
- Cordell, B. Auto-Brewery Syndrome: Diagnosis and Treatment of This Little-Known Condition. AJN Am. J. Nurs. 2025, 125, 30–37. [Google Scholar] [CrossRef]
- Prescott, S.L.; Logan, A.C. Each meal matters in the exposome: Biological and community considerations in fast-food-socioeconomic associations. Econ. Hum. Biol. 2017, 27, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Lucinian, Y.A.; Gagnon, C.; Latour, É.; Hamel, V.; Iglesies-Grau, J.; Nigam, A.; Harel, F.; Nozza, A.; Juneau, M.; Moubarac, J.-C. Effect of a single ultra processed meal on myocardial endothelial function, adenosine mediated effects and cognitive performances. Sci. Rep. 2025, 15, 28671. [Google Scholar] [CrossRef]
- Osborn, L.J.; Orabi, D.; Goudzari, M.; Sangwan, N.; Banerjee, R.; Brown, A.L.; Kadam, A.; Gromovsky, A.D.; Linga, P.; Cresci, G.A.M.; et al. A Single Human-Relevant Fast Food Meal Rapidly Reorganizes Metabolomic and Transcriptomic Signatures in a Gut Microbiota-Dependent Manner. Immunometabolism 2021, 3, e210029. [Google Scholar] [CrossRef]
- Gibson, R.; Eriksen, R.; Singh, D.; Vergnaud, A.C.; Heard, A.; Chan, Q.; Elliott, P.; Frost, G. A cross-sectional investigation into the occupational and socio-demographic characteristics of British police force employees reporting a dietary pattern associated with cardiometabolic risk: Findings from the Airwave Health Monitoring Study. Eur. J. Nutr. 2018, 57, 2913–2926. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Burch, J.; Shivappa, N.; Violanti, J.M.; Burchfiel, C.M.; Fekedulegn, D.; Andrew, M.E.; Hartley, T.A.; Miller, D.B.; Mnatsakanova, A.; et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J. Occup. Environ. Med. 2014, 56, 986–989. [Google Scholar] [CrossRef]
- Sackey, J.D.; Potter, D.; Ortiz, A.; Lewis, K.; Lefebre, A.; Rosen, M.D.; Valera, P. Examining the Association Between Food and Nutrition Insecurity and Diet Quality in Correctional Officers In and Around New Jersey. Curr. Dev. Nutr. 2025, 9, 160322. [Google Scholar] [CrossRef]
- Leung, C.W.; Fulay, A.P.; Parnarouskis, L.; Martinez-Steele, E.; Gearhardt, A.N.; Wolfson, J.A. Food insecurity and ultra-processed food consumption: The modifying role of participation in the Supplemental Nutrition Assistance Program (SNAP). Am. J. Clin. Nutr. 2022, 116, 197–205. [Google Scholar] [CrossRef]
- Mohr, A.E.; Jasbi, P.; van Woerden, I.; Bowes, D.A.; Chi, J.; Gu, H.; Bruening, M.; Whisner, C.M. Longitudinal assessment of food insecurity status on the gut microbiome and metabolome of first-year college students. Br. J. Nutr. 2025, 133, 1473–1486. [Google Scholar] [CrossRef]
- Palazzi, C.M.; Ciampaglia, G.; Binato, B.; Ragazzini, M.; Bertuccioli, A.; Cavecchia, I.; Matera, M.; Cazzaniga, M.; Zonzini, G.B.; Zerbinati, N.; et al. Position Statement of the Microbiota International Clinical Society (MICS). Front. Microbiomes 2025, 4, 1657750. [Google Scholar] [CrossRef]
- Anderson, D.A. The aggregate cost of crime in the United States. J. Law Econ. 2021, 64, 857–885. [Google Scholar] [CrossRef]
- Haber, L.A.; Boudin, C.; Williams, B.A. Criminal justice reform is health care reform. J. Am. Med. Assoc. 2024, 331, 21–22. [Google Scholar] [CrossRef] [PubMed]

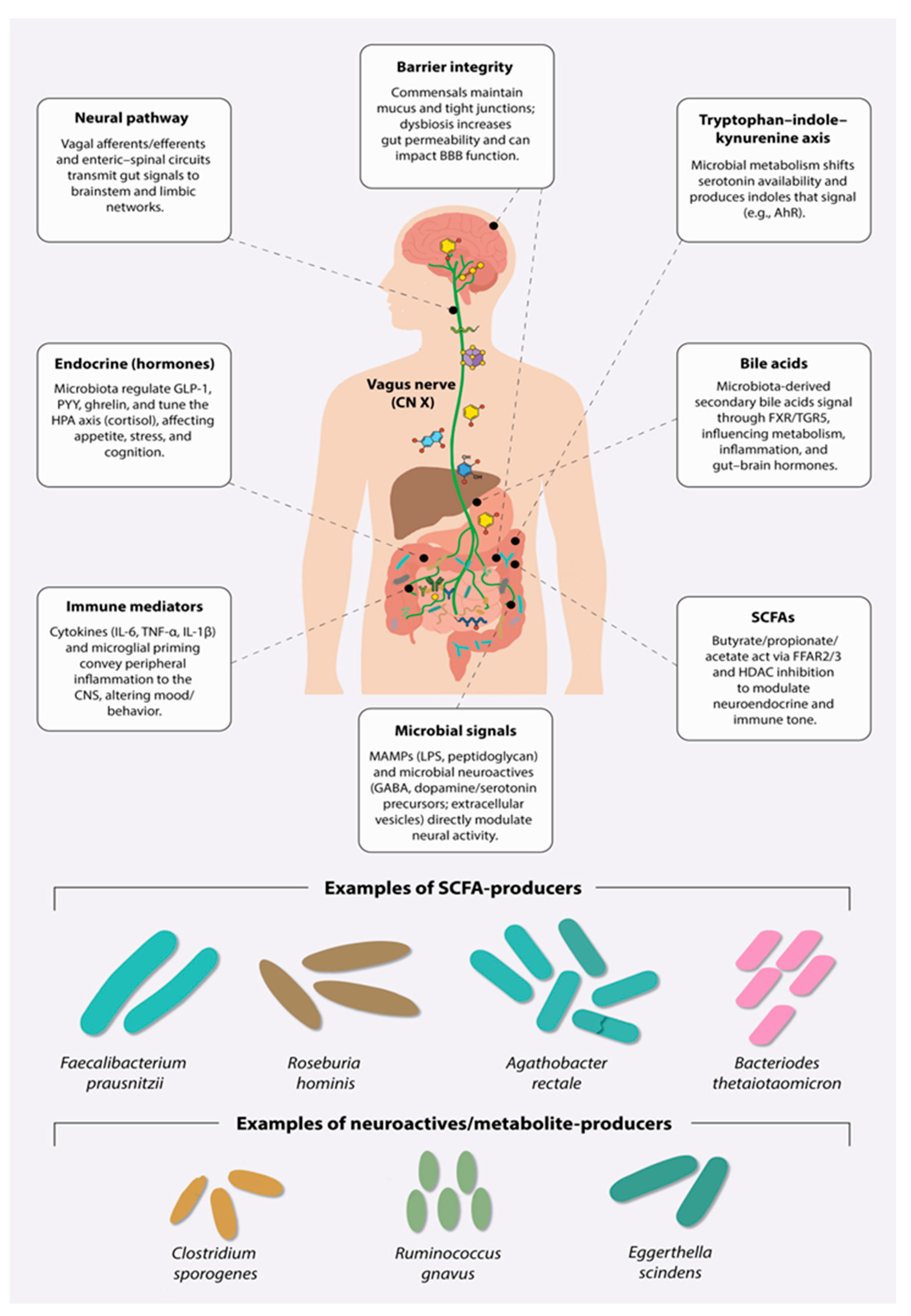
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logan, A.C.; Cordell, B.; Pillai, S.D.; Robinson, J.M.; Prescott, S.L. From Bacillus Criminalis to the Legalome: Will Neuromicrobiology Impact 21st Century Criminal Justice? Brain Sci. 2025, 15, 984. https://doi.org/10.3390/brainsci15090984
Logan AC, Cordell B, Pillai SD, Robinson JM, Prescott SL. From Bacillus Criminalis to the Legalome: Will Neuromicrobiology Impact 21st Century Criminal Justice? Brain Sciences. 2025; 15(9):984. https://doi.org/10.3390/brainsci15090984
Chicago/Turabian StyleLogan, Alan C., Barbara Cordell, Suresh D. Pillai, Jake M. Robinson, and Susan L. Prescott. 2025. "From Bacillus Criminalis to the Legalome: Will Neuromicrobiology Impact 21st Century Criminal Justice?" Brain Sciences 15, no. 9: 984. https://doi.org/10.3390/brainsci15090984
APA StyleLogan, A. C., Cordell, B., Pillai, S. D., Robinson, J. M., & Prescott, S. L. (2025). From Bacillus Criminalis to the Legalome: Will Neuromicrobiology Impact 21st Century Criminal Justice? Brain Sciences, 15(9), 984. https://doi.org/10.3390/brainsci15090984






