Sleep Disorders in Children with Autism Spectrum Disorder: Developmental Impact and Intervention Strategies
Abstract
1. Introduction
2. Neurobiology of Sleep in Typical and Atypical Development
3. Neurodevelopmental Role of Sleep in Infancy
4. Sleep as Prognostic Marker of Neurodevelopmental Disorders
5. Heterogeneity of Sleep Disturbances in ASD
6. Clinical Approach to Sleep Disturbances in ASD
6.1. Non-Pharmacological Interventions for Sleep Disorders in ASD
6.2. Oral Non-Prescription Treatments and Nutritional Supplements
6.2.1. Tryptophan/5-Hydroxytryptophan
6.2.2. Carnosine
6.2.3. Iron
6.2.4. Magnesium
6.2.5. Vitamin D
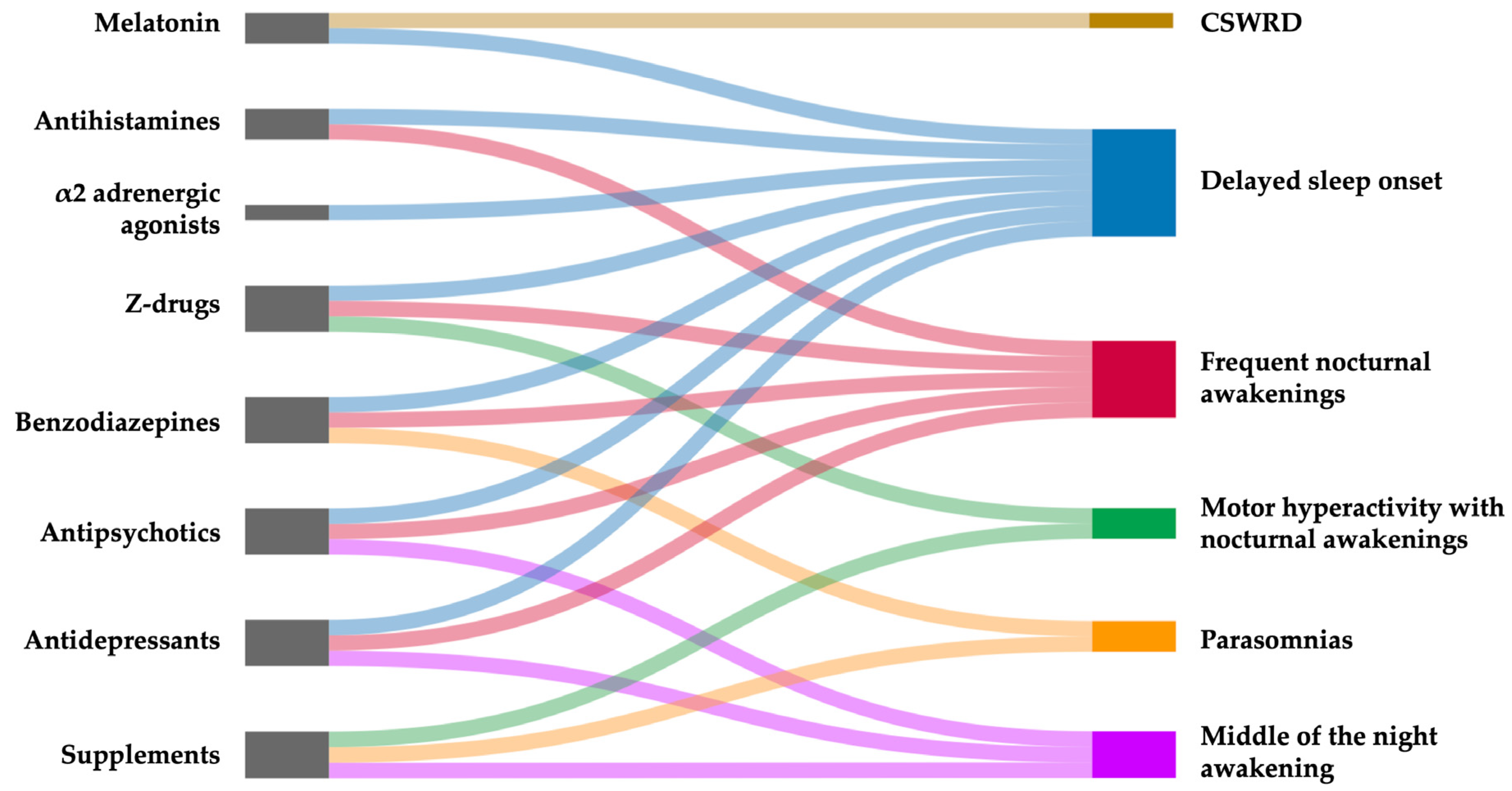
6.3. Pharmacological Therapy
6.3.1. Melatonin
6.3.2. α2-Adrenergic Agonists
6.3.3. Antihistamines
6.3.4. Antipsychotics
6.3.5. Benzodiazepines and Z-Drugs
6.3.6. Antidepressants
6.3.7. Anti-Seizure Medications
6.3.8. Orexin Receptor Antagonists
7. Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-HTP | 5-Hydroxitryptophan |
| ADHD | Attention Deficit Hyperactivity Disorder |
| ASD | Autism Spectrum Disorder |
| CSWRD | Circadian Sleep–Wake Rhythm Disorder |
| CSHQ | Children’s Sleep Habits Questionnaire |
| DLMO | Dim Light Melatonin Onset |
| DORAs | Dual Orexin Receptor Antagonists |
| EEG | Electroencephalographic |
| EMG | Electromyogram |
| FDA | U.S. Food and Drug Administration |
| FR | Fast Release |
| GABA | Gamma-aminobutyric acid |
| IDD | Intellectual Developmental Disorder |
| IQ | Intelligence Quotient |
| MT | Melatonin receptor |
| NDDs | Neurodevelopmental Disorders |
| NREM | Non-Rapid Eye Movement |
| ORAs | Orexin Receptor Antagonists |
| OXR | Orexin Receptor |
| PedPRM | Pediatric Prolonged Release Melatonin |
| PLMD | Periodic Limb Movement Disorder |
| RCTs | Randomized Controlled Trials |
| REM | Rapid Eye Movement |
| RLS | Restless Legs Syndrome |
| RSD | Restless Sleep Disorder |
| SE | Sleep Efficiency |
| SoL | Sleep onset Latency |
| SORAs | Selective Orexin Receptor Antagonists |
| SSP | Short Sensory Profile |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| SWA | Slow-Wave Activity |
| SWR | Sharp Wave Ripple |
| Trp | Tryptophan |
| TST | Total Sleep Time |
| UVB | Ultraviolet B |
| WASO | Wake After Sleep Onset |
References
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Butler, M.G. The Autism Spectrum: Behavioral, Psychiatric and Genetic Associations. Genes 2023, 14, 677. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.; Thiruvahindrapuram, B.; Chan, A.J.S.; Engchuan, W.; Higginbotham, E.J.; Howe, J.L.; Loureiro, L.O.; Reuter, M.S.; Roshandel, D.; Whitney, J.; et al. Genomic architecture of autism from comprehensive whole-genome sequence annotation. Cell 2022, 185, 4409–4427.e18. [Google Scholar] [CrossRef] [PubMed]
- Spoto, G.; Di Rosa, G.; Nicotera, A.G. The Impact of Genetics on Cognition: Insights into Cognitive Disorders and Single Nucleotide Polymorphisms. J. Pers. Med. 2024, 14, 156. [Google Scholar] [CrossRef]
- Christensen, D.L.; Baio, J.; Van Naarden Braun, K.; Bilder, D.; Charles, J.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2016, 65, 1–23. [Google Scholar] [CrossRef]
- Fombonne, E.; Morotti, H.; Mastel, S.; Keller, K.; Barnard, R.A.; Hall, T.; O’Roak, B.J. Autism questionnaire scores do not only rise because of autism. Dev. Med. Child Neurol. 2021, 63, 235–236. [Google Scholar] [CrossRef]
- Bauman, M.L. Medical comorbidities in autism: Challenges to diagnosis and treatment. Neurother. J. Am. Soc. Exp. Neurother. 2010, 7, 320–327. [Google Scholar] [CrossRef]
- Devnani, P.A.; Hegde, A.U. Autism and sleep disorders. J. Pediatr. Neurosci. 2015, 10, 304–307. [Google Scholar] [CrossRef]
- Liu, X.; Hubbard, J.A.; Fabes, R.A.; Adam, J.B. Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry Hum. Dev. 2006, 37, 179–191. [Google Scholar] [CrossRef]
- Rattaz, C.; Michelon, C.; Munir, K.; Baghdadli, A. Challenging behaviours at early adulthood in autism spectrum disorders: Topography, risk factors and evolution. J. Intellect. Disabil. Res. JIDR 2018, 62, 637–649. [Google Scholar] [CrossRef]
- Cohen, S.; Fulcher, B.D.; Rajaratnam, S.M.W.; Conduit, R.; Sullivan, J.P.; St Hilaire, M.A.; Phillips, A.J.K.; Loddenkemper, T.; Kothare, S.V.; McConnell, K.; et al. Sleep patterns predictive of daytime challenging behavior in individuals with low-functioning autism. Autism Res. 2018, 11, 391–403. [Google Scholar] [CrossRef]
- DeVincent, C.J.; Gadow, K.D.; Delosh, D.; Geller, L. Sleep disturbance and its relation to DSM-IV psychiatric symptoms in preschool-age children with pervasive developmental disorder and community controls. J. Child Neurol. 2007, 22, 161–169. [Google Scholar] [CrossRef]
- Tudor, M.E.; Hoffman, C.D.; Sweeney, D.P. Children with Autism: Sleep Problems and Symptom Severity. Focus Autism Other Dev. Disabil. 2012, 27, 254–262. [Google Scholar] [CrossRef]
- Cohen, S.; Conduit, R.; Lockley, S.W.; Rajaratnam, S.M.; Cornish, K.M. The relationship between sleep and behavior in autism spectrum disorder (ASD): A review. J. Neurodev. Disord. 2014, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Hirata, I.; Mohri, I.; Kato-Nishimura, K.; Tachibana, M.; Kuwada, A.; Kagitani-Shimono, K.; Ohno, Y.; Ozono, K.; Taniike, M. Sleep problems are more frequent and associated with problematic behaviors in preschoolers with autism spectrum disorder. Res. Dev. Disabil. 2016, 49–50, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.O.; Dovgan, K.; Neumeyer, A.M.; Malow, B.A. Course and Predictors of Sleep and Co-occurring Problems in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2019, 49, 2101–2115. [Google Scholar] [CrossRef]
- Park, S.; Cho, S.C.; Cho, I.H.; Kim, B.N.; Kim, J.W.; Shin, M.S.; Chung, U.-S.; Park, T.-W.; Son, J.-W.; Yoo, H.J. Sleep problems and their correlates and comorbid psychopathology of children with autism spectrum disorders. Res. Autism Spectr. Disord. 2012, 6, 1068–1072. [Google Scholar] [CrossRef]
- Schroder, C.M.; Malow, B.A.; Maras, A.; Melmed, R.D.; Findling, R.L.; Breddy, J.; Nir, T.; Shahmoon, S.; Zisapel, N.; Gringras, P. Pediatric Prolonged-Release Melatonin for Sleep in Children with Autism Spectrum Disorder: Impact on Child Behavior and Caregiver’s Quality of Life. J. Autism Dev. Disord. 2019, 49, 3218–3230. [Google Scholar] [CrossRef]
- Schreck, K.A.; Mulick, J.A.; Smith, A.F. Sleep problems as possible predictors of intensified symptoms of autism. Res. Dev. Disabil. 2004, 25, 57–66. [Google Scholar] [CrossRef]
- Jin, C.S.; Hanley, G.P.; Beaulieu, L. An individualized and comprehensive approach to treating sleep problems in young children. J. Appl. Behav. Anal. 2013, 46, 161–180. [Google Scholar] [CrossRef]
- McLay, L.; France, K.; Blampied, N.; Hunter, J. Using functional behavioral assessment to treat sleep problems in two children with autism and vocal stereotypy. Int. J. Dev. Disabil. 2017, 65, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E.; McLay, L.K.; France, K.G.; Blampied, N.M. Sleep and stereotypy in children with autism: Effectiveness of function-based behavioral treatment. Sleep Med. 2021, 80, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, L.; Postorino, V.; Siracusano, M.; Riccioni, A.; Curatolo, P. The Relationship between Sleep Problems, Neurobiological Alterations, Core Symptoms of Autism Spectrum Disorder, and Psychiatric Comorbidities. J. Clin. Med. 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, M.E.; Blanken, L.M.E.; Kocevska, D.; Mileva-Seitz, V.R.; Jaddoe, V.W.V.; White, T.; Verhulst, F.; Luijk, M.P.C.M.; Tiemeier, H. The bidirectional association between sleep problems and autism spectrum disorder: A population-based cohort study. Mol. Autism 2018, 9, 8. [Google Scholar] [CrossRef]
- Reynolds, S.; Lane, S.J.; Thacker, L. Sensory processing, physiological stress, and sleep behaviors in children with and without autism spectrum disorders. OTJR Occup. Particip. Health 2012, 32, 246–257. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Malow, B.A. Sleep and autism spectrum disorders. Pediatr. Clin. 2011, 58, 685–698. [Google Scholar] [CrossRef]
- Dewald, J.F.; Meijer, A.M.; Oort, F.J.; Kerkhof, G.A.; Bögels, S.M. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: A meta-analytic review. Sleep Med. Rev. 2010, 14, 179–189. [Google Scholar] [CrossRef]
- Reynolds, K.C.; Patriquin, M.; Alfano, C.A.; Loveland, K.A.; Pearson, D.A. Parent-Reported Problematic Sleep Behaviors in Children with Comorbid Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder. Res. Autism Spectr. Disord. 2017, 39, 20–32. [Google Scholar] [CrossRef]
- Maski, K.; Holbrook, H.; Manoach, D.; Hanson, E.; Kapur, K.; Stickgold, R. Sleep Dependent Memory Consolidation in Children with Autism Spectrum Disorder. Sleep 2015, 38, 1955–1963. [Google Scholar] [CrossRef]
- Gisbert Gustemps, L.; Lugo Marín, J.; Setien Ramos, I.; Ibañez Jimenez, P.; Romero Santo-Tomás, O.; Jurado Luque, M.J.; Ballester Navarro, P.; Esteve Cruella, A.; Díez Villoria, E.; Canal Bedia, R.; et al. Sleep disturbances in autism spectrum disorder without intellectual impairment: Relationship with executive function and psychiatric symptoms. Sleep Med. 2021, 83, 106–114. [Google Scholar] [CrossRef]
- Howes, O.D.; Rogdaki, M.; Findon, J.L.; Wichers, R.H.; Charman, T.; King, B.H.; Loth, E.; McAlonan, G.M.; McCracken, J.T.; Parr, J.R.; et al. Autism spectrum disorder: Consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J. Psychopharmacol. 2018, 32, 3–29. [Google Scholar] [CrossRef]
- Mainieri, G.; Montini, A.; Nicotera, A.; Di Rosa, G.; Provini, F.; Loddo, G. The Genetics of Sleep Disorders in Children: A Narrative Review. Brain Sci. 2011, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Kryger, M.H.; Roth, T.; Dement, W.C. Principles and Practice of Sleep Medicine, 5th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Anafi, R.C.; Kayser, M.S.; Raizen, D.M. Exploring phylogeny to find the function of sleep. Nat. Rev. Neurosci. 2019, 20, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ednick, M.; Cohen, A.P.; McPhail, G.L.; Beebe, D.; Simakajornboon, N.; Amin, R.S. A review of the effects of sleep during the first year of life on cognitive, psychomotor, and temperament development. Sleep 2009, 32, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Renouard, L.; Hayworth, C.; Rempe, M.; Clegern, W.; Wisor, J.; Frank, M.G. REM sleep promotes bidirectional plasticity in developing visual cortex in vivo. Neurobiol. Sleep Circadian Rhythm. 2022, 12, 100076. [Google Scholar] [CrossRef]
- Siegel, J.M. The neurobiology of sleep. Semin. Neurol. 2009, 29, 277–296. [Google Scholar] [CrossRef]
- Szymusiak, R.; McGinty, D. Hypothalamic regulation of sleep and arousal. Ann. N.Y. Acad. Sci. 2008, 1129, 275–286. [Google Scholar] [CrossRef]
- Ballester, P.; Richdale, A.L.; Baker, E.K.; Peiró, A.M. Sleep in autism: A biomolecular approach to aetiology and treatment. Sleep Med. Rev. 2020, 54, 101357. [Google Scholar] [CrossRef]
- Ellis, C.M.; Lemmens, G.; Parkes, J.D. Melatonin and insomnia. J. Sleep Res. 1996, 5, 61–65. [Google Scholar] [CrossRef]
- Goldman, S.E.; Adkins, K.W.; Calcutt, M.W.; Carter, M.D.; Goodpaster, R.L.; Wang, L.; Shi, Y.; Burgess, H.J.; Hachey, D.L.; Malow, B.A. Melatonin in children with autism spectrum disorders: Endogenous and pharmacokinetic profiles in relation to sleep. J. Autism Dev. Disord. 2014, 44, 2525–2535. [Google Scholar] [CrossRef]
- Baker, E.K.; Richdale, A.L.; Hazi, A.; Prendergast, L.A. Assessing the Dim Light Melatonin Onset in Adults with Autism Spectrum Disorder and No Comorbid Intellectual Disability. J. Autism Dev. Disord. 2017, 47, 2120–2137. [Google Scholar] [CrossRef]
- Mulder, E.J.; Anderson, G.M.; Kema, I.P.; de Bildt, A.; van Lang, N.D.; den Boer, J.A.; Minderaa, R.B. Platelet serotonin levels in pervasive developmental disorders and mental retardation: Diagnostic group differences, within-group distribution, and behavioral correlates. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 491–499. [Google Scholar] [CrossRef]
- Masi, A.; Moni, M.A.; Azim, S.I.; Choi, B.; Heussler, H.; Lin, P.I.; Diaz, A.M.; Eapen, V. Clinical and behavioral attributes leading to sleep disorders in children on the autism spectrum. Autism Res. 2022, 15, 1274–1287. [Google Scholar] [CrossRef]
- Cortese, S.; Wang, F.; Angriman, M.; Masi, G.; Bruni, O. Sleep Disorders in Children and Adolescents with Autism Spectrum Disorder: Diagnosis, Epidemiology, and Management. CNS Drugs 2020, 34, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.A.; Richdale, A.L. Sleep problems, behavior, and psychopathology in autism: Inter-relationships across the lifespan. Curr. Opin. Psychol. 2020, 34, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Simakajornboon, N. Sleep-related movement disorders in children: Recent updates. World J. Pediatr. WJP 2024, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- DelRosso, L.M.; Bruni, O.; Ferri, R. Restless sleep disorder in children: A pilot study on a tentative new diagnostic category. Sleep 2018, 41, zsy102. [Google Scholar] [CrossRef]
- Hollway, J.A.; Aman, M.G.; Butter, E. Correlates and Risk Markers for Sleep Disturbance in Participants of the Autism Treatment Network. J. Autism Dev. Disord. 2013, 43, 2830–2843. [Google Scholar] [CrossRef]
- Mazurek, M.O.; Petroski, G.F. Sleep problems in children with autism spectrum disorder: Examining the contributions of sensory over-responsivity and anxiety. Sleep Med. 2014. [Google Scholar] [CrossRef]
- Tzischinsky, O.; Meiri, G.; Manelis, L.; Bar-Sinai, A.; Flusser, H.; Michaelovski, A.; Zivan, O.; Ilan, M.; Faroy, M.; Menashe, I.; et al. Sleep disturbances are associated with specific sensory sensitivities in children with autism. Mol. Autism. 2018, 9, 22. [Google Scholar] [CrossRef]
- Kılıç, B.K.; Kayıhan, H.; Çifci, A. Sensory processing in typically developing toddlers with and without sleep problems. Infant Behav. Dev. 2024, 76, 101981. [Google Scholar] [CrossRef] [PubMed]
- Ozonoff, S.; Iosif, A.M.; Baguio, F.; Cook, I.C.; Hill, M.M.; Hutman, T.; Rogers, S.J.; Rozga, A.; Sangha, S.; Sigman, M.; et al. A prospective study of the emergence of early behavioral signs of autism. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 256–266.e662. [Google Scholar] [PubMed]
- Ozonoff, S.; Iosif, A.M. Changing conceptualizations of regression: What prospective studies reveal about the onset of autism spectrum disorder. Neurosci. Biobehav. Rev. 2019, 100, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.; Dounavi, K. The Emergence of Autism Symptoms Prior to 18 Months of Age: A Systematic Literature Review. J. Autism Dev. Disord. 2021, 51, 973–993. [Google Scholar] [CrossRef]
- Hazlett, H.C.; Gu, H.; Munsell, B.C.; Kim, S.H.; Styner, M.; Wolff, J.J.; Elison, J.T.; Swanson, M.R.; Zhu, H.; Botteron, K.N.; et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 2017, 542, 348–351. [Google Scholar] [CrossRef]
- Hadders-Algra, M. Early Diagnostics and Early Intervention in Neurodevelopmental Disorders-Age-Dependent Challenges and Opportunities. J. Clin. Med. 2021, 10, 861. [Google Scholar] [CrossRef]
- Magee, J.C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef]
- Spoto, G.; Valentini, G.; Saia, M.C.; Butera, A.; Amore, G.; Salpietro, V.; Nicotera, A.G.; Di Rosa, G. Synaptopathies in Developmental and Epileptic Encephalopathies: A Focus on Pre-synaptic Dysfunction. Front. Neurol. 2021, 13, 826211. [Google Scholar] [CrossRef]
- Nicotera, A.G.; Amore, G.; Saia, M.C.; Vinci, M.; Musumeci, A.; Chiavetta, V.; Federico, C.; Spoto, G.; Saccone, S.; Di Rosa, G.; et al. Fibroblast Growth Factor Receptor 2 (FGFR2), a New Gene Involved in the Genesis of Autism Spectrum Disorder. Neuromolecular Med. 2023, 25, 650–656. [Google Scholar] [CrossRef]
- Medina, E.; Peterson, S.; Ford, K.; Singletary, K.; Peixoto, L. Critical periods and Autism Spectrum Disorders, a role for sleep. Neurobiol. Sleep Circadian Rhythm. 2022, 14, 100088. [Google Scholar] [CrossRef]
- Spoto, G.; Butera, A.; Albertini, M.L.; Consoli, C.; Ceraolo, G.; Nicotera, A.G.; Rosa, G.D. The Ambiguous Role of Growth Factors in Autism: What Do We Really Know? Int. J. Mol. Sci. 2025, 26, 1607. [Google Scholar] [CrossRef]
- Di Rosa, G.; Cavallaro, T.; Alibrandi, A.; Marseglia, L.; Lamberti, M.; Giaimo, E.; Nicotera, A.; Bonsignore, M.; Gagliano, A. Predictive role of early milestones-related psychomotor profiles and long-term neurodevelopmental pitfalls in preterm infants. Early Hum. Dev. 2016, 101, 49–55. [Google Scholar] [CrossRef]
- LeBlanc, J.J.; Fagiolini, M. Autism: A “critical period” disorder? Neural Plast. 2011, 2011, 921680. [Google Scholar] [CrossRef]
- Manti, S.; Spoto, G.; Nicotera, A.G.; Di Rosa, G.; Piedimonte, G. Impact of respiratory viral infections during pregnancy on the neurological outcomes of the newborn: Current knowledge. Front. Neurosci. 2024, 17, 1320319. [Google Scholar] [CrossRef]
- Kinney, H.C.; Brody, B.A.; Kloman, A.S.; Gilles, F.H. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J. Neuropathol. Exp. Neurol. 1988, 47, 217–234. [Google Scholar] [CrossRef]
- Spoto, G.; Nicotera, A.G.; Butera, A.; Di Rosa, G. Editorial: Neurodevelopment and preterm birth. Front. Neurol. 2024, 15, 1412711. [Google Scholar] [CrossRef] [PubMed]
- Roffwarg, H.P.; Muzio, J.N.; Dement, W.C. Ontogenetic development of the human sleep-dream cycle. Science 1966, 152, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, A.; Eisermann, M.; Plouin, P. Child EEG (and maturation). Handb. Clin. Neurol. 2019, 160, 125–142. [Google Scholar] [CrossRef] [PubMed]
- McVea, D.A.; Murphy, T.H.; Mohajerani, M.H. Large Scale Cortical Functional Networks Associated with Slow-Wave and Spindle-Burst-Related Spontaneous Activity. Front. Neural Circuits 2016, 10, 103. [Google Scholar] [CrossRef]
- Lindemann, C.; Ahlbeck, J.; Bitzenhofer, S.H.; Hanganu-Opatz, I.L. Spindle Activity Orchestrates Plasticity during Development and Sleep. Neural Plast. 2016, 2016, 5787423. [Google Scholar] [CrossRef]
- Di Bella, P.; Attardi, A.G.; Butera, A.; Mancini, A.; Calabrò, N.; Lo Re, E.G.; Trimarchi, G.; Nicotera, A.G.; Di Rosa, G.; Giudice, D.L. Semi-Automatic Analysis of Specific Electroencephalographic Patterns during NREM2 Sleep in a Pediatric Population after SARS-CoV-2 Infection. J. Pers. Med. 2024, 14, 152. [Google Scholar] [CrossRef]
- Tononi, G.; Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Bridi, M.C.D.; Peixoto, L. Excitatory/Inhibitory imbalance as a mechanism linking autism and sleep problems. Curr. Opin. Neurobiol. 2025, 90, 102968. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Norimoto, H. Sleep sharp wave ripple and its functions in memory and synaptic plasticity. Neurosci. Res. 2023, 189, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 2015, 25, 1073–1188. [Google Scholar] [CrossRef] [PubMed]
- Paterno, R.; Marafiga, J.R.; Ramsay, H.; Li, T.; Salvati, K.A.; Baraban, S.C. Hippocampal gamma and sharp-wave ripple oscillations are altered in a Cntnap2 mouse model of autism spectrum disorder. Cell Rep. 2021, 37, 109970. [Google Scholar] [CrossRef]
- Leontiadis, L.J.; Trompoukis, G.; Tsotsokou, G.; Miliou, A.; Felemegkas, P.; Papatheodoropoulos, C. Rescue of sharp wave-ripples and prevention of network hyperexcitability in the ventral but not the dorsal hippocampus of a rat model of fragile X syndrome. Front. Cell. Neurosci. 2023, 17, 1296235. [Google Scholar] [CrossRef]
- Tomisaki, E.; Tanaka, E.; Watanabe, T.; Shinohara, R.; Hirano, M.; Onda, Y.; Mochizuki, Y.; Yato, Y.; Yamakawa, N.; Anme, T.; et al. The relationship between the development of social competence and sleep in infants: A longitudinal study. Child Adolesc. Psychiatry Ment. Health 2018, 12, 53. [Google Scholar] [CrossRef]
- Bernier, A.; Beauchamp, M.H.; Bouvette-Turcot, A.A.; Carlson, S.M.; Carrier, J. Sleep and cognition in preschool years: Specific links to executive functioning. Child Dev. 2013, 84, 1542–1553. [Google Scholar] [CrossRef]
- Huhdanpää, H.; Morales-Muñoz, I.; Aronen, E.T.; Pölkki, P.; Saarenpää-Heikkilä, O.; Paunio, T.; Kylliäinen, A.; Paavonen, E.J. Sleep Difficulties in Infancy Are Associated with Symptoms of Inattention and Hyperactivity at the Age of 5 Years: A Longitudinal Study. J. Dev. Behav. Pediatr. JDBP 2019, 40, 432–440. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, F.V.; Al Mamun, A.; O’Callaghan, M.; Clavarino, A.; Williams, G.M.; Bor, W.; Heussler, H.; Najman, J.M. The link between sleep problems in infancy and early childhood and attention problems at 5 and 14 years: Evidence from a birth cohort study. Early Hum. Dev. 2010, 86, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A.; De Marcas, G.; Guri, Y.; Berger, A.; Tikotzky, L.; Bar-Haim, Y. Infant Sleep Predicts Attention Regulation and Behavior Problems at 3-4 Years of Age. Dev. Neuropsychol. 2015, 40, 122–137. [Google Scholar] [CrossRef]
- Dicanio, D.; Spoto, G.; Alibrandi, A.; Minutoli, R.; Nicotera, A.G.; Di Rosa, G. Long-term predictivity of early neurological assessment and developmental trajectories in low-risk preterm infants. Front. Neurol. 2022, 13, 958682. [Google Scholar] [CrossRef]
- Geva, R.; Yaron, H.; Kuint, J. Neonatal Sleep Predicts Attention Orienting and Distractibility. J. Atten. Disord. 2016, 20, 138–150. [Google Scholar] [CrossRef]
- Panda, S.; Hogenesch, J.B.; Kay, S.A. Circadian rhythms from flies to human. Nature 2002, 417, 329–335. [Google Scholar] [CrossRef]
- Elkhatib Smidt, S.D.; Ghorai, A.; Taylor, S.C.; Gehringer, B.N.; Dow, H.C.; Langer, A.; Rawot, E.; Zhang, J.; Mitchell, J.A.; Rader, D.J.; et al. The relationship between autism spectrum and sleep-wake traits. Autism Res. 2022, 15, 641–652. [Google Scholar] [CrossRef]
- Begum-Ali, J.; Gossé, L.K.; Mason, L.; Pasco, G.; Charman, T.; Johnson, M.H.; Jones, E.J.H.; STAARS Team. Infant sleep predicts trajectories of social attention and later autism traits. J. Child Psychol. Psychiatry Allied Discip. 2023, 64, 1200–1211. [Google Scholar] [CrossRef]
- MacDuffie, K.E.; Shen, M.D.; Dager, S.R.; Styner, M.A.; Kim, S.H.; Paterson, S.; Pandey, J.; St John, T.; Elison, J.T.; Wolff, J.J.; et al. Sleep Onset Problems and Subcortical Development in Infants Later Diagnosed with Autism Spectrum Disorder. Am. J. Psychiatry 2020, 177, 518–525. [Google Scholar] [CrossRef]
- Holding, B.C.; Sundelin, T.; Lekander, M.; Axelsson, J. Sleep deprivation and its effects on communication during individual and collaborative tasks. Sci. Rep. 2019, 9, 3131. [Google Scholar] [CrossRef]
- Calhoun, S.L.; Pearl, A.M.; Fernandez-Mendoza, J.; Durica, K.C.; Mayes, S.D.; Murray, M.J. Sleep Disturbances Increase the Impact of Working Memory Deficits on Learning Problems in Adolescents with High-Functioning Autism Spectrum Disorder. J. Autism Dev. Disord. 2020, 50, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Pathways in ASD Study Team; Georgiades, S.; Szatmari, P.; Duku, E.; Zwaigenbaum, L.; Bryson, S.; Roberts, W.; Fombonne, E.; Mirenda, P.; Smith, I.; et al. Phenotypic overlap between core diagnostic features and emotional/behavioral problems in preschool children with autism spectrum disorder. J. Autism Dev. Disord. 2011, 41, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Deutz, M.H.; Geeraerts, S.B.; van Baar, A.L.; Deković, M.; Prinzie, P. The Dysregulation Profile in middle childhood and adolescence across reporters: Factor structure, measurement invariance, and links with self-harm and suicidal ideation. Eur. Child Adolesc. Psychiatry 2016, 25, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.C.; Gorman, K. Development of Emotion Self-Regulation Among Young Children with Autism Spectrum Disorders: The Role of Parents. J. Autism Dev. Disord. 2018, 48, 1249–1260. [Google Scholar] [CrossRef]
- Favole, I.; Davico, C.; Marcotulli, D.; Sodero, R.; Svevi, B.; Amianto, F.; Ricci, F.S.; Arduino, G.M.; Vitiello, B. Sleep disturbances and emotional dysregulation in young children with autism spectrum, intellectual disability, or global developmental delay. Sleep Med. 2023, 105, 45–52. [Google Scholar] [CrossRef]
- Zaidman-Zait, A.; Zwaigenbaum, L.; Duku, E.; Bennett, T.; Szatmari, P.; Mirenda, P.; Smith, I.; Vaillancourt, T.; Volden, J.; Waddell, C.; et al. Factor analysis of the children’s sleep habits questionnaire among preschool children with autism spectrum disorder. Res. Dev. Disabil. 2020, 97, 103548. [Google Scholar] [CrossRef]
- Belli, A.; Breda, M.; Di Maggio, C.; Esposito, D.; Marcucci, L.; Bruni, O. Children with neurodevelopmental disorders: How do they sleep? Curr. Opin. Psychiatry 2022, 35, 345–351. [Google Scholar] [CrossRef]
- Whelan, S.; Mannion, A.; Madden, A.; Berger, F.; Costello, R.; Ghadiri, S.; Leader, G. Examining the Relationship Between Sleep Quality, Social Functioning, and Behavior Problems in Children with Autism Spectrum Disorder: A Systematic Review. Nat. Sci. Sleep 2022, 14, 675–695. [Google Scholar] [CrossRef]
- Bangerter, A.; Chatterjee, M.; Manyakov, N.V.; Ness, S.; Lewin, D.; Skalkin, A.; Boice, M.; Goodwin, M.S.; Dawson, G.; Hendren, R.; et al. Relationship Between Sleep and Behavior in Autism Spectrum Disorder: Exploring the Impact of Sleep Variability. Front. Neurosci. 2020, 14, 211. [Google Scholar] [CrossRef]
- Han, G.T.; Trevisan, D.A.; Abel, E.A.; Cummings, E.M.; Carlos, C.; Bagdasarov, A.; Kala, S.; Parker, T.; Canapari, C.; McPartland, J.C. Associations between sleep problems and domains relevant to daytime functioning and clinical symptomatology in autism: A meta-analysis. Autism Res. 2022, 15, 1249–1260. [Google Scholar] [CrossRef]
- Goldman, S.E.; Surdyka, K.; Cuevas, R.; Adkins, K.; Wang, L.; Malow, B.A. Defining the sleep phenotype in children with autism. Dev. Neuropsychol. 2009, 34, 560–573. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, F.E.; Foster-Owens, M.D.; Conduit, R.; Rinehart, N.J.; Riby, D.M.; Cornish, K.M. The developmental trajectory of parent-report and objective sleep profiles in autism spectrum disorder: Associations with anxiety and bedtime routines. Autism 2017, 21, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Fadini, C.C.; Lamônica, D.A.; Fett-Conte, A.C.; Osório, E.; Zuculo, G.M.; Giacheti, C.M.; Pinato, L. Influence of sleep disorders on the behavior of individuals with autism spectrum disorder. Front. Hum. Neurosci. 2015, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.K.D.; Murphy, L.E.; Kocak, M.; Tylavsky, F.A.; Pagani, L.S. Prospective associations between infant sleep at 12-months and autism spectrum disorder screening scores at 24-months in a community-based birth cohort. J. Clin. Psychiatry 2018, 79, 16m11127. [Google Scholar] [CrossRef]
- Hollway, J.A.; Aman, M.G. Pharmacological treatment of sleep disturbance in developmental disabilities: A review of the literature. Res. Dev. Disabil. 2011, 32, 939–962. [Google Scholar] [CrossRef]
- Esposito, D.; Belli, A.; Ferri, R.; Bruni, O. Sleeping without Prescription: Management of Sleep Disorders in Children with Autism with Non-Pharmacological Interventions and Over-the-Counter Treatments. Brain Sci. 2020, 10, 441. [Google Scholar] [CrossRef]
- Johnson, K.P.; Zarrinnegar, P. Autism Spectrum Disorder and Sleep. Child Adolesc. Psychiatr. Clin. 2021, 30, 195–208. [Google Scholar] [CrossRef]
- Wang, Z.; Ding, R.; Wang, J. The Association between Vitamin D Status and Autism Spectrum Disorder (ASD): A Systematic Review and Meta-Analysis. Nutrients 2020, 13, 86. [Google Scholar] [CrossRef]
- Chen, L.; Guo, X.; Hou, C.; Tang, P.; Zhang, X.; Chong, L.; Li, R. The causal association between iron status and the risk of autism: A Mendelian randomization study. Front. Nutr. 2022, 9, 957600. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Zhong, L.; Zeng, L.; Li, L.; Yao, P. Understanding autism: Causes, diagnosis, and advancing therapies. Brain Res. Bull. 2025, 227, 111411. [Google Scholar] [CrossRef]
- Di Rosa, G.; Nicotera, A.G.; Lenzo, P.; Spanò, M.; Tortorella, G. Long-term neuropsychiatric follow-up in hyperprolinemia type I. Psychiatr. Genet. 2014, 24, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Nutritional and metabolic status of children with autism vs. neurotypical children, and the association with autism severity. Nutr. Metab. 2011, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Smith-Hicks, C.; Wright, D.; Kenny, A.; Stowe, R.C.; McCormack, M.; Stanfield, A.C.; Holder, J.L., Jr. Sleep Abnormalities in the Synaptopathies-SYNGAP1-Related Intellectual Disability and Phelan-McDermid Syndrome. Brain Sci. 2021, 11, 1229. [Google Scholar] [CrossRef] [PubMed]
- Elia, M.; Ferri, R.; Musumeci, S.A.; Del Gracco, S.; Bottitta, M.; Scuderi, C.; Miano, G.; Panerai, S.; Bertrand, T.; Grubar, J.C. Sleep in subjects with autistic disorder: A neurophysiological and psychological study. Brain Dev. 2000, 22, 88–92. [Google Scholar] [CrossRef]
- Spoto, G.; Accetta, A.S.; Grella, M.; Di Modica, I.; Nicotera, A.G.; Di Rosa, G. Respiratory Disorders in Rett Syndrome. Curr. Respir. Med. Rev. 2024, 21, 72–80. [Google Scholar] [CrossRef]
- Wei, S.; Smits, M.G.; Tang, X.; Kuang, L.; Meng, H.; Ni, S.; Xiao, M.; Zhou, X. Efficacy and safety of melatonin for sleep onset insomnia in children and adolescents: A meta-analysis of randomized controlled trials. Sleep Med. 2020, 68, 1–8. [Google Scholar] [CrossRef]
- Sealy, J.; Glovinsky, I.P. Strengthening the reflective functioning capacities of parents who have a child with a neurodevelopmental disability through a brief, relationship-focused intervention. Infant Ment. Health J. 2016, 37, 115–124. [Google Scholar] [CrossRef]
- Williams Buckley, A.; Hirtz, D.; Oskoui, M.; Armstrong, M.J.; Batra, A.; Bridgemohan, C.; Coury, D.; Dawson, G.; Donley, D.; Findling, R.L.; et al. Practice guideline: Treatment for insomnia and disrupted sleep behavior in children and adolescents with autism spectrum disorder: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2020, 94, 392–404. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Louw, J.S.; Leader, G. Efficacy of parent training incorporated in behavioral sleep interventions for children with autism spectrum disorder and/or intellectual disabilities: A systematic review. Sleep Med. 2019, 53, 141–152. [Google Scholar] [CrossRef]
- Malow, B.A.; Adkins, K.W.; Reynolds, A.; Weiss, S.K.; Loh, A.; Fawkes, D.; Katz, T.; Goldman, S.E.; Madduri, N.; Hundley, R.; et al. Parent-based sleep education for children with autism spectrum disorders. J. Autism Dev. Disord. 2014, 44, 216–228. [Google Scholar] [CrossRef]
- Gringras, P.; Green, D.; Wright, B.; Rush, C.; Sparrowhawk, M.; Pratt, K.; Allgar, V.; Hooke, N.; Moore, D.; Zaiwalla, Z.; et al. Weighted Blankets and Sleep in Autistic Children—A Randomized Controlled Trial. Pediatrics. 2014, 134, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.M.T.; Cignolini, A.; Warren, R.; Budden, S.; Skowron-Gooch, A. Improvement in Sensory Impairment and Social Interaction in Young Children with Autism Following Treatment with an Original Qigong Massage Methodology. Am. J. Chin. Med. 2007, 35, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.W.; Krishna, J.; Klingemier, E.; Beukemann, M.; Nawabit, R.; Ibrahim, S. A Randomized, Crossover Trial of a Novel Sound-to-Sleep Mattress Technology in Children with Autism and Sleep Difficulties. J. Clin. Sleep Med. 2017, 13, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bruni, O.; Angriman, M.; Calisti, F.; Comandini, A.; Esposito, G.; Cortese, S.; Ferri, R. Practitioner Review: Treatment of chronic insomnia in children and adolescents with neurodevelopmental disabilities. J. Child Psychol. Psychiatry Allied Discip. 2018, 59, 489–508. [Google Scholar] [CrossRef]
- Bruni, O.; Angriman, M.; Melegari, M.G.; Ferri, R. Pharmacotherapeutic management of sleep disorders in children with neurodevelopmental disorders. Expert Opin. Pharmacother. 2019, 20, 2257–2271. [Google Scholar] [CrossRef]
- Schneider-Helmert, D.; Spinweber, C.L. Evaluation of L-tryptophan for treatment of insomnia: A review. Psychopharmacology 1986, 89, 1–7. [Google Scholar] [CrossRef]
- Monti, J.M. Serotonin control of sleep-wake behavior. Sleep Med. Rev. 2011, 15, 269–281. [Google Scholar] [CrossRef]
- van Zyl, L.T.; Chung, S.A.; Shahid, A.; Shapiro, C.M. L-Tryptophan As Treatment for Pediatric Non-Rapid Eye Movement Parasomnia. J. Child Adolesc. Psychopharmacol. 2018, 28, 395–401. [Google Scholar] [CrossRef]
- Bruni, O.; Ferri, R.; Miano, S.; Verrillo, E. L -5-Hydroxytryptophan treatment of sleep terrors in children. Eur. J. Pediatr. 2004, 163, 402–407. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Effects and side effects associated with the non-nutritional use of tryptophan by humans. J. Nutr. 2012, 142, 2236S–2244S. [Google Scholar] [CrossRef]
- Aziz-Zadeh, L.; Ringold, S.M.; Jayashankar, A.; Kilroy, E.; Butera, C.; Jacobs, J.P.; Tanartkit, S.; Mahurkar-Joshi, S.; Bhatt, R.R.; Dapretto, M.; et al. Relationships between brain activity, tryptophan-related gut metabolites, and autism symptomatology. Nat. Commun. 2025, 16, 3465. [Google Scholar] [CrossRef]
- Li, J.; Zhai, P.; Bi, L.; Wang, Y.; Yang, X.; Yang, Y.; Li, N.; Dang, W.; Feng, G.; Li, P.; et al. Associations between amino acid levels and autism spectrum disorder severity. BMC Psychiatry 2025, 25, 332. [Google Scholar] [CrossRef]
- Kałużna-Czaplińska, J.; Jóźwik-Pruska, J.; Chirumbolo, S.; Bjørklund, G. Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metab. Brain Dis. 2017, 32, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Daly, E.; Ecker, C.; Hallahan, B.; Deeley, Q.; Craig, M.; Murphy, C.; Johnston, P.; Spain, D.; Gillan, N.; Gudbrandsen, M.; et al. Response inhibition and serotonin in autism: A functional MRI study using acute tryptophan depletion. Brain 2014, 137 Pt 9, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Daly, E.M.; Deeley, Q.; Ecker, C.; Craig, M.; Hallahan, B.; Murphy, C.; Johnston, P.; Spain, D.; Gillan, N.; Brammer, M.; et al. Serotonin and the neural processing of facial emotions in adults with autism: An fMRI study using acute tryptophan depletion. Arch. Gen. Psychiatry 2012, 69, 1003–1013. [Google Scholar] [CrossRef]
- McDougle, C.J.; Naylor, S.T.; Cohen, D.J.; Aghajanian, G.K.; Heninger, G.R.; Price, L.H. Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch. Gen. Psychiatry 1996, 53, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Smolik, C.M.; Barba-Escobedo, P.A.; Gamez, M.; Sanchez, J.J.; Javors, M.A.; Daws, L.C.; Gould, G.G. Acute dietary tryptophan manipulation differentially alters social behavior, brain serotonin and plasma corticosterone in three inbred mouse strains. Neuropharmacology 2015, 90, 1–8. [Google Scholar] [CrossRef]
- Mehrazad-Saber, Z.; Kheirouri, S.; Noorazar, S.G. Effects of l-Carnosine Supplementation on Sleep Disorders and Disease Severity in Autistic Children: A Randomized, Controlled Clinical Trial. Basic Clin. Pharmacol. Toxicol. 2018, 123, 72–77. [Google Scholar] [CrossRef]
- Ming, X.; Stein, T.P.; Barnes, V.; Rhodes, N.; Guo, L. Metabolic perturbance in autism spectrum disorders: A metabolomics study. J. Proteome Res. 2012, 11, 5856–5862. [Google Scholar] [CrossRef]
- Chez, M.G.; Buchanan, C.P.; Aimonovitch, M.C.; Becker, M.; Schaefer, K.; Black, C.; Komen, J. Double-blind, placebo-controlled study of L-carnosine supplementation in children with autistic spectrum disorders. J. Child Neurol. 2002, 17, 833–837. [Google Scholar] [CrossRef]
- Ann Abraham, D.; Narasimhan, U.; Christy, S.; Muhasaparur Ganesan, R. Effect of L-Carnosine as adjunctive therapy in the management of children with autism spectrum disorder: A randomized controlled study. Amino Acids 2020, 52, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, A.; Lentini, G.; Rapacchietta, S.; Cinnirella, P.; Elia, M.; Ferri, R.; Bruni, O. The Role of Supplements and Over-the-Counter Products to Improve Sleep in Children: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 7821. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.; Ayton, S.; Bush, A.; Lei, P. A delicate balance: Iron metabolism and diseases of the brain. Front. Aging Neurosci. 2013, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Earley, C.J.; Allen, R.P.; Beard, J.L.; Connor, J.R. Insight into the pathophysiology of restless legs syndrome. J. Neurosci. Res. 2000, 62, 623–628. [Google Scholar] [CrossRef]
- Peirano, P.D.; Algarín, C.R.; Garrido, M.I.; Lozoff, B. Iron deficiency anemia in infancy is associated with altered temporal organization of sleep states in childhood. Pediatr. Res. 2007, 62, 715–719. [Google Scholar] [CrossRef]
- Kordas, K.; Siegel, E.H.; Olney, D.K.; Katz, J.; Tielsch, J.M.; Chwaya, H.M.; Kariger, P.K.; Leclerq, S.C.; Khatry, S.K.; Stoltzfus, R.J. Maternal reports of sleep in 6-18month-old infants from Nepal and Zanzibar: Association with iron deficiency anemia and stunting. Early Hum. Dev. 2008, 84, 389–398. [Google Scholar] [CrossRef]
- Youssef, J.; Singh, K.; Huntington, N.; Becker, R.; Kothare, S.V. Relationship of serum ferritin levels to sleep fragmentation and periodic limb movements of sleep on polysomnography in autism spectrum disorders. Pediatr. Neurol. 2013, 49, 274–278. [Google Scholar] [CrossRef]
- Dosman, C.F.; Brian, J.A.; Drmic, I.E.; Senthilselvan, A.; Harford, M.M.; Smith, R.W.; Sharieff, W.; Zlotkin, S.H.; Moldofsky, H.; Roberts, S.W. Children with autism: Effect of iron supplementation on sleep and ferritin. Pediatr. Neurol. 2007, 36, 152–158. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Connolly, H.V.; Katz, T.; Goldman, S.E.; Weiss, S.K.; Halbower, A.C.; Shui, A.M.; Macklin, E.A.; Hyman, S.L.; Malow, B.A. Randomized, Placebo-Controlled Trial of Ferrous Sulfate to Treat Insomnia in Children With Autism Spectrum Disorders. Pediatr. Neurol. 2020, 104, 30–39. [Google Scholar] [CrossRef]
- Gorantla, S.; Ravisankar, A.; Trotti, L.M. Magnesium citrate monotherapy improves restless legs syndrome symptoms and multiple suggested immobilization test scores in an open-label pilot study. J. Clin. Sleep Med. 2024, 20, 1357–1361. [Google Scholar] [CrossRef]
- Jadidi, A.; Rezaei Ashtiani, A.; Khanmohamadi Hezaveh, A.; Aghaepour, S.M. Therapeutic effects of magnesium and vitamin B6 in alleviating the symptoms of restless legs syndrome: A randomized controlled clinical trial. BMC Complement. Med. Ther. 2022, 23, 1. [Google Scholar] [CrossRef]
- Marshall, N.S.; Serinel, Y.; Killick, R.; Child, J.M.; Raisin, I.; Berry, C.M.; Lallukka, T.; Wassing, R.; Lee, R.W.; Ratnavadivel, R.; et al. Magnesium supplementation for the treatment of restless legs syndrome and periodic limb movement disorder: A systematic review. Sleep Med. Rev. 2019, 48, 101218. [Google Scholar] [CrossRef]
- DelRosso, L.M.; Picchietti, D.L.; Spruyt, K.; Bruni, O.; Garcia-Borreguero, D.; Kotagal, S.; Owens, J.A.; Simakajornboon, N.; Ferri, R.; International Restless Legs Syndrome Study Group (IRLSSG). Restless sleep in children: A systematic review. Sleep Med. Rev. 2021, 56, 101406. [Google Scholar] [CrossRef]
- Almalki, A.H.; Bamaga, A.K.; Alharbi, A.; Abduljabbar, M.H.; Alnemari, R.M.; Baali, F.H.; Algarni, M.A.; Ahmed, M.F.; Ramzy, S. Exploring the association between serum magnesium level and autism spectrum disorder using validated spectrofluorimetric method. Anal. Biochem. 2025, 699, 115755. [Google Scholar] [CrossRef]
- Skalny, A.V.; Mazaletskaya, A.L.; Ajsuvakova, O.P.; Bjørklund, G.; Skalnaya, M.G.; Chernova, L.N.; Skalny, A.A.; Tinkov, A.A. Magnesium Status in Children with Attention-Deficit/Hyperactivity Disorder and/or Autism Spectrum Disorder. J. Korean Acad. Child Adolesc. Psychiatry 2020, 31, 41–45. [Google Scholar] [CrossRef] [PubMed]
- LeClerc, S.; Easley, D. Pharmacological therapies for autism spectrum disorder: A review. Pharm. Ther. 2015, 40, 389–397. [Google Scholar]
- Pelayo, R.; Dubik, M. Pediatric sleep pharmacology. Semin. Pediatr. Neurol. 2008, 15, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Heussler, H.; Chan, P.; Price, A.M.; Waters, K.; Davey, M.J.; Hiscock, H. Pharmacological and non-pharmacological management of sleep disturbance in children: An Australian Paediatric Research Network survey. Sleep Med. 2013, 14, 189–194. [Google Scholar] [CrossRef]
- Efron, D.; Lycett, K.; Sciberras, E. Use of sleep medication in children with ADHD. Sleep Med. 2014, 15, 472–475. [Google Scholar] [CrossRef]
- Zambrelli, E.; Lividini, A.; Spadavecchia, S.; Turner, K.; Canevini, M.P. Effects of Supplementation With Antioxidant Agents on Sleep in Autism Spectrum Disorder: A Review. Front. Psychiatry 2021, 12, 689277. [Google Scholar] [CrossRef]
- Gringras, P.; Nir, T.; Breddy, J.; Frydman-Marom, A.; Findling, R.L. Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children With Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 948–957.e4. [Google Scholar] [CrossRef]
- Nogueira, H.A.; de Castro, C.T.; da Silva, D.C.G.; Pereira, M. Melatonin for sleep disorders in people with autism: Systematic review and meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 123, 110695. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.J.; Revell, V.L.; Molina, T.A.; Eastman, C.I. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J. Clin. Endocrinol. Metab. 2010, 95, 3325–3331. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Mishima, K.; Fukumizu, M.; Takahashi, H.; Ishikawa, Y.; Hamada, I.; Sugioka, H.; Yotsuya, O.; Yamashita, Y. Melatonin Treatment and Adequate Sleep Hygiene Interventions in Children with Autism Spectrum Disorder: A Randomized Controlled Trial. J. Autism Dev. Disord. 2022, 52, 2784–2793. [Google Scholar] [CrossRef] [PubMed]
- Braam, W.; van Geijlswijk, I.; Keijzer, H.; Smits, M.G.; Didden, R.; Curfs, L.M. Loss of response to melatonin treatment is associated with slow melatonin metabolism. J. Intellect. Disabil. Res. JIDR 2010, 54, 547–555. [Google Scholar] [CrossRef]
- Yang, H.; Lu, F.; Zhao, X. Factors influencing the effect of melatonin on sleep quality in children with autism spectrum disorder: A systematic review and meta-analysis. Sleep Breath. 2025, 29, 1–12. [Google Scholar] [CrossRef]
- Ming, X.; Gordon, E.; Kang, N.; Wagner, G.C. Use of clonidine in children with autism spectrum disorders. Brain Dev. 2008, 30, 454–460. [Google Scholar] [CrossRef]
- Nguyen, M.; Tharani, S.; Rahmani, M.; Shapiro, M. A review of the use of clonidine as a sleep aid in the child and adolescent population. Clin. Pediatr. 2014, 53, 211–216. [Google Scholar] [CrossRef]
- Jaselskis, C.A.; Cook, E.H.; Fletcher, K.E., Jr.; Leventhal, B.L. Clonidine treatment of hyperactive and impulsive children with autistic disorder. J. Clin. Psychopharmacol. 1992, 12, 322–327. [Google Scholar] [CrossRef]
- Fankhauser, M.P.; Karumanchi, V.C.; German, M.L.; Yates, A.; Karumanchi, S.D. A double-blind, placebo-controlled study of the efficacy of transdermal clonidine in autism. J. Clin. Psychiatry 1992, 53, 77–82. [Google Scholar]
- Politte, L.C.; Scahill, L.; Figueroa, J.; McCracken, J.T.; King, B.; McDougle, C.J. A randomized, placebo-controlled trial of extended-release guanfacine in children with autism spectrum disorder and ADHD symptoms: An analysis of secondary outcome measures. Neuropsychopharmacology 2018, 43, 1772–1778. [Google Scholar] [CrossRef]
- Scahill, L.; McCracken, J.T.; King, B.H.; Rockhill, C.; Shah, B.; Politte, L.; Sanders, R.; Minjarez, M.; Cowen, J.; Mullett, J.; et al. Extended-Release Guanfacine for Hyperactivity in Children With Autism Spectrum Disorder. Am. J. Psychiatry 2015, 172, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Posey, D.J.; Puntney, J.I.; Sasher, T.M.; Kem, D.L.; McDougle, C.J. Guanfacine treatment of hyperactivity and inattention in pervasive developmental disorders: A retrospective analysis of 80 cases. J. Child Adolesc. Psychopharmacol. 2004, 14, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.M.; Gururaj, V.J.; Allen, J.E. The effectiveness of diphenhydramine HCI in pediatric sleep disorders. J. Clin. Pharmacol. 1976, 16, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Mignot, E.; Taheri, S.; Nishino, S. Sleeping with the hypothalamus: Emerging therapeutic targets for sleep disorders. Nat. Neurosci. 2002, 5 (Suppl. S11), 1071–1075. [Google Scholar] [CrossRef]
- Ottaviano, S.; Giannotti, F.; Cortesi, F. The effect of niaprazine on some common sleep disorders in children. A double-blind clinical trial by means of continuous home-videorecorded sleep. Child’s Nerv. Syst. 1991, 7, 332–335. [Google Scholar] [CrossRef]
- Magera, B.E.; Betlach, C.J.; Sweatt, A.P.; Derrick, C.W., Jr. Hydroxyzine intoxication in a 13-month-old child. Pediatrics 1981, 67, 280–283. [Google Scholar] [CrossRef]
- France, K.G.; Blampied, N.M.; Wilkinson, P. A multiple-baseline, double-blind evaluation of the effects of trimeprazine tartrate on infant sleep disturbance. Exp. Clin. Psychopharmacol. 1999, 7, 502–513. [Google Scholar] [CrossRef]
- Simonoff, E.A.; Stores, G. Controlled trial of trimeprazine tartrate for night waking. Arch. Dis. Child. 1987, 62, 253–257. [Google Scholar] [CrossRef]
- France, K.G.; Blampied, N.M.; Wilkinson, P. Treatment of infant sleep disturbance by trimeprazine in combination with extinction. J. Dev. Behav. Pediatr. JDBP 1991, 12, 308–314. [Google Scholar] [CrossRef]
- Borowy, C.S.; Mukherji, P. Antihistamine Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Owens, J.A.; Rosen, C.L.; Mindell, J.A. Medication use in the treatment of pediatric insomnia: Results of a survey of community-based pediatricians. Pediatrics 2003, 111 Pt 1, e628–e635. [Google Scholar] [CrossRef] [PubMed]
- Gringras, P. When to use drugs to help sleep. Arch. Dis. Child. 2008, 93, 976–981. [Google Scholar] [CrossRef]
- Ginovart, N.; Kapur, S. Role of dopamine D(2) receptors for antipsychotic activity. Handb. Exp. Pharmacol. 2012, 212, 27–52. [Google Scholar] [CrossRef]
- Kaar, S.J.; Natesan, S.; McCutcheon, R.; Howes, O.D. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology 2020, 172, 107704. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Corves, C.; Arbter, D.; Engel, R.R.; Li, C.; Davis, J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 2009, 373, 31–41. [Google Scholar] [CrossRef]
- Ishitobi, M.; Kosaka, H.; Takahashi, T.; Yatuga, C.; Asano, M.; Tanaka, Y.; Ueno, K.; Okazaki, R.; Omori, M.; Hiratani, M.; et al. Effectiveness and tolerability of switching to aripiprazole from risperidone in subjects with autism spectrum disorders: A prospective open-label study. Clin. Neuropharmacol. 2013, 36, 151–156. [Google Scholar] [CrossRef]
- Masi, G.; Cosenza, A.; Millepiedi, S.; Muratori, F.; Pari, C.; Salvadori, F. Aripiprazole monotherapy in children and young adolescents with pervasive developmental disorders: A retrospective study. CNS Drugs 2009, 23, 511–521. [Google Scholar] [CrossRef]
- Golubchik, P.; Sever, J.; Weizman, A. Low-dose quetiapine for adolescents with autistic spectrum disorder and aggressive behavior: Open-label trial. Clin. Neuropharmacol. 2011, 34, 216–219. [Google Scholar] [CrossRef]
- Schnoes, C.J.; Kuhn, B.R.; Workman, E.F.; Ellis, C.R. Pediatric prescribing practices for clonidine and other pharmacologic agents for children with sleep disturbance. Clin. Pediatr. 2006, 45, 229–238. [Google Scholar] [CrossRef]
- Kent, J.M.; Hough, D.; Singh, J.; Karcher, K.; Pandina, G. An open-label extension study of the safety and efficacy of risperidone in children and adolescents with autistic disorder. J. Child Adolesc. Psychopharmacol. 2013, 23, 676–686. [Google Scholar] [CrossRef]
- Lloyd, R.; Tippmann-Peikert, M.; Slocumb, N.; Kotagal, S. Characteristics of REM sleep behavior disorder in childhood. J. Clin. Sleep Med. 2012, 8, 127–131. [Google Scholar] [CrossRef]
- Gunja, N. The clinical and forensic toxicology of Z-drugs. J. Med. Toxicol. 2013, 9, 155–162. [Google Scholar] [CrossRef]
- Dolder, C.; Nelson, M.; McKinsey, J. Use of non-benzodiazepine hypnotics in the elderly: Are all agents the same? CNS Drugs 2007, 21, 389–405. [Google Scholar] [CrossRef]
- Arens, R.; Wright, B.; Elliott, J.; Zhao, H.; Wang, P.P.; Brown, L.W.; Namey, T.; Kaplan, P. Periodic limb movement in sleep in children with Williams syndrome. J. Pediatr. 1998, 133, 670–674. [Google Scholar] [CrossRef]
- Thirumalai, S.S.; Shubin, R.A.; Robinson, R. Rapid eye movement sleep behavior disorder in children with autism. J. Child Neurol. 2002, 17, 173–178. [Google Scholar] [CrossRef]
- Blumer, J.L.; Reed, M.D.; Steinberg, F.; O’Riordan, M.A.; Rosen, C.L.; Springer, M.A.; Christensen, M.; Glaze, D.; NICHD PPRU Network. Potential pharmacokinetic basis for zolpidem dosing in children with sleep difficulties. Clin. Pharmacol. Ther. 2008, 83, 551–558. [Google Scholar] [CrossRef]
- Blumer, J.L.; Findling, R.L.; Shih, W.J.; Soubrane, C.; Reed, M.D. Controlled clinical trial of zolpidem for the treatment of insomnia associated with attention-deficit/ hyperactivity disorder in children 6 to 17 years of age. Pediatrics 2009, 123, e770–e776. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05540574 (accessed on 30 April 2025).
- Walsh, J.K.; Pollak, C.P.; Scharf, M.B.; Schweitzer, P.K.; Vogel, G.W. Lack of residual sedation following middle-of-the-night zaleplon administration in sleep maintenance insomnia. Clin. Neuropharmacol. 2000, 23, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Kalachnik, J.E.; Hanzel, T.E.; Sevenich, R.; Harder, S.R. Brief report: Clonazepam behavioral side effects with an individual with mental retardation. J. Autism Dev. Disord. 2003, 33, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.E.; Tanzi, M.G.; Gabay, M. Paradoxical reactions to benzodiazepines: Literature review and treatment options. Pharmacotherapy 2004, 24, 1177–1185. [Google Scholar] [CrossRef]
- Vaquerizo-Serrano, J.; De Pablo, G.S.; Singh, J.; Santosh, P. Catatonia in autism spectrum disorders: A systematic review and meta-analysis. Eur. Psychiatry. 2021, 65, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A. Update in pediatric sleep medicine. Curr. Opin. Pulm. Med. 2011, 17, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Garg, A.; Rataboli, P.V. Role of zolpidem in the management of insomnia. CNS Neurosci. Ther. 2011, 17, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Wagner, M.L. Non-benzodiazepines for the treatment of insomnia. Sleep Med. Rev. 2000, 4, 551–581. [Google Scholar] [CrossRef]
- Valtuille, Z.; Acquaviva, E.; Trebossen, V.; Ouldali, N.; Bourmaud, A.; Sclison, S.; Gomez, A.; Revet, A.; Peyre, H.; Delorme, R.; et al. Prescription Trends of Medications Used to Treat Sleep Disturbances in School-Aged Children: An Interrupted Time-Series Analysis in France, 2016–2023. J. Pediatr. 2025, 280, 114502. [Google Scholar] [CrossRef]
- Wichniak, A.; Wierzbicka, A.; Jernajczyk, W. Sleep and antidepressant treatment. Curr. Pharm. Des. 2012, 18, 5802–5817. [Google Scholar] [CrossRef]
- Posey, D.J.; Guenin, K.D.; Kohn, A.E.; Swiezy, N.B.; McDougle, C.J. A naturalistic open-label study of mirtazapine in autistic and other pervasive developmental disorders. J. Child Adolesc. Psychopharmacol. 2001, 11, 267–277. [Google Scholar] [CrossRef]
- Owens, J.A.; Rosen, C.L.; Mindell, J.A.; Kirchner, H.L. Use of pharmacotherapy for insomnia in child psychiatry practice: A national survey. Sleep Med. 2010, 11, 692–700. [Google Scholar] [CrossRef]
- Pranzatelli, M.R.; Tate, E.D.; Dukart, W.S.; Flint, M.J.; Hoffman, M.T.; Oksa, A.E. Sleep disturbance and rage attacks in opsoclonus-myoclonus syndrome: Response to trazodone. J. Pediatr. 2005, 147, 372–378. [Google Scholar] [CrossRef]
- Bossini, L.; Coluccia, A.; Casolaro, I.; Benbow, J.; Amodeo, G.; De Giorgi, R.; Fagiolini, A. Off-Label Trazodone Prescription: Evidence, Benefits and Risks. Curr. Pharm. Des. 2015, 21, 3343–3351. [Google Scholar] [CrossRef]
- Rosenberg, R.P.; Hull, S.G.; Lankford, D.A.; Mayleben, D.W.; Seiden, D.J.; Furey, S.A.; Jayawardena, S.; Roth, T. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J. Clin. Sleep Med. 2014, 10, 1093–1100. [Google Scholar] [CrossRef]
- Robinson, A.A.; Malow, B.A. Gabapentin shows promise in treating refractory insomnia in children. J. Child Neurol. 2013, 28, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Xu, Y.; Ding, W.; Tang, Q.; Zhang, B.; Yuan, Y.; Jin, J. Research progress on melatonin, 5-HT, and orexin in sleep disorders of children with autism spectrum disorder. Biomol. Biomed. 2025, 25, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Kostansek, J.A., IV; Latona, G.J.; Heruye, S.H.; Matthews, S.; Bockman, C.S.; Simeone, K.A.; Simeone, T.A. Orexin receptors regulate hippocampal sharp wave-ripple complexes in ex vivo slices. Eur. J. Pharmacol. 2023, 950, 175763. [Google Scholar] [CrossRef]
- de Gans, C.J.; Burger, P.; van den Ende, E.S.; Hermanides, J.; Nanayakkara, P.W.B.; Gemke, R.J.B.J.; Rutters, F.; Stenvers, D.J. Sleep assessment using EEG-based wearables-A systematic review. Sleep Med. Rev. 2024, 76, 101951. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; AMAli, A.; Aslam, H.; AAbd-Alrazaq, A.; AlSaad, R.; Alajlani, M.; Ahmad, R.; Khalil, L.; Ahmed, A.; Sheikh, J. Wearable Artificial Intelligence for Sleep Disorders: Scoping Review. J. Med. Internet Res. 2025, 27, e65272. [Google Scholar] [CrossRef]
- Chae, S.M.; Yeo, J.Y.; Han, S.Y.; Chung, N.R.; Hwang, J.H. Infant sleep interventions with sleep measurements using actigraphy: A systematic review. Int. J. Nurs. Pract. 2024, 30, e13196. [Google Scholar] [CrossRef]
- Lee, J.; Lim, J.; Kang, S.; Kim, S.; Jung, S.Y.; Kim, S.; Hong, S.B.; Park, Y.R. Mobile App-Assisted Parent Training Intervention for Behavioral Problems in Children with Autism Spectrum Disorder: Pilot Randomized Controlled Trial. JMIR Hum. Factors 2024, 11, e52295. [Google Scholar] [CrossRef]

| Behavioral Intervention | Description of the Intervention | Type of Sleep Disorder |
|---|---|---|
| Extinction—planned ignoring | Caregiver ignores undesirable, sleep-disruptive behavior (e.g., crying), encouraging self-soothing. | Delayed sleep onset, nocturnal awakenings, co-sleeping, and bedtime resistance |
| Gradual extinction | Caregiver ignores bedtime disruption only for a predetermined amount of time before intervening. | |
| Scheduled awakenings | Caregiver interrupts the sleep cycle and prevents the disruptive episode by waking the child shortly before the typical onset of the event. | Arousal disorders such as nocturnal awakenings and sleep terrors |
| Bedtime fading | Gradually delay bedtime to match the child’s natural sleep-onset time. | Bedtime resistance |
| Stimulus fading | Caregiver progressively increases their physical distance from the child at bedtime. | Co-sleeping |
| Chronotherapy | Caregiver gradually moves bedtime and wake time later each day to shift and stabilize the child’s circadian rhythm. | CSWRD |
| Bedtime pass | The child receives a bedtime pass allowing one room exit or parent visit per night. After use, further exits are ignored, with a silent return to bed. Unused passes can be traded for a weekly reward. | Nocturnal awakenings |
| Agents | Pharmacological Class | Dosage | Mechanisms of Action | Effect on Sleep Structure | Adverse Effects |
|---|---|---|---|---|---|
| Melatonin | Serotonin-derived neurohormone | 0.5–4 mg/day up to 6 mg in adolescents | MT1/MT2 R agonist, contributing to sleep-promoting and chronobiotic effects | ↓ SoL, ↑ TST, regulate circadian rhythms | Daytime drowsiness, headache, nausea |
| Clonidine | α2-adrenergic agonist | 0.05–0.10 mg/day up to 0.30 mg/day | α2-adrenergic R agonist, inhibiting noradrenergic transmission | ↑ REM latency | Daytime drowsiness, orthostatic hypotension, GI disorder, skin irritation (due to transdermal formulations) |
| Diphenhydramine | Antihistamine | 0.5 mg/kg up to 25 mg/day | H1 R antagonist | ↓ SoL, ↓ arousal threshold | Daytime drowsiness, GI disorder; overdose leading to catatonic stupor, anxiety, hallucinations, respiratory failure |
| Trimeprazine tartrate | Antihistamine | 6 mg/kg/day | H1 R and D2 R antagonist | ↓ WASO and NA | Daytime drowsiness, irritability |
| Hydroxyzine | Antihistamine | 0.5–1 mg/kg/day | H1 R antagonist | ↓ SoL, ↓ arousal threshold | Daytime drowsiness, headache |
| Niaprazine | Antihistamine | 1 mg/kg/day | H1 R antagonist | ↓ SoL, ↓ arousal threshold | Daytime drowsiness |
| Clonazepam | Benzodiazepine | 0.25–0.5 mg/day | GABA Rs agonist | ↓ SoL, ↓ arousal threshold | Daytime drowsiness, rebound insomnia, anterograde amnesia (dose-dependent); respiratory depression |
| Zolpidem | Z-drug | 0.25 mg/kg/day up to 10 mg/day | GABA Rs agonist | ↓ SoL and WASO, ↑ TST | Headache, GI disorders, dizziness |
| Mirtazapine | Antidepressant | 7.5–45 mg/day | Presynaptic α2-adrenergic R and 5-HT2/3 R antagonist | ↑ SWS and ↓ SoL | Sedation, dry mouth, ↑ appetite, GI disorders, myalgia, dizziness, tremor, irritability, confusion |
| Trazodone | Antidepressant | 25–150 mg/day | 5-HT2A/C R and H1 R antagonist | ↓ SoL, ↑ sleep maintenance, ↓ REM, ↑ SWS | Daytime drowsiness, nausea, morning grogginess, serotonin syndrome, hypotension |
| Amitriptyline | Antidepressant | 5–50 mg/day | 5-HT2A R, α-adrenergic, H1 R, and mACh R antagonist | ↓ SoL, ↑ REM latency | Anxiety, constipation, sedation, anticholinergic effects, cardiotoxicity |
| Gabapentin | Anti-seizure medication | 3–15 mg/kg/day | GABA Rs agonist | ↑ SWS and ↓ WASO | Irritability and delayed sleep onset |
| Risperidone | Atypical antipsychotic | 0.5–3.5 mg/day | D2 R, and 5-HT2 R antagonist | ↓ SoL and WASO, ↑ TST and SWS | Weight gain, DM, hyperlipidemia, neuroleptic malignant syndrome, tardive dyskinesia, MD |
| Aripiprazole | Atypical antipsychotic | 1–5 mg/day | D2 R antagonist or partial agonist; 5-HT1A/2C R partial agonist, 5HT2A R antagonist | ↓ SoL and WASO, ↑ TST and SWS | Weight gain, DM, hyperlipidemia, neuroleptic malignant syndrome, tardive dyskinesia, MD |
| Olanzapine | Atypical antipsychotic | 2–10 mg/day | D2/D4 R antagonist and 5-HT2A/2C R antagonist | ↓ SoL and WASO, ↑ TST and SWS | Weight gain, daytime drowsiness, hypercholesterolemia, DM |
| Quetiapine | Atypical antipsychotic | 25–150 mg/day | D2 R, 5-HT1A/2A R antagonist | ↓ SoL and WASO, ↑ TST and SWS | Weight gain, dizziness, headache, sedation, orthostatic hypotension, hyperglycemia, dyslipidemia, tardive dyskinesia |
| Suvorexant | Orexin antagonist | 10 mg/day up to 20 mg | OX1/2 R antagonist | ↓ SoL and WASO | Daytime drowsiness, headache, abnormal dreams, narcolepsy/cataplexy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albertini, M.L.; Spoto, G.; Ceraolo, G.; Fichera, M.F.; Consoli, C.; Nicotera, A.G.; Di Rosa, G. Sleep Disorders in Children with Autism Spectrum Disorder: Developmental Impact and Intervention Strategies. Brain Sci. 2025, 15, 983. https://doi.org/10.3390/brainsci15090983
Albertini ML, Spoto G, Ceraolo G, Fichera MF, Consoli C, Nicotera AG, Di Rosa G. Sleep Disorders in Children with Autism Spectrum Disorder: Developmental Impact and Intervention Strategies. Brain Sciences. 2025; 15(9):983. https://doi.org/10.3390/brainsci15090983
Chicago/Turabian StyleAlbertini, Maria Ludovica, Giulia Spoto, Graziana Ceraolo, Maria Flavia Fichera, Carla Consoli, Antonio Gennaro Nicotera, and Gabriella Di Rosa. 2025. "Sleep Disorders in Children with Autism Spectrum Disorder: Developmental Impact and Intervention Strategies" Brain Sciences 15, no. 9: 983. https://doi.org/10.3390/brainsci15090983
APA StyleAlbertini, M. L., Spoto, G., Ceraolo, G., Fichera, M. F., Consoli, C., Nicotera, A. G., & Di Rosa, G. (2025). Sleep Disorders in Children with Autism Spectrum Disorder: Developmental Impact and Intervention Strategies. Brain Sciences, 15(9), 983. https://doi.org/10.3390/brainsci15090983








