Dopaminergic Inhibition of the Inwardly Rectifying Potassium Current in Direct Pathway Medium Spiny Neurons in Normal and Parkinsonian Striatum
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Patch Clamp Electrophysiology
2.3. Unilateral Intrastriatal Microinjection and Contralateral Rotation
2.4. Immunohistochemistry
2.5. Drugs and Chemicals
2.6. Statistical Analysis
3. Results
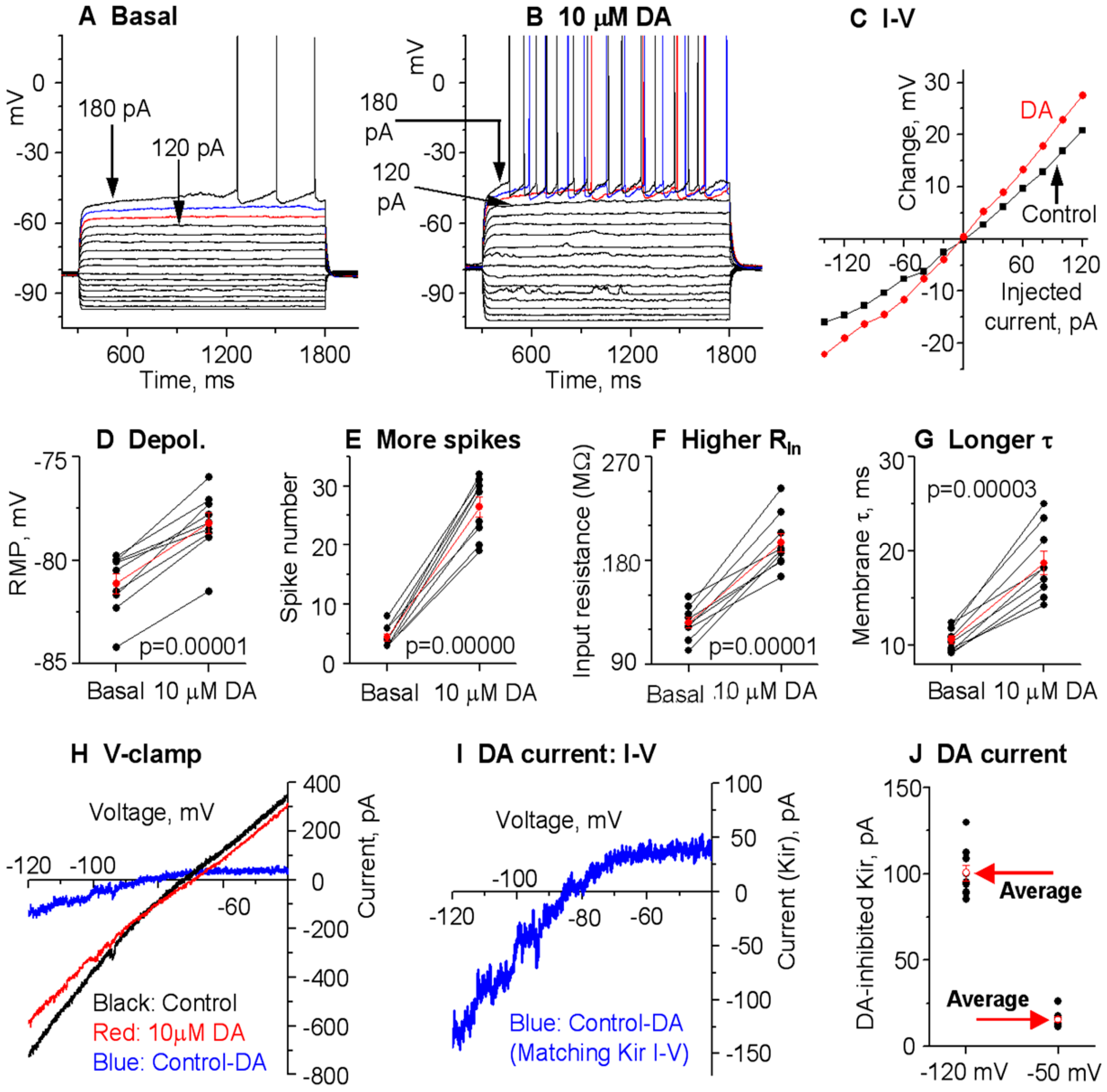
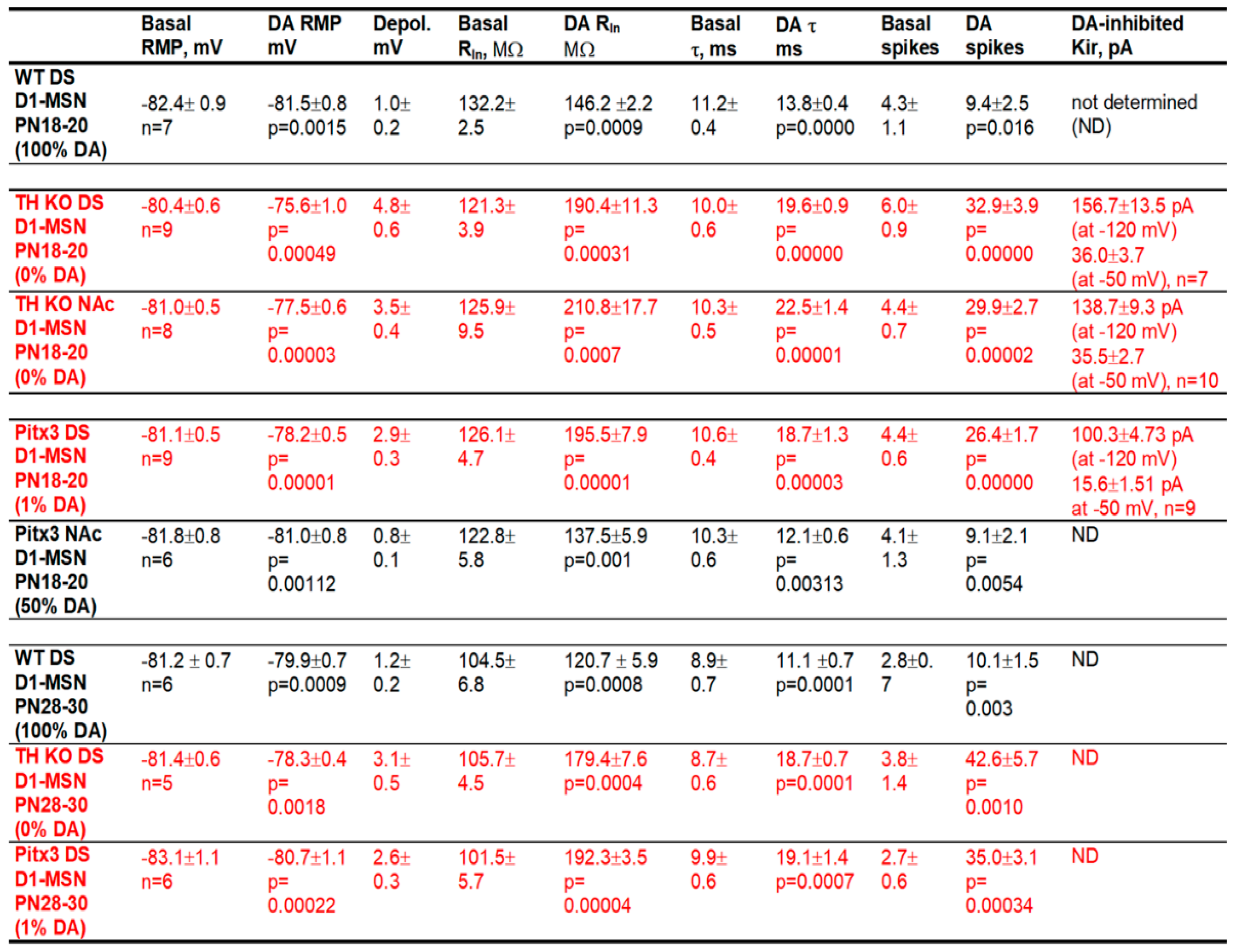
3.1. DA Modestly Increases D1-MSN Excitability in the Dorsal Striatum (DS) in Normal Mice with Intact DA Innervation
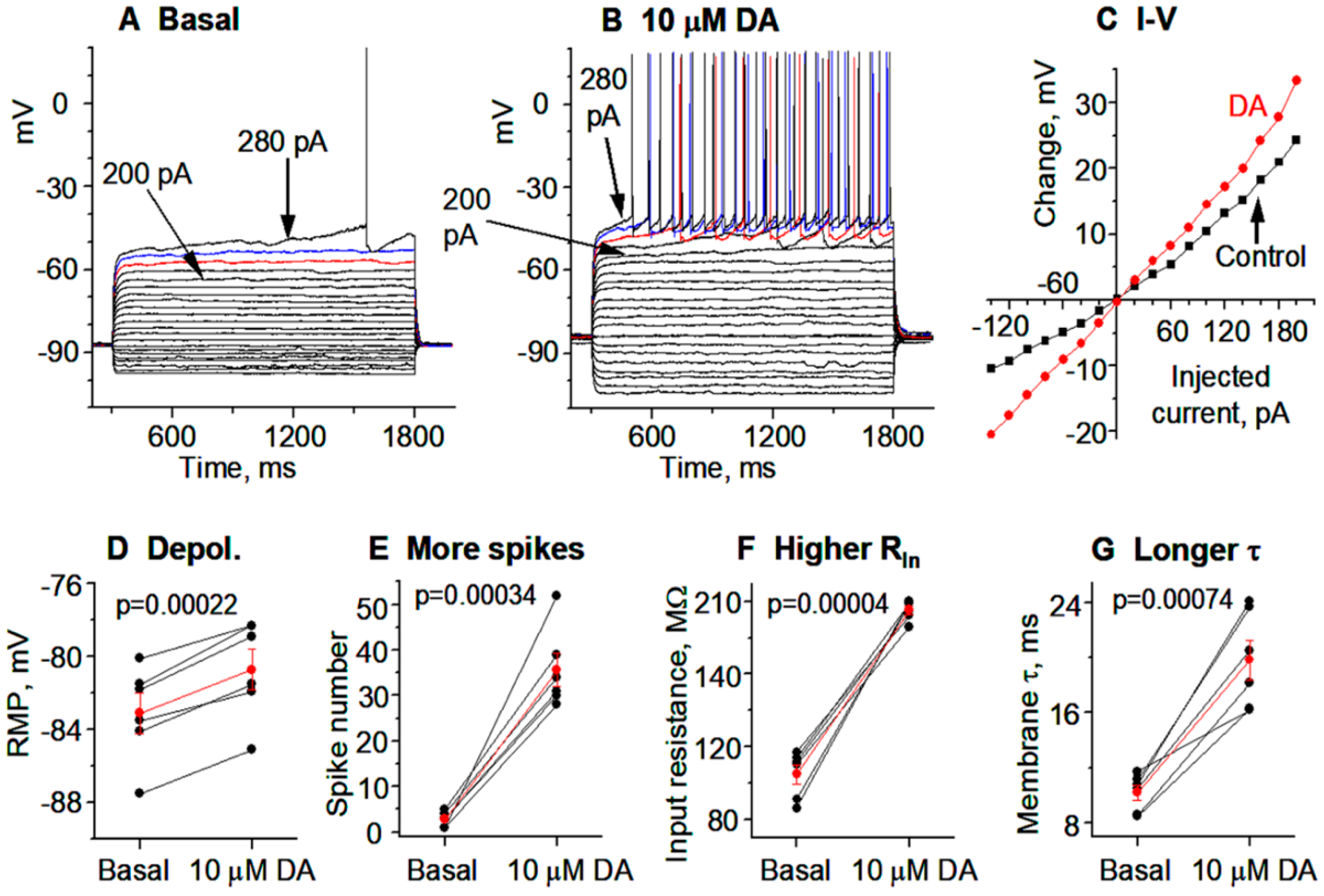
3.2. DA Hyperactively Increases D1-MSN Excitability in the DS in TH KO Mice with Total DA Loss
3.3. DA Agonism Hyperactively Increases D1-MSN Excitability in the NAc in TH KO Mice with Total DA Loss
3.4. Hyperactive DA Responses in D1-MSNs Remain in More Mature TH KO Mice
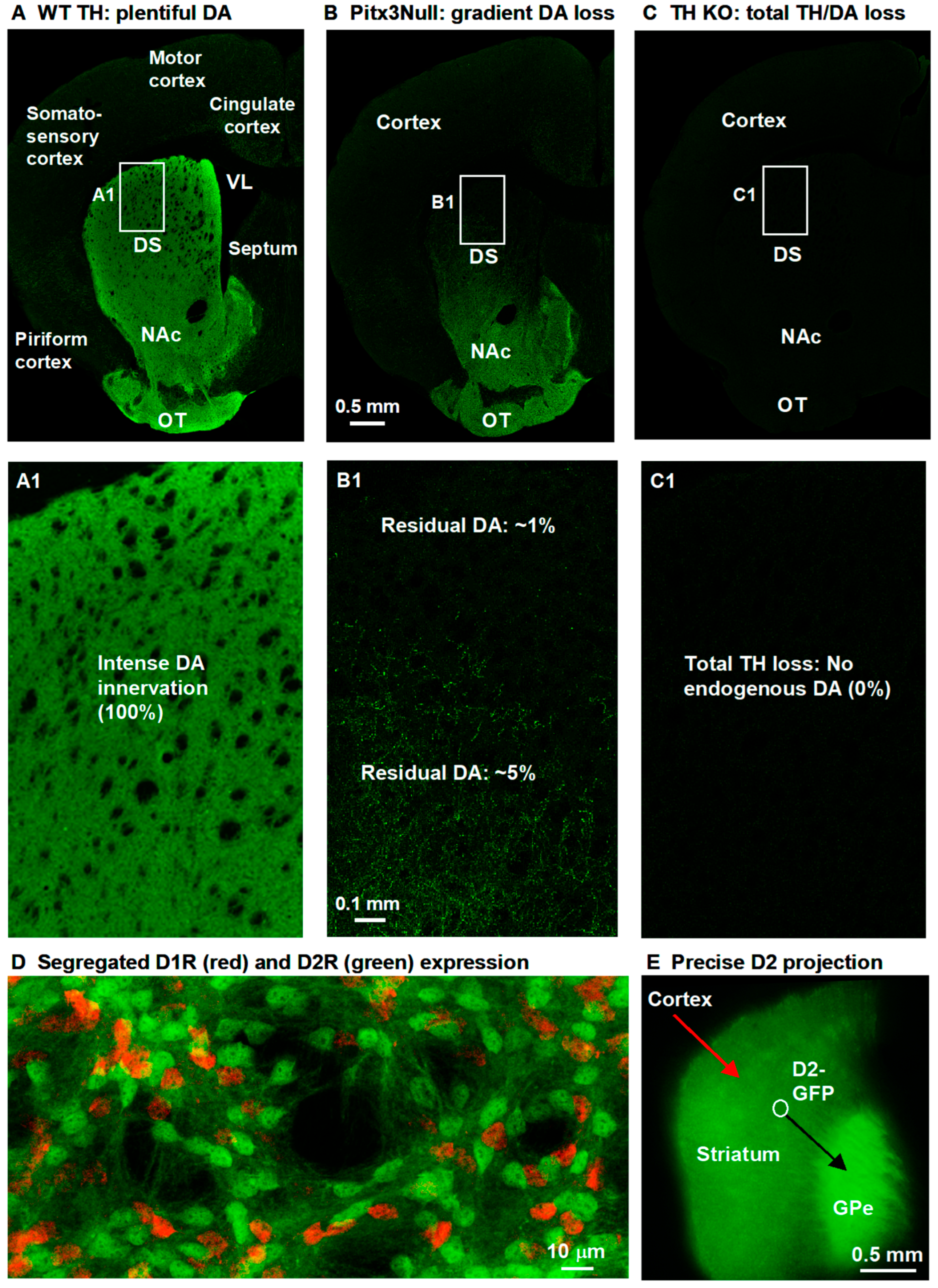
3.5. Gradient Hyperactive DA Response in D1-MSNs in Pitx3Null Mice with Gradient DA Denervation
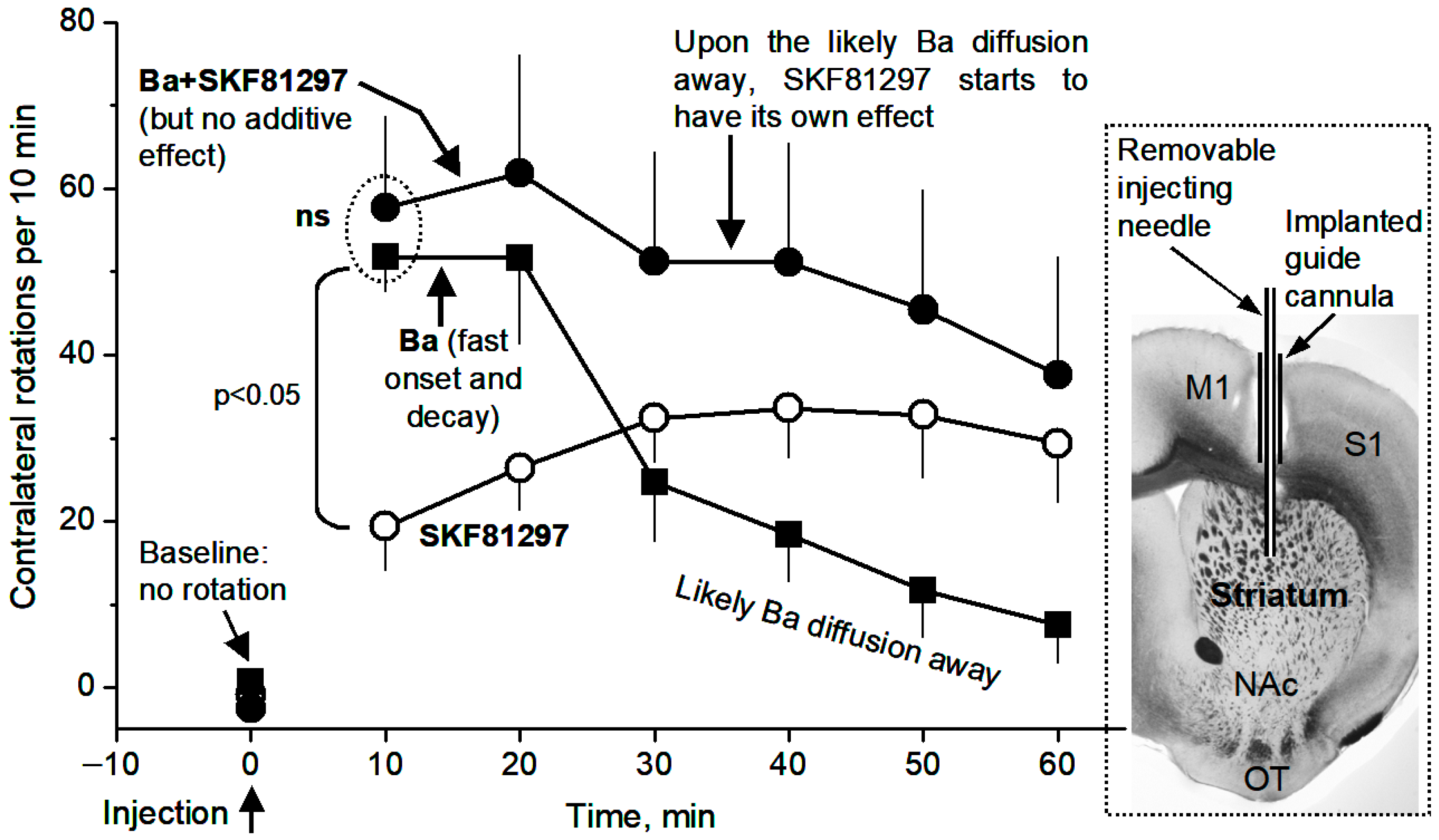
3.6. Local Microinjection of BaCl2, a Kir Inhibitor, into the Dorsal Striatum Stimulates Movement and Occludes the Motor Stimulation of Microinjected D1 Agonist SKF81297
4. Discussion
4.1. Hyperactive D1R Activation Inhibits Kir in D1-MSNs and Increases Their Intrinsic Excitability in Parkinsonian Animals Displaying Hyperactive D1R Agonistic Motor Response
4.2. DA Response Intensity in D1-MSNs Is Dependent on DA Denervation Severity
4.3. D1-MSN Basal Intrinsic Membrane Excitability Is Slightly Low or Nearly Normal in Parkinsonian Striatum in a Brain Slice Preparation
4.4. Kir Is a Key Ion Channel Mediating DA/D1R Agonism’s Profound Motor Stimulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, F.M. Whole-Brain Confocal Imaging Provides an Accurate Global View of the Nigral Dopamine System. Diagnostics 2025, 15, 1436. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Bolam, J.P. The Neuroanatomical Organization of the Basal Ganglia. In Handbook of Behavioral Neuroscience; Heinz Steiner, K.Y.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 24, pp. 3–32. [Google Scholar]
- LeWitt, P.A.; Fahn, S. Levodopa therapy for Parkinson disease: A look backward and forward. Neurology 2016, 86, S3–S12. [Google Scholar] [CrossRef]
- Li, L.; Zhou, F.M. Parallel dopamine D1 receptor activity dependence of l-Dopa-induced normal movement and dyskinesia in mice. Neuroscience 2013, 236, 66–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mailman, R.; Huang, X.; Nichols, D.E. Parkinson’s disease and D1 dopamine receptors. Curr. Opin. Investig. Drugs 2001, 2, 1582–1591. [Google Scholar] [PubMed][Green Version]
- Rascol, O.; Blin, O.; Thalamas, C.; Descombes, S.; Soubrouillard, C.; Azulay, P.; Fabre, N.; Viallet, F.; Lafnitzegger, K.; Wright, S.; et al. ABT-431, a D1 receptor agonist prodrug, has efficacy in Parkinson’s disease. Ann. Neurol. 1999, 45, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.D.; Prescott, T.J. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog. Neurobiol. 2010, 90, 385–417. [Google Scholar] [CrossRef]
- Li, C.; Elabi, O.F.; Fieblinger, T.; Cenci, M.A. Structural-functional properties of direct-pathway striatal neurons at early and chronic stages of dopamine denervation. Eur. J. Neurosci. 2024, 59, 1227–1241. [Google Scholar] [CrossRef]
- Nicola, S.M.; Surmeier, J.; Malenka, R.C. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu. Rev. Neurosci. 2000, 23, 185–215. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, J.; Dai, D.; Xing, J.; He, J.; Fu, Z.; Zhang, L.; Li, Z.; Wang, W. Differential dopaminergic regulation of inwardly rectifying potassium channel mediated subthreshold dynamics in striatal medium spiny neurons. Neuropharmacology 2016, 107, 396–410. [Google Scholar] [CrossRef]
- Friend, D.M.; Kravitz, A.V. Working together: Basal ganglia pathways in action selection. Trends Neurosci. 2014, 37, 301–303. [Google Scholar] [CrossRef]
- Sippy, T.; Lapray, D.; Crochet, S.; Petersen, C.C. Cell-Type-Specific Sensorimotor Processing in Striatal Projection Neurons during Goal-Directed Behavior. Neuron 2015, 88, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Nisenbaum, E.S.; Wilson, C.J. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J. Neurosci. 1995, 15, 4449–4463. [Google Scholar] [CrossRef] [PubMed]
- Ariano, M.A.; Cepeda, C.; Calvert, C.R.; Flores-Hernandez, J.; Hernandez-Echeagaray, E.; Klapstein, G.J.; Chandler, S.H.; Aronin, N.; DiFiglia, M.; Levine, M.S. Striatal potassium channel dysfunction in Huntington’s disease transgenic mice. J. Neurophysiol. 2005, 93, 2565–2574. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Tian, X.; Day, M.; Ulrich, S.; Tkatch, T.; Nathanson, N.M.; Surmeier, D.J. Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat. Neurosci. 2007, 10, 1458–1466. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Cazorla, M.; Shegda, M.; Ramesh, B.; Harrison, N.L.; Kellendonk, C. Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J. Neurosci. 2012, 32, 2398–2409. [Google Scholar] [CrossRef]
- Higashi, H.; Inanaga, K.; Nishi, S.; Uchimura, N. Enhancement of dopamine actions on rat nucleus accumbens neurones in vitro after methamphetamine pre-treatment. J. Physiol. 1989, 408, 587–603. [Google Scholar] [CrossRef]
- Pacheco-Cano, M.T.; Bargas, J.; Hernandez-Lopez, S.; Tapia, D.; Galarraga, E. Inhibitory action of dopamine involves a subthreshold Cs(+)-sensitive conductance in neostriatal neurons. Exp. Brain Res. 1996, 110, 205–211. [Google Scholar] [CrossRef]
- Uchimura, N.; Higashi, H.; Nishi, S. Hyperpolarizing and depolarizing actions of dopamine via D-1 and D-2 receptors on nucleus accumbens neurons. Brain Res. 1986, 375, 368–372. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Freeze, B.S.; Parker, P.R.; Kay, K.; Thwin, M.T.; Deisseroth, K.; Kreitzer, A.C. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010, 466, 622–626. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, F.M. Striatal But Not Extrastriatal Dopamine Receptors Are Critical to Dopaminergic Motor Stimulation. Front. Pharmacol. 2017, 8, 935. [Google Scholar] [CrossRef]
- Lahiri, A.K.; Bevan, M.D. Dopaminergic Transmission Rapidly and Persistently Enhances Excitability of D1 Receptor-Expressing Striatal Projection Neurons. Neuron 2020, 106, 277–290.e6. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, O.J.; McGuirt, A.F.; Mosharov, E.V.; Pigulevskiy, I.; Hobson, B.D.; Choi, S.; Frier, M.D.; Santini, E.; Borgkvist, A.; Sulzer, D. Dopamine Triggers the Maturation of Striatal Spiny Projection Neuron Excitability during a Critical Period. Neuron 2018, 99, 540–554.e4. [Google Scholar] [CrossRef] [PubMed]
- Suarez, L.M.; Alberquilla, S.; Garcia-Montes, J.R.; Moratalla, R. Differential Synaptic Remodeling by Dopamine in Direct and Indirect Striatal Projection Neurons in Pitx3(-/-) Mice, a Genetic Model of Parkinson’s Disease. J. Neurosci. 2018, 38, 3619–3630. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Li, L.; Zhou, F.M. Nigral dopamine loss induces a global upregulation of presynaptic dopamine D1 receptor facilitation of the striatonigral GABAergic output. J. Neurophysiol. 2015, 113, 1697–1711. [Google Scholar] [CrossRef]
- Li, L.; Qiu, G.; Ding, S.; Zhou, F.M. Serotonin hyperinnervation and upregulated 5-HT2A receptor expression and motor-stimulating function in nigrostriatal dopamine-deficient Pitx3 mutant mice. Brain Res. 2013, 1491, 236–250. [Google Scholar] [CrossRef]
- Li, L.; Sagot, B.; Zhou, F.M. Similar L-dopa-stimulated motor activity in mice with adult-onset 6-hydroxydopamine-induced symmetric dopamine denervation and in transcription factor Pitx3 null mice with perinatal-onset symmetric dopamine denervation. Brain Res. 2015, 1615, 12–21. [Google Scholar] [CrossRef]
- Sagot, B.; Li, L.; Zhou, F.M. Hyperactive Response of Direct Pathway Striatal Projection Neurons to L-dopa and D1 Agonism in Freely Moving Parkinsonian Mice. Front. Neural Circuits 2018, 12, 57. [Google Scholar] [CrossRef]
- Wei, W.; Li, L.; Yu, G.; Ding, S.; Li, C.; Zhou, F.M. Supersensitive presynaptic dopamine D2 receptor inhibition of the striatopallidal projection in nigrostriatal dopamine-deficient mice. J. Neurophysiol. 2013, 110, 2203–2216. [Google Scholar] [CrossRef]
- Wei, W.; Ding, S.; Zhou, F.M. Dopaminergic treatment weakens medium spiny neuron collateral inhibition in the parkinsonian striatum. J. Neurophysiol. 2017, 117, 987–999. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, Y.; Lin, G.; Liao, F.F.; Zhou, F.M. Dopamine-independent development and maintenance of mouse striatal medium spiny neuron dendritic spines. Neurobiol. Dis. 2023, 181, 106096. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.-M.; Li, L.; Yue, J.; Dani, J.A. Transcription factor Pitx3 mutant mice as a model for Parkinson’s disease. Front. Biol. 2016, 11, 427–438. [Google Scholar] [CrossRef]
- Ding, S.; Li, L.; Zhou, F.M. Robust presynaptic serotonin 5-HT(1B) receptor inhibition of the striatonigral output and its sensitization by chronic fluoxetine treatment. J. Neurophysiol. 2015, 113, 3397–3409. [Google Scholar] [CrossRef]
- van den Munckhof, P.; Gilbert, F.; Chamberland, M.; Levesque, D.; Drouin, J. Striatal neuroadaptation and rescue of locomotor deficit by L-dopa in aphakia mice, a model of Parkinson’s disease. J. Neurochem. 2006, 96, 160–170. [Google Scholar] [CrossRef]
- Pons, R.; Syrengelas, D.; Youroukos, S.; Orfanou, I.; Dinopoulos, A.; Cormand, B.; Ormazabal, A.; Garzia-Cazorla, A.; Serrano, M.; Artuch, R. Levodopa-induced dyskinesias in tyrosine hydroxylase deficiency. Mov. Disord. 2013, 28, 1058–1063. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Palmiter, R.D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 1995, 83, 1197–1209. [Google Scholar] [CrossRef]
- Kim, D.S.; Szczypka, M.S.; Palmiter, R.D. Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J. Neurosci. 2000, 20, 4405–4413. [Google Scholar] [CrossRef]
- Seeman, P.; Weinshenker, D.; Quirion, R.; Srivastava, L.K.; Bhardwaj, S.K.; Grandy, D.K.; Premont, R.T.; Sotnikova, T.D.; Boksa, P.; El-Ghundi, M.; et al. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc. Natl. Acad. Sci. USA 2005, 102, 3513–3518. [Google Scholar] [CrossRef]
- Kim, D.S.; Froelick, G.J.; Palmiter, R.D. Dopamine-dependent desensitization of dopaminergic signaling in the developing mouse striatum. J. Neurosci. 2002, 22, 9841–9849. [Google Scholar] [CrossRef]
- Wang, Y.; Bouabid, S.; Darvas, M.; Zhou, F.M. The antiparkinson drug ropinirole inhibits movement in a Parkinson’s disease mouse model with residual dopamine neurons. Exp. Neurol. 2020, 333, 113427. [Google Scholar] [CrossRef]
- Gong, S.; Zheng, C.; Doughty, M.L.; Losos, K.; Didkovsky, N.; Schambra, U.B.; Nowak, N.J.; Joyner, A.; Leblanc, G.; Hatten, M.E.; et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 2003, 425, 917–925. [Google Scholar] [CrossRef]
- Valjent, E.; Bertran-Gonzalez, J.; Herve, D.; Fisone, G.; Girault, J.A. Looking BAC at striatal signaling: Cell-specific analysis in new transgenic mice. Trends Neurosci. 2009, 32, 538–547. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, F.M. cAMP-producing chemogenetic and adenosine A2a receptor activation inhibits the inwardly rectifying potassium current in striatal projection neurons. Neuropharmacology 2019, 148, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J. Neurosci. 1993, 13, 4908–4923. [Google Scholar] [CrossRef] [PubMed]
- Koos, T.; Tepper, J.M. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci. 1999, 2, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Tepper, J.M.; Koos, T.; Wilson, C.J. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004, 27, 662–669. [Google Scholar] [CrossRef]
- Zhou, F.W.; Jin, Y.; Matta, S.G.; Xu, M.; Zhou, F.M. An ultra-short dopamine pathway regulates basal ganglia output. J. Neurosci. 2009, 29, 10424–10435. [Google Scholar] [CrossRef]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0.05”. Am. Stat. 2019, 73, 1–19. [Google Scholar] [CrossRef]
- Kubo, Y.; Adelman, J.P.; Clapham, D.E.; Jan, L.Y.; Karschin, A.; Kurachi, Y.; Lazdunski, M.; Nichols, C.G.; Seino, S.; Vandenberg, C.A. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol. Rev. 2005, 57, 509–526. [Google Scholar] [CrossRef]
- Perez, M.F.; White, F.J.; Hu, X.T. Dopamine D2 receptor modulation of K+ channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. J. Neurophysiol. 2006, 96, 2217–2228. [Google Scholar] [CrossRef]
- Keefe, K.A.; Gerfen, C.R. D1 dopamine receptor-mediated induction of zif268 and c-fos in the dopamine-depleted striatum: Differential regulation and independence from NMDA receptors. J. Comp. Neurol. 1996, 367, 165–176. [Google Scholar] [CrossRef]
- Paul, M.L.; Graybiel, A.M.; David, J.C.; Robertson, H.A. D1-like and D2-like dopamine receptors synergistically activate rotation and c-fos expression in the dopamine-depleted striatum in a rat model of Parkinson’s disease. J. Neurosci. 1992, 12, 3729–3742. [Google Scholar] [CrossRef] [PubMed]
- Alagem, N.; Dvir, M.; Reuveny, E. Mechanism of Ba2+ block of a mouse inwardly rectifying K+ channel: Differential contribution by two discrete residues. J. Physiol. 2001, 534, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.G.; Marshall, J.D.; Ahanonu, B.; Wu, Y.W.; Kim, T.H.; Grewe, B.F.; Zhang, Y.; Li, J.Z.; Ding, J.B.; Ehlers, M.D.; et al. Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature 2018, 557, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.B.; Bair-Marshall, C.; Nelson, A.B. Aberrant Striatal Activity in Parkinsonism and Levodopa-Induced Dyskinesia. Cell Rep. 2018, 23, 3438–3446.e5. [Google Scholar] [CrossRef]
- Singh, A.; Liang, L.; Kaneoke, Y.; Cao, X.; Papa, S.M. Dopamine regulates distinctively the activity patterns of striatal output neurons in advanced parkinsonian primates. J. Neurophysiol. 2015, 113, 1533–1544. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef]
- Corvol, J.C.; Studler, J.M.; Schonn, J.S.; Girault, J.A.; Herve, D. Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J. Neurochem. 2001, 76, 1585–1588. [Google Scholar] [CrossRef]
- Herve, D. Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front. Neuroanat. 2011, 5, 48. [Google Scholar] [CrossRef]
- Lee, K.W.; Hong, J.H.; Choi, I.Y.; Che, Y.; Lee, J.K.; Yang, S.D.; Song, C.W.; Kang, H.S.; Lee, J.H.; Noh, J.S.; et al. Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase. J. Neurosci. 2002, 22, 7931–7940. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Stoof, J.C.; Kebabian, J.W. Opposing roles for D-1 and D-2 dopamine receptors in efflux of cyclic AMP from rat neostriatum. Nature 1981, 294, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yoshimoto, J.; Kannon, T.; Kuroda, K.; Kaibuchi, K. Phosphorylation Signals in Striatal Medium Spiny Neurons. Trends Pharmacol. Sci. 2016, 37, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Planert, H.; Berger, T.K.; Silberberg, G. Membrane properties of striatal direct and indirect pathway neurons in mouse and rat slices and their modulation by dopamine. PLoS ONE 2013, 8, e57054. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.V.; Riccardi, E.; D’Ascenzo, M.; Azzena, G.B.; Grassi, C. Dopamine D1-like receptor activation depolarizes medium spiny neurons of the mouse nucleus accumbens by inhibiting inwardly rectifying K+ currents through a cAMP-dependent protein kinase A-independent mechanism. Neuroscience 2010, 167, 678–690. [Google Scholar] [CrossRef]
- Rice, M.E.; Patel, J.C.; Cragg, S.J. Dopamine release in the basal ganglia. Neuroscience 2011, 198, 112–137. [Google Scholar] [CrossRef]
- Marcott, P.F.; Gong, S.; Donthamsetti, P.; Grinnell, S.G.; Nelson, M.N.; Newman, A.H.; Birnbaumer, L.; Martemyanov, K.A.; Javitch, J.A.; Ford, C.P. Regional Heterogeneity of D2-Receptor Signaling in the Dorsal Striatum and Nucleus Accumbens. Neuron 2018, 98, 575–587.e4. [Google Scholar] [CrossRef]
- Kish, S.J.; Shannak, K.; Hornykiewicz, O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease; Pathophysiologic and clinical implications. N. Engl. J. Med. 1988, 318, 876–880. [Google Scholar] [CrossRef]
- Hornykiewicz, O. Chemical neuroanatomy of the basal ganglia--normal and in Parkinson’s disease. J. Chem. Neuroanat. 2001, 22, 3–12. [Google Scholar] [CrossRef]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Fieblinger, T.; Graves, S.M.; Sebel, L.E.; Alcacer, C.; Plotkin, J.L.; Gertler, T.S.; Chan, C.S.; Heiman, M.; Greengard, P.; Cenci, M.A.; et al. Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nat. Commun. 2014, 5, 5316. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Ling, G.Y.; Gajendiran, M.; Xu, Z.C. Enhanced excitatory synaptic transmission in spiny neurons of rat striatum after unilateral dopamine denervation. Neurosci. Lett. 2001, 308, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Tseng, K.Y.; Kasanetz, F.; Kargieman, L.; Riquelme, L.A.; Murer, M.G. Cortical slow oscillatory activity is reflected in the membrane potential and spike trains of striatal neurons in rats with chronic nigrostriatal lesions. J. Neurosci. 2001, 21, 6430–6439. [Google Scholar] [CrossRef]
- Kaneko, S.; Hikida, T.; Watanabe, D.; Ichinose, H.; Nagatsu, T.; Kreitman, R.J.; Pastan, I.; Nakanishi, S. Synaptic integration mediated by striatal cholinergic interneurons in basal ganglia function. Science 2000, 289, 633–637. [Google Scholar] [CrossRef]
- Xu, M.; Li, L.; Pittenger, C. Ablation of fast-spiking interneurons in the dorsal striatum, recapitulating abnormalities seen post-mortem in Tourette syndrome, produces anxiety and elevated grooming. Neuroscience 2016, 324, 321–329. [Google Scholar] [CrossRef]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Gutman, G.A.; Chandy, K.G.; Grissmer, S.; Lazdunski, M.; McKinnon, D.; Pardo, L.A.; Robertson, G.A.; Rudy, B.; Sanguinetti, M.C.; Stuhmer, W.; et al. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol. Rev. 2005, 57, 473–508. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Fabbro, D.; Kelly, E.; Mathie, A.A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Davies, J.A.; et al. The Concise Guide to PHARMACOLOGY 2023/24: Enzymes. Br. J. Pharmacol. 2023, 180 (Suppl. 2), S289–S373. [Google Scholar] [CrossRef]
- Taglialatela, M.; Drewe, J.A.; Brown, A.M. Barium blockade of a clonal potassium channel and its regulation by a critical pore residue. Mol. Pharmacol. 1993, 44, 180–190. [Google Scholar] [CrossRef]
- Marcott, P.F.; Mamaligas, A.A.; Ford, C.P. Phasic dopamine release drives rapid activation of striatal D2-receptors. Neuron 2014, 84, 164–176. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, Y.; Liao, F.-F.; Zhou, F.-M. Dopaminergic Inhibition of the Inwardly Rectifying Potassium Current in Direct Pathway Medium Spiny Neurons in Normal and Parkinsonian Striatum. Brain Sci. 2025, 15, 979. https://doi.org/10.3390/brainsci15090979
Wang Q, Wang Y, Liao F-F, Zhou F-M. Dopaminergic Inhibition of the Inwardly Rectifying Potassium Current in Direct Pathway Medium Spiny Neurons in Normal and Parkinsonian Striatum. Brain Sciences. 2025; 15(9):979. https://doi.org/10.3390/brainsci15090979
Chicago/Turabian StyleWang, Qian, Yuhan Wang, Francesca-Fang Liao, and Fu-Ming Zhou. 2025. "Dopaminergic Inhibition of the Inwardly Rectifying Potassium Current in Direct Pathway Medium Spiny Neurons in Normal and Parkinsonian Striatum" Brain Sciences 15, no. 9: 979. https://doi.org/10.3390/brainsci15090979
APA StyleWang, Q., Wang, Y., Liao, F.-F., & Zhou, F.-M. (2025). Dopaminergic Inhibition of the Inwardly Rectifying Potassium Current in Direct Pathway Medium Spiny Neurons in Normal and Parkinsonian Striatum. Brain Sciences, 15(9), 979. https://doi.org/10.3390/brainsci15090979






