The High-Risk Model of Threat Perception Modulates Learning of Placebo and Nocebo Effects and Functional Somatic Disorders
Abstract
1. Introduction
2. Is Paradoxical Skin Temperature Increase (PTI) During Threat Perception in Chronic Pain Linked to Dysregulation of the ANS?
3. The HRMTP Modulates Functional Somatic Disorders and Placebo and Nocebo Somatic Effects
“Sometimes it is more important to know what kind of patient has a disease than what kind of disease the patient has.”—Sir William Osler
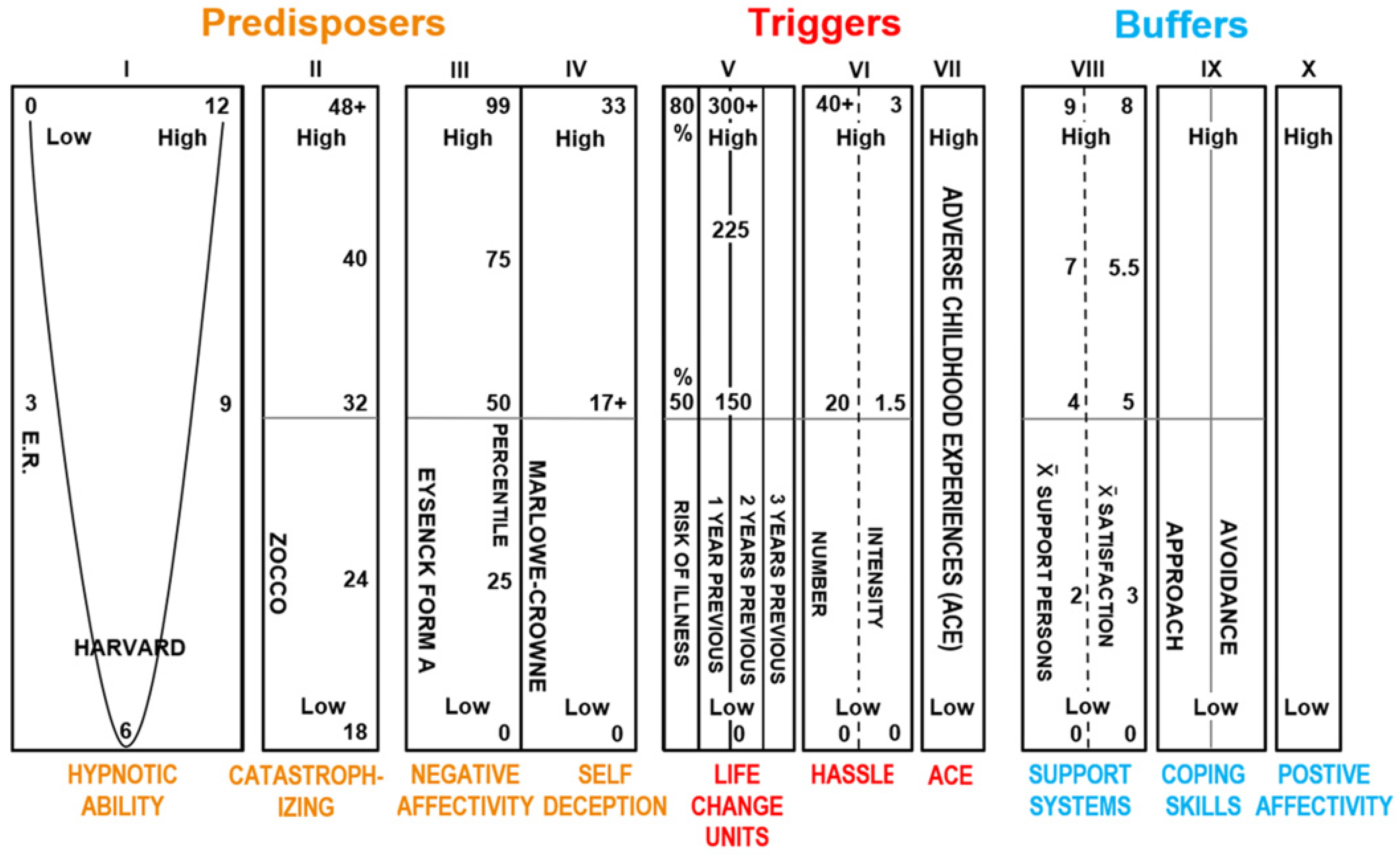
- A. Predisposing Risk Factors Modulating Threat and Pain Perception
- 1.
- Trait hypnotizability–suggestibility [65,66,67,71,110], related trait Absorption [111] and trait Alexithymia [27,72,75,78] are three closely related personality trait factors that are postulated by the HRMTP to be psychosocial personality mechanisms modulating (1) ANS reactivity during threat (HPAA) and pain perception, (2) the generation of somatic placebo and nocebo effects and (3) FSD [55]. Specifically, it is postulated [24,26,27,33] that these three related personality traits, (1) hypnotizability–suggestibility, (2) Absorption and (3) Alexithymia, despite varied labels, all empirically converge to modulate human threat perception (HPAA) and pain perception and to modulate FSD [55].
- 2.
- High learned catastrophizing [24,28,30,112,113,114,115,116,117] is explicit or implicit verbal responses to threat that reliably amplifies threat and pain perception and is measured by verbal report psychometric scales [27,114] and also apparently manifests in EEG effective connectivity measures [113]. Amygdala functional connectivity in fMRI data mediates the association between catastrophizing and threat-safety learning in chronic pain in youth [16].
- 3.
- High trait Neuroticism or Negative Affectivity (NA) [24,30,36,38,86,112,118,119] is a predisposition to amplified threat perception, independent of objective negative events. Trait or state NA can be measured with psychometric measures [86] or by the electrodermal response—EDR [36,38,83], and state NA appears associated with the default mode network (DMN) in chronic low back pain [85]. Nocebo effects are linked to high NA as a risk factor [116]. High NA is robustly related experimentally, even to the common cold ([120], NEJM) and medical illness with identified pathophysiology [121].
- 4.
- High trait Self-Deception (SD) is a trait tendency to interpret negative events or threats positively [30,33,112]. SD is measured with a High Marlowe Crowne score [122,123] or another psychometric measure of social desirability. High SD is a >17 score on the Marlowe Crowne psychometric scale and is a measure of repressed threat perception unrelated to the complex Weinberger hypothesis [122,124].
- B. Triggering Risk Factors Modulating Threat or Pain Perception
- 1.
- 2.
- The density and intensity of Daily Hassles: Accumulation of Daily Hassles inducing threat in some people activates the hypothalamic–pituitary–adrenal axis and can be associated with mortality and morbidity [24,30,33,129]. Hassles are measured with several psychometric scales of known reliability and validity.
- 3.
- C. Buffering Risk Factors Modulating Threat or Pain Perception
- 1.
- High social support [24,26,30,33,99,100,133] is measured by several verbal report psychometric scales and is reliably correlated with many physical diseases, including cardiovascular and cancer diseases, in replicated studies of morbidity and mortality mediated by threat (HPAA) and other neuroendocrine measures.
- 2.
- Approach and Avoidance Coping Skills [133,134,135] are measured by Approach and Avoidance psychometric scales [136] and are related to amplified Approach Coping Skills in positive mental and physical health outcomes [133]. Two-factor learning theory [27] predicts that avoidance behavior [137] is associated with many mental disorders and avoidance coping is postulated to be associated with FSD, specifically chronic pain [27,32,138]. Approach coping and placebo effects are associated with “safety cues” and fear extinction [27,139] and reduced somatization and reduced FSD.
- 3.
- High positive affectivity [30,140,141,142] is measured with several psychometric verbal report scales and is reliably related to positive mental and physical clinical health outcomes [140]. High positive affectivity appears to buffer nocebo effects [141]. Two studies [141,142] recently confirmed the HRMTP’s prediction that experimentally induced trait or state positive affectivity would buffer or reduce nocebo effects.
4. Primary Predisposing Risk Factor Trait Hypnotizability–Suggestibility Modulates Threat Perception (HPAA) and the Expression of Other Predisposing, Triggering and Buffering Risk Factors
4.1. Predisposing Risk Factor Trait Hypnotizaiblity and Blood Pressure Reactivity in the ICU from Cadiac Bypass Surgery
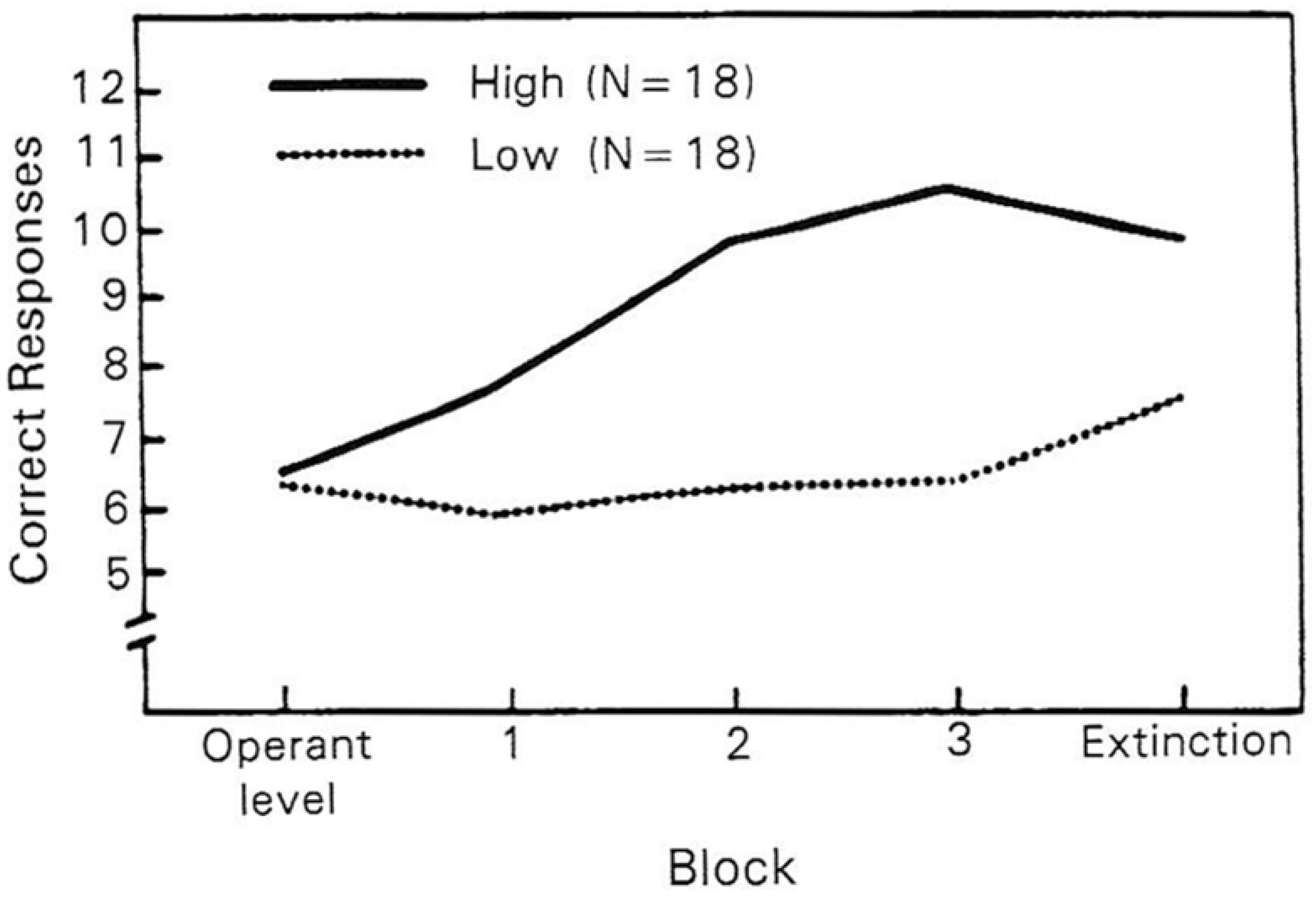
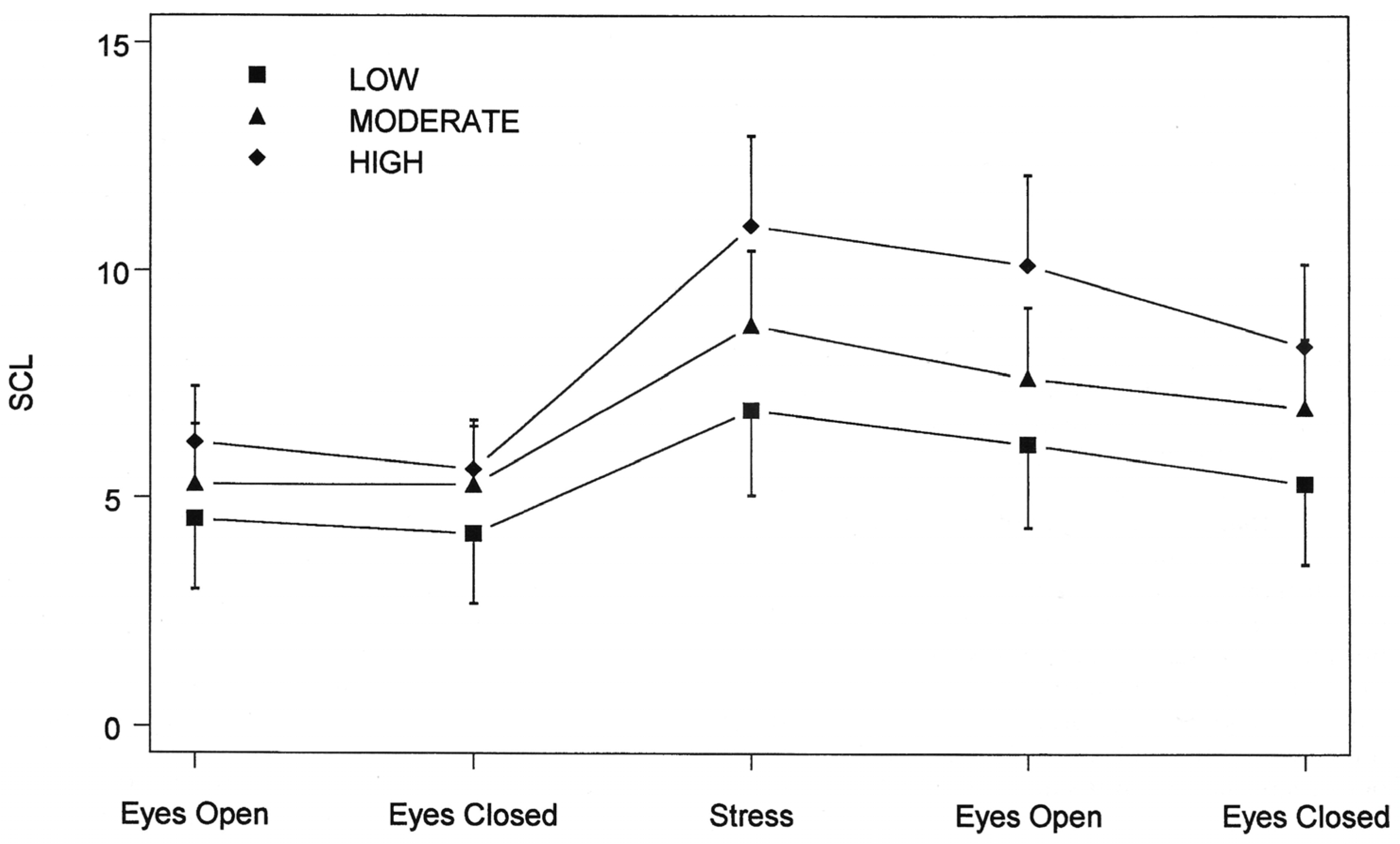
4.2. Predisposing Risk Factor High and Low Hypnotizability Is Associated with Distinct Patterns of ANS Reactivity to Threat Induction
4.3. Primary Predisposing Risk Factor Trait Hypnotizability–Suggestibility, Definition and Parameters
4.4. ANS Reactivity and Functional Somatic Disorders (FSDs)
5. Can Automaticity and Reduced Sense of Self Agency in High Hypnotizable (HH) Patients Exponentially Amplify Threat Perception?
5.1. Trait Hypnotizability–Suggestibility and Related Trait Absorption (TAS) Modulate Learned Placebo and Nocebo Effects and Functional Somatic Disorders
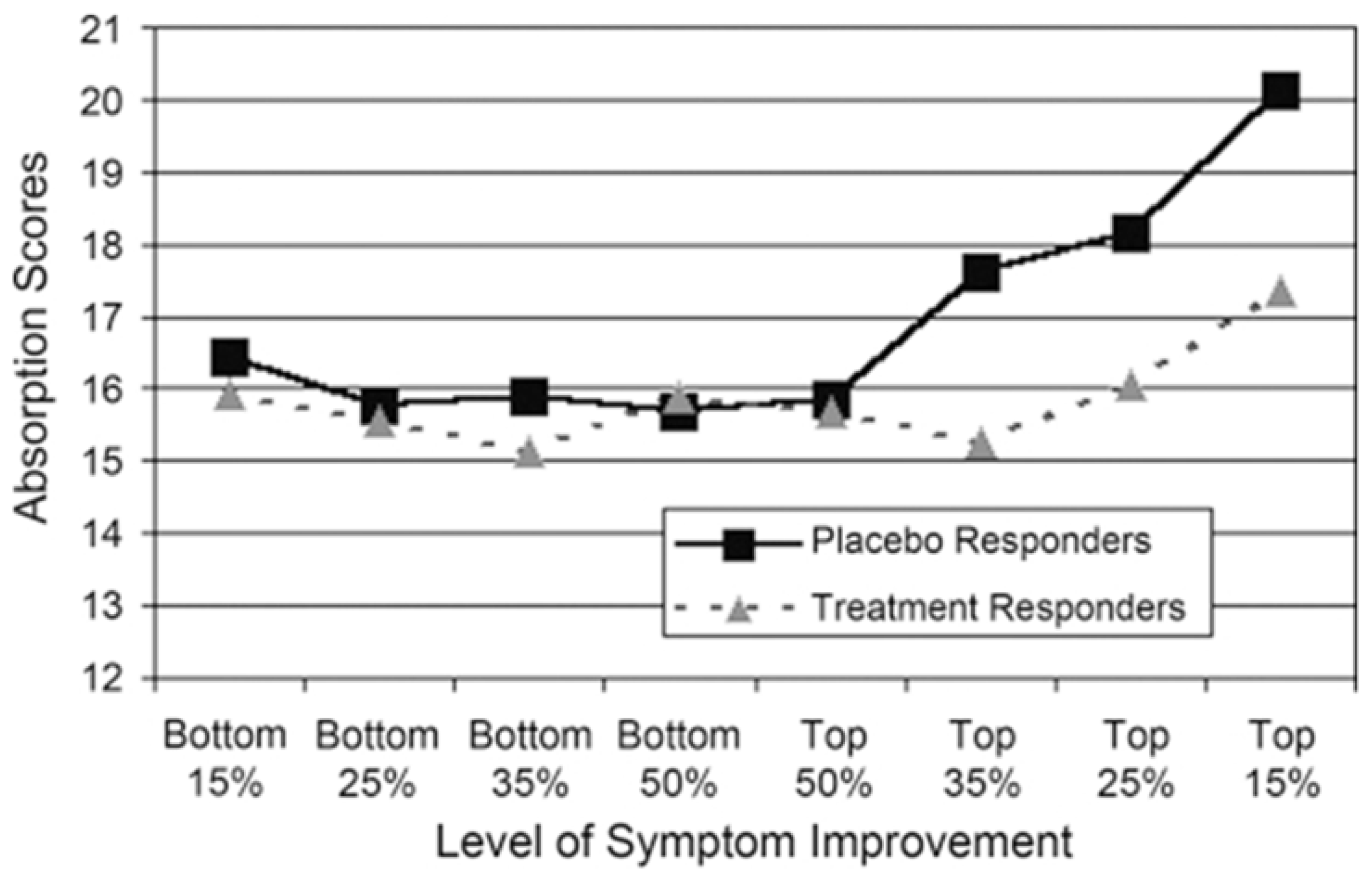
5.2. Trait Absorption (TAS) and Functional Somatic Disorders
5.3. Predisposing Risk Factor Trait High Alexithymia Is Correlated Negatively with Low Hypnotizability and During Threat-Induced Functional Somatic Disorders
6. Is Pavlovian Learning of Biologically Embedded Threat Perception Modulated by the Predisposing, Triggering and Buffering Risk Factors of the HRMTP?
“Stress is a state of mind, involving both the brain and body as well as their interactions; … it also reflects stable epigenetic modifications in development that set lifelong patterns of physiological reactivity and behavior through biological embedding of early environments interacting with cumulative change from experiences over the lifespan.”(McEwen, 2012, PNAS, p. 17180 [6])
7. Are Predisposing Risk Factors Linked to Triggering Risk Factors of the HRMTP?
Respiratory Heart Rate Variability [19] Learning Activates and Modulates the Vagal Brake in Emotional Learning
8. Frequency of Functional Somatic Disorders in Primary Care Medicine and the Risk of Unintentional Iatrogenic Injury in the Invasive Medical–Surgical Hospital Context
“Pain syndrome patients, in their desperate search for the elusive cure, often chase “windmills” and convince their doctors to perform a myriad of invasive tests, and procedures. As a result of their pain behaviors, many experience iatrogenic complications, suffering and disability.”—G.M. Aronoff, MD Editor, Clinical Journal of Pain, 1(1) 1985 [251].
Two Subsets of Patients at Risk for Unintentional Iatrogenic Injury
9. The Conditioned Response Model (CRM) of Emotional Learning of Placebo and Nocebo Somatic Effects and FSD
- Positive or negative verbal suggestion is postulated by the CRM to induce placebo or nocebo somatic effects and FSD, and all invasive verbal suggestion is postulated to be modulated by the innately effective (US-UR) psychosocial active ingredient trait hypnotizability–suggestibility [33,35,36,53,54,61,154,267,270]. Two large meta-analyses found that trait hypnotizability–suggestibility modulates, in a dose–response or linear manner, the efficacy of verbal suggestions to reduce clinical and experimental threat and pain perception [39,40].
- Interpersonal delivery of rapport through accurate verbal Empathy and Warmth, are innately effective (US-UR) psychosocial active stimuli to induce and modulate placebo and nocebo somatic effects and FSD. Empirically, empathy and warmth can modulate anxiety, threat and pain perception [27,264,271,272,273,274,275,276]. It also appears that empathy is modulated by trait hypnotizability-suggestibility [247].
- The CRM predicts that the identification and application of innately effective [53,54,61,197] psychosocially active stimuli (US-UR), in the context of increasingly invasive [278] and threatening medical–surgical procedures (US-UR) on patients, can amplify the magnitude of future placebo effects in clinical trials [61,197,278,279,280,281,282,283].
- Associative learning can, through neutral stimuli (CS), automatically and unconsciously [222,223] trigger activation or deactivation of previously learned (e. g., adverse childhood experiences and traumatic major life changes like the injury or death of a parent) and biologically embedded nocebo effects [6,7,28,29,31,35,37,54,56,228] through epigenetic mechanisms, like heterochromatin and Euchromatin [284,285,286].
- Trait hypnotizability–suggestibility is postulated to be an innately effective (US-UR) modulator of empathy learning [247], verbal suggestion learning and associative learning of placebo somatic effects [101,102,103,104,105,106], nocebo somatic effects [106,107,108,109] and functional somatic disorders [23,30,33,34,41,44,45].
- It is predicted by the CRM and HRMTP that chronic anomalies and incongruities [27,69,70,71] in emotional perception and emotional learning and ANS reactivity in trait low hypnotizability–suggestibility and High Alexithymia can reduce HRV [19] and increase risk of ANS dysregulation [28,30,31,35,36,79,80,81] as postulated in chronic threat and pain perception associated with allostasis and biological embedding [6,7,82].
- It is predicted that trait high hypnotizability (HH) amplifies threat perception, and that HH is a risk factor for FSD, unless the patient’s HH is mobilized specifically by verbal hypnotherapy or non-specifically by psychosocial therapies, like pain reprocessing therapy, CBT, EMDR, or biofeedback therapy.
10. Clinical vs. Experimental Contexts
11. The Psychophysiology of the Clinician–Patient Relationship and Associative Emotional Learning Modulated by Trait Hypnotizability–Suggestibility and Social Support
12. General Predictions from the CRM and the HRMTP
- The CRM predicted that as new innate specific active biological ingredients (US-UR), including psychosocial active ingredients (e.g., trait hypnotizability, empathy and warmth) for “healing” are isolated, paradoxically the placebo response can get stronger in future randomized placebo-controlled gold standard clinical trials [53,54,61]. This early prediction of amplified placebo effects in future clinical trials [53,54,61] appears to be supported today by the high reported correlation (r = 0.73) between placebo effects and pharmacological effects in recent clinical randomized controlled trials [281] predicted by Wickramasekera in 1980 and 1985. The increased magnitude of placebo effects in US clinical trials of pain and especially of neuropathic pain [282,296] support the CRM prediction. The progressive mean increases in placebo effects (40%) in Irritable Bowel Syndrome—IBS [297] are also supportive of the CRM prediction. The recent review of treatment effects in pharmacological randomized controlled clinical trials of five diseases [281] stated that the placebo effect is the “major driver of treatment effects in clinical trials that alone explains 69% of the variance” [281]. This review [281] of the efficacy of 150 pharmacological randomized controlled clinical trials found that 72% of the variance in treatment effects could be attributed to placebo effects or context effects.
- The HRMTP predicts that, in FSD patients with chronic pain, intermittent threat perception can drive dysregulation of the ANS and PTI. This prediction was apparently confirmed by the association of experimental threat induction and Paradoxical Increase in hand Temperature (PTI) in approximately 50% of 224 of chronic pain patients of both genders. This PTI was first experimentally demonstrated by Wickramasekera et al. [37] and replicated independently in a PhD dissertation [96].
- The HRMTP predicts that the primary Predisposing risk factors (a) trait hypnotizability–suggestibility, if it implicates threat learning, interacting with other HRMTP risk factors (e.g., high catastrophizing, high ACEs and low social support) can maladaptively amplify threat perception in an exponential manner and launch a progressive trajectory of severe morbidity, if not mortality.
13. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HRMTP | High Risk Model of Threat Perception |
| CRM | Conditioned Response Model |
| FSD | Functional Somatic Disorder |
| HPAA | Hypothalamus–Pituitary–Adrenal Axis |
| ANS | Autonomic Nervous System |
| PTI | Paradoxical Hand Temperature Increase |
| SRSSs | Stress-Related Somatic Symptoms |
| US | Unconditioned Stimulus |
| UCR | Unconditioned Response |
| CS | Conditioned Stimulus |
| CR | Conditioned Response |
References
- Nicholson, L.B. The Immune System. Essays Biochem. 2016, 60, 275–301. [Google Scholar] [CrossRef]
- Ader, R. Conditioned Immunopharmacological Effects in Animals: Implications for a Conditioning Model of Pharmacotherapy. In Placebo: Theory, Research, and Mechanisms; White, L., Tursky, B., Schwartz, G.E., Eds.; Guilford Press: New York, NY, USA, 1985; pp. 306–323. [Google Scholar]
- Hadamitzky, M.; Lückemann, L.; Pacheco-López, G.; Schedlowski, M. Pavlovian Conditioning of Immunological and Neuroendocrine Functions. Physiol. Rev. 2020, 100, 357–405. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J.E. Coming to Terms with Fear. Proc. Natl. Acad. Sci. USA 2014, 111, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Fiorio, M.; Braga, M.; Marotta, A.; Villa-Sánchez, B.; Edwards, M.J.; Tinazzi, M.; Barbiani, D. Functional Neurological Disorder and Placebo and Nocebo Effects: Shared Mechanisms. Nat. Rev. Neurol. 2022, 18, 624–635. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Brain on Stress: How the Social Environment Gets under the Skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17180–17185. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Chapter 5—Central Role of the Brain in Stress and Adaptation: Allostasis, Biological Embedding, and Cumulative Change. In Stress: Concepts, Cognition, Emotion, and Behavior; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 39–55. ISBN 978-0-12-800951-2. [Google Scholar]
- Smith, S.M.; Vale, W.W. The Role of the Hypothalamic-Pituitary-Adrenal Axis in Neuroendocrine Responses to Stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Benedetti, F.; Pollo, A.; Lopiano, L.; Lanotte, M.; Vighetti, S.; Rainero, I. Conscious Expectation and Unconscious Conditioning in Analgesic, Motor, and Hormonal Placebo/Nocebo Responses. J. Neurosci. 2003, 23, 4315–4323. [Google Scholar] [CrossRef]
- Botvinik-Nezer, R.; Petre, B.; Ceko, M.; Lindquist, M.A.; Friedman, N.P.; Wager, T.D. Placebo Treatment Affects Brain Systems Related to Affective and Cognitive Processes, but Not Nociceptive Pain. Nat. Commun. 2024, 15, 6017. [Google Scholar] [CrossRef]
- Branco, P.; Vachon-Presseau, E.; Hall, K.T.; Wager, T.D.; Vania Apkarian, A. C2.1Predictability of Placebo Responses and Their Clinical Implications. In Placebo Effects Through the Lens of Translational Research; Colloca, L., Noel, J., Franklin, P.D., Seneviratne, C., Eds.; Oxford University Press: Oxford, UK, 2023. [Google Scholar]
- Colloca, L. Emotional Modulation of Placebo Analgesia. Pain 2014, 155, 651. [Google Scholar] [CrossRef]
- Colloca, L.; Raghuraman, N.; Wang, Y.; Akintola, T.; Brawn-Cinani, B.; Colloca, G.; Kier, C.; Varshney, A.; Murthi, S. Virtual Reality: Physiological and Behavioral Mechanisms to Increase Individual Pain Tolerance Limits. Pain 2020, 161, 2010–2021. [Google Scholar] [CrossRef]
- Berens, A.E.; Jensen, S.K.G.; Nelson, C.A. Biological Embedding of Childhood Adversity: From Physiological Mechanisms to Clinical Implications. BMC Med. 2017, 15, 135. [Google Scholar] [CrossRef]
- Timmers, I.; Quaedflieg, C.W.E.M.; Hsu, C.; Heathcote, L.C.; Rovnaghi, C.R.; Simons, L.E. The Interaction between Stress and Chronic Pain through the Lens of Threat Learning. Neurosci. Biobehav. Rev. 2019, 107, 641–655. [Google Scholar] [CrossRef]
- Timmers, I.; López-Solà, M.; Heathcote, L.C.; Heirich, M.; Rush, G.Q.; Shear, D.; Borsook, D.; Simons, L.E. Amygdala Functional Connectivity Mediates the Association between Catastrophizing and Threat-Safety Learning in Youth with Chronic Pain. Pain 2022, 163, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Woda, A.; Picard, P.; Dutheil, F. Dysfunctional Stress Responses in Chronic Pain. Psychoneuroendocrinology 2016, 71, 127–135. [Google Scholar] [CrossRef]
- Algra, A.; Tijssen, J.G.; Roelandt, J.R.; Pool, J.; Lubsen, J. Heart Rate Variability from 24-Hour Electrocardiography and the 2-Year Risk for Sudden Death. Circulation 1993, 88, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Menuet, C.; Ben-Tal, A.; Linossier, A.; Allen, A.M.; Machado, B.H.; Moraes, D.J.A.; Farmer, D.G.S.; Paterson, D.J.; Mendelowitz, D.; Lakatta, E.G.; et al. Redefining Respiratory Sinus Arrhythmia as Respiratory Heart Rate Variability: An International Expert Recommendation for Terminological Clarity. Nat. Rev. Cardiol. 2025, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shanks, J.; Abukar, Y.; Lever, N.A.; Pachen, M.; LeGrice, I.J.; Crossman, D.J.; Nogaret, A.; Paton, J.F.R.; Ramchandra, R. Reverse Re-Modelling Chronic Heart Failure by Reinstating Heart Rate Variability. Basic Res. Cardiol. 2022, 117, 4. [Google Scholar] [CrossRef]
- Jorgensen, T.; Dantoft, T.M.; Petersen, M.W.; Gormsen, L.; Winter-Jensen, M.; Fink, P.; Linneberg, A.; Benros, M.E.; Eplov, L.F.; Bjerregaard, A.A.; et al. Is Reduced Heart Rate Variability Associated with Functional Somatic Disorders? A Cross-Sectional Population-Based Study; DanFunD. BMJ Open 2024, 14, e073909. [Google Scholar] [CrossRef]
- Greenleaf, M.; Fisher, S.; Miaskowski, C.; DuHamel, K. Hypnotizability and Recovery from Cardiac Surgery. Am. J. Clin. Hypn. 1992, 35, 119–128. [Google Scholar] [CrossRef]
- Jorgensen, M.M.; Zachariae, R. Autonomic Reactivity to Cognitive and Emotional Stress of Low, Medium, and High Hypnotizable Healthy Subjects: Testing Predictions from the High Risk Model of Threat Perception. Int. J. Clin. Exp. Hypn. 2002, 50, 248–275. [Google Scholar] [CrossRef]
- Wickramasekera, I. A Model of the Patient at High Risk for Chronic Stress Related Disorders: Do Beliefs Have Biological Consequences? In Proceedings of the Annual Convention of the Biofeedback Society of America, San Diego, CA, USA, 23–27 February 1979; Biofeedback Society of America: Wheat Ridge, CO, USA, 1979. [Google Scholar]
- Wickramasekera, I. A Model of People at High Risk to Develop Chronic Stress-Related Symptoms. In Stress and Tension Control; McGuigan, F.J., Ed.; Plenum: New York, NY, USA, 1984. [Google Scholar]
- Wickramasekera, I. Risk Factors That Lead to the Development of Chronic Stress Related Symptoms. Advances 1986, 4, 9–35. [Google Scholar]
- Wickramasekera, I. Clinical Behavioral Medicine: Some Concepts and Procedures; Plenum: New York, NY, USA, 1988. [Google Scholar]
- Wickramasekera, I. Assessment and Treatment of Somatization Disorders: The High-Risk Model of Threat Perception. In Handbook of Clinical Hypnosis; Lynn, S., Rhue, J., Kirsh, I., Eds.; American Psychological Association: Washington, DC, USA, 1993. [Google Scholar]
- Wickramasekera, I. Somatic to Psychological Symptoms and Information Transfer from Implicit to Explicit Memory: A Controlled Study with Predictions from the High Risk Model of Threat Perception. Dissociation Prog. Dissociative Disord. 1994, 7, 153–166. [Google Scholar]
- Wickramasekera, I. Somatization. Concepts, Data, and Predictions from the High Risk Model of Threat Perception. J. Nerv. Ment. Dis. 1995, 183, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wickramasekera, I. Secrets Kept from the Mind but Not the Body or Behavior: The Unsolved Problems of Identifying and Treating Somatization and Psychophysiological Disease. Adv. Mind-Body Med. 1998, 14, 81–98. [Google Scholar]
- Wickramasekera, I. The High Risk Model of Threat Perception and Trojan Horse Role Induction: Somatization and Psychophysiological Disease. In Handbook of Mind-Body Medicine for Primary Care; Moss, D., McGrady, A., Davies, T., Wickramasekera, I., Eds.; Sage: Thousand Oaks, CA, USA, 2003; pp. 19–42. [Google Scholar]
- Wickramasekera, I. Specific Predictions from the Conditioned Response Model (CR) of the Placebo and Empirical Data Consistent with These Specific Predictions. In Proceedings of the 3rd International Conference of the Society for Interdisciplinary Placebo Studies, Baltimore, MD, USA, 26–28 May 2021. [Google Scholar]
- Younger, J.W.; Rossetti, G.C.; Borckardt, J.J.; Smith, A.R.; Tasso, A.F.; Nash, M.R. Hypnotizability and Somatic Complaints: A Gender-Specific Phenomenon. Int. J. Clin. Exp. Hypn. 2007, 55, 1–13. [Google Scholar] [CrossRef]
- Wickramasekera, I.; Davies, T.E.; Davies, S.M. Applied Psychophysiology: A Bridge between the Biomedical Model and the Biopsychosocial Model in Family Medicine. Prof. Psychol. Res. Pract. 1996, 27, 221–233. [Google Scholar] [CrossRef]
- Wickramasekera, I.; Pope, A.T.; Kolm, P. On the Interaction of Hypnotizability and Negative Affect in Chronic Pain. Implications for the Somatization of Trauma. J. Nerv. Ment. Dis. 1996, 184, 628–635. [Google Scholar] [CrossRef]
- Wickramasekera, I.; Kolm, P.; Pope, A.; Turner, M. Observation of a Paradoxical Temperature Increase during Cognitive Stress in Some Chronic Pain Patients. Appl. Psychophysiol. Biofeedback 1998, 23, 233–241. [Google Scholar] [CrossRef]
- Perlstrom, J.R.; Wickramasekera, I. Insomnia, Hypnotic Ability, Negative Affectivity, and the High Risk Model of Threat Perception. J. Nerv. Ment. Dis. 1998, 186, 437–440. [Google Scholar] [CrossRef]
- Thompson, T.; Terhune, D.B.; Oram, C.; Sharangparni, J.; Rouf, R.; Solmi, M.; Veronese, N.; Stubbs, B. The Effectiveness of Hypnosis for Pain Relief: A Systematic Review and Meta-Analysis of 85 Controlled Experimental Trials. Neurosci. Biobehav. Rev. 2019, 99, 298–310. [Google Scholar] [CrossRef]
- Milling, L.S.; Valentine, K.E.; LoStimolo, L.M.; Nett, A.M.; McCarley, H.S. Hypnosis and the Alleviation of Clinical Pain: A Comprehensive Meta-Analysis. Int. J. Clin. Exp. Hypn. 2021, 69, 297–322. [Google Scholar] [CrossRef]
- Wieder, L.; Brown, R.; Thompson, T.; Terhune, D.B. Suggestibility in Functional Neurological Disorder: A Meta-Analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 150–157. [Google Scholar] [CrossRef]
- Challis, G.B.; Stam, H.J. A Longitudinal Study of the Development of Anticipatory Nausea and Vomiting in Cancer Chemotherapy Patients: The Role of Absorption and Autonomic Perception. Health Psychol. 1992, 11, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Zachariae, R.; Paulsen, K.; Mehlsen, M.; Jensen, A.B.; Johansson, A.; von der Maase, H. Anticipatory Nausea: The Role of Individual Differences Related to Sensory Perception and Autonomic Reactivity. Ann. Behav. Med. 2007, 33, 69–79. [Google Scholar] [CrossRef]
- Spinhoven, P.; ter Kuile, M.M. Treatment Outcome Expectancies and Hypnotic Susceptibility as Moderators of Pain Reduction in Patients with Chronic Tension-Type Headache. Int. J. Clin. Exp. Hypn. 2000, 48, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, K.; Hoogduin, K.A.L.; Keijsers, G.P.J.; Näring, G.W.B.; Moene, F.C.; Sandijck, P. Hypnotic Susceptibility in Patients with Conversion Disorder. J. Abnorm. Psychol. 2002, 111, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.S. Hypnotizability as a Factor in the Hypnotic Treatment of Obesity. Int. J. Clin. Exp. Hypn. 1985, 33, 150–159. [Google Scholar] [CrossRef]
- Wickramasekera, I.; Price, D.C. Morbid Obesity, Absorption, Neuroticism, and the High Risk Model of Threat Perception. Am. J. Clin. Hypn. 1997, 39, 291–301. [Google Scholar] [CrossRef]
- Gick, M.; Mcleod, C.; Hulihan, D. Absorption, Social Desirability, and Symptoms in a Behavioral Medicine Population. J. Nerv. Ment. Dis. 1997, 185, 454–458. [Google Scholar] [CrossRef]
- Kermit, K.; Devine, D.A.; Tatman, S.M. High Risk Model of Threat Perception in Chronic Pain Patients: Implications for Primary Care and Chronic Pain Programs. J. Nerv. Ment. Dis. 2000, 188, 577–582. [Google Scholar] [CrossRef]
- McGrady, A.V.; Andrasik, F.; Davies, T.; Striefel, S.; Wickramasekera, I.; Baskin, S.M.; Penzien, D.B.; Tietjen, G. Psychophysiologic Therapy for Chronic Headache in Primary Care. Prim. Care Companion J. Clin. Psychiatry 1999, 1, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A.; Guthrie, R.M.; Moulds, M.L. Hypnotizability in Acute Stress Disorder. Am. J. Psychiatry 2001, 158, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Engel, G.L. The Need for a New Medical Model: A Challenge for Biomedicine. Science 1977, 196, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wickramasekera, I. A Conditioned Response Model of the Placebo Effect Predictions from the Model. Biofeedback Self Regul. 1980, 5, 5–18. [Google Scholar] [CrossRef]
- Wickramasekera, I. A Conditioned Response Model of the Placebo Effect: Predictions from the Model. In Placebo: Theory, Research, and Mechanisms; White, L., Tursky, B., Schwartz, G.E., Eds.; Guilford Press: New York, NY, USA, 1985; pp. 255–287. [Google Scholar]
- Burton, C.; Fink, P.; Henningsen, P.; Löwe, B.; Rief, W. EURONET-SOMA Group Functional Somatic Disorders: Discussion Paper for a New Common Classification for Research and Clinical Use. BMC Med. 2020, 18, 34. [Google Scholar] [CrossRef]
- Aristizabal, M.J.; Anreiter, I.; Halldorsdottir, T.; Odgers, C.L.; McDade, T.W.; Goldenberg, A.; Mostafavi, S.; Kobor, M.S.; Binder, E.B.; Sokolowski, M.B.; et al. Biological Embedding of Experience: A Primer on Epigenetics. Proc. Natl. Acad. Sci. USA 2020, 117, 23261–23269. [Google Scholar] [CrossRef]
- Schury, K.; Kolassa, I.-T. Biological Memory of Childhood Maltreatment: Current Knowledge and Recommendations for Future Research. Ann. N. Y. Acad. Sci. 2012, 1262, 93–100. [Google Scholar] [CrossRef]
- Haller, H.; Cramer, H.; Lauche, R.; Dobos, G. Somatoform Disorders and Medically Unexplained Symptoms in Primary Care. Dtsch. Arztebl. Int. 2015, 112, 279–287. [Google Scholar] [CrossRef]
- Husain, M.; Chalder, T. Medically Unexplained Symptoms: Assessment and Management. Clin. Med. 2021, 21, 13–18. [Google Scholar] [CrossRef]
- Benedetti, F.; Amanzio, M.; Giovannelli, F.; Craigs-Brackhahn, K.; Arduino, C.; Shaibani, A. Are Nocebo Effects in Adulthood Linked to Prenatal Maternal Cortisol Levels? Clin. Neuropsychiatry 2022, 19, 298–306. [Google Scholar] [CrossRef]
- Wickramasekera, I. How Does Biofeedback Reduce Clinical Symptoms and Do Memories and Beliefs Have Biological Consequences? Toward a Model of Mind-Body Healing. Appl. Psychophysiol. Biofeedback 1999, 24, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Wickramasekera, I. Adjunctive Hypnotherapy. In Essentials of Complementary and Alternative Medicine; Jonas, W.B., Levin, J.S., Eds.; Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 426–443. ISBN 978-0-683-30674-3. [Google Scholar]
- Hilgard, E.R.; Hilgard, J.R. Hypnosis in the Relief of Pain, 1st ed.; William Kaufmann, Inc.: Los Altos, CA, USA, 1975; ISBN 978-0-913232-16-3. [Google Scholar]
- Zachariae, R.; Jørgensen, M.M.; Bjerring, P.; Svendsen, G. Autonomic and Psychological Responses to an Acute Psychological Stressor and Relaxation: The Influence of Hypnotizability and Absorption. Int. J. Clin. Exp. Hypn. 2000, 48, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Terhune, D.B.; Cleeremans, A.; Raz, A.; Lynn, S.J. Hypnosis and Top-down Regulation of Consciousness. Neurosci. Biobehav. Rev. 2017, 81, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, S.W.G.; Whalley, M.G.; Stenger, V.A.; Oakley, D.A. Cerebral Activation during Hypnotically Induced and Imagined Pain. NeuroImage 2004, 23, 392–401. [Google Scholar] [CrossRef]
- Derbyshire, S.W.G.; Whalley, M.G.; Oakley, D.A. Fibromyalgia Pain and Its Modulation by Hypnotic and Non-Hypnotic Suggestion: An fMRI Analysis. Eur. J. Pain 2009, 13, 542–550. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Zhou, E.S. If It Goes up, Must It Come down? Chronic Stress and the Hypothalamic-Pituitary-Adrenocortical Axis in Humans. Psychol. Bull. 2007, 133, 25–45. [Google Scholar] [CrossRef]
- Pomerantz, B. Stress and Relaxation Effects in High and Low Hypnotizables: Physiological and Subjective Measures. Doctoral Dissertation, Virginia Consortium for Professional Psychology, Norfolk, VA, USA, 1986. [Google Scholar]
- Pomerantz, B.; Wickramasekera, I. High and Low Trait Hypnotizability and Their Psychophysiological Correlates Under High and Low Stress. Washington, DC, USA, 1992. [Google Scholar]
- Wickramasekera, I. On Attempts to Modify Hypnotic Susceptibility: Some Psychophysiological Procedures and Promising Directions. Ann. N. Y. Acad. Sci. 1977, 296, 143–153. [Google Scholar] [CrossRef]
- Frankel, F.H.; Apfel-Savitz, R.; Nemiah, J.C.; Sifneos, P.E. The Relationship between Hypnotizability and Alexithymia. Psychother. Psychosom. 1977, 28, 172–178. [Google Scholar] [CrossRef]
- Luminet, O.; Nielson, K.A. Alexithymia: Toward an Experimental, Processual Affective Science with Effective Interventions. Annu. Rev. Psychol. 2025, 76, 741–769. [Google Scholar] [CrossRef]
- Költő, A. Hypnotic Susceptibility and Mentalization Skills in the Context of Parental Behavior. Ph.D. Thesis, ELTE Eötvös Loránd University, Budapest, Hungary, 2015. [Google Scholar]
- Költő, A.; Banyai, E. Hypnotizability and Alexithymia: Preliminary Findings; Targu Mures, Romania, 2014. Available online: https://real.mtak.hu/37714/1/mental.15.2014.004.pdf (accessed on 23 August 2025).
- Porges, S.W. Polyvagal Theory: A Science of Safety. Front. Integr. Neurosci. 2022, 16, 871227. [Google Scholar] [CrossRef]
- Kano, M.; Yoshizawa, M.; Kono, K.; Muratsubaki, T.; Morishita, J.; Van Oudenhove, L.; Yagihashi, M.; Mugikura, S.; Dupont, P.; Takase, K.; et al. Parasympathetic Activity Correlates with Subjective and Brain Responses to Rectal Distension in Healthy Subjects but Not in Non-Constipated Patients with Irritable Bowel Syndrome. Sci. Rep. 2019, 9, 7358. [Google Scholar] [CrossRef]
- Lane, R.D.; Solms, M.; Weihs, K.L.; Hishaw, A.; Smith, R. Affective Agnosia: A Core Affective Processing Deficit in the Alexithymia Spectrum. Biopsychosoc. Med. 2020, 14, 20. [Google Scholar] [CrossRef]
- Kano, M.; Endo, Y.; Fukudo, S. Association Between Alexithymia and Functional Gastrointestinal Disorders. Front. Psychol. 2018, 9, 599. [Google Scholar] [CrossRef]
- Lane, R.D. Alexithymia 3.0: Reimagining Alexithymia from a Medical Perspective. Biopsychosoc. Med. 2020, 14, 21. [Google Scholar] [CrossRef]
- Taylor, G.J.; Porcelli, P.; Bagby, R.M. Alexithymia: A Defense of the Original Conceptualization of the Construct and a Critique of the Attention-Appraisal Model. Clin. Neuropsychiatry 2024, 21, 329–357. [Google Scholar] [CrossRef]
- Benarroch, E. Autonomic Nervous System and Neuroimmune Interactions. Neurology 2019, 92, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Norris, C.J.; Larsen, J.T.; Cacioppo, J.T. Neuroticism Is Associated with Larger and More Prolonged Electrodermal Responses to Emotionally Evocative Pictures. Psychophysiology 2007, 44, 823–826. [Google Scholar] [CrossRef]
- Dawson, M.E.; Schell, A.M.; Filion, D.L. The Electrodermal System. In Handbook of Psychophysiology, 4th ed.; Cambridge handbooks in psychology; Cambridge University Press: New York, NY, USA, 2017; pp. 217–243. ISBN 978-1-107-05852-1. [Google Scholar]
- Letzen, J.E.; Robinson, M.E. Negative Mood Influences Default Mode Network Functional Connectivity in Chronic Low Back Pain Patients: Implications for Functional Neuroimaging Biomarkers. Pain 2017, 158, 48–57. [Google Scholar] [CrossRef]
- Watson, D. Neuroticism/Negative Affectivity. In The Cambridge Handbook of Anxiety and Related Disorders; Cambridge University Press: New York, NY, USA, 2019; pp. 91–120. ISBN 978-1-107-19306-2. [Google Scholar]
- Boucsein, W. Electrodermal Activity; Plenum: New York, NY, USA, 1992. [Google Scholar]
- Blomqvist, N. On the Relation between Change and Initial Value. J. Am. Stat. Assoc. 1977, 72, 746–749. [Google Scholar] [CrossRef]
- Hilgard, E.R. Divided Consciousness: Multiple Controls in Human Thought and Action, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1986. [Google Scholar]
- Wickramasekera, I. Invited Presentation—On the Interaction of Two Orthogonal Risk Factors: 1) Hypnotic Ability, 2) Negative Affectivity (Threat Perception-HPAA) for Psychophysiological Dysregulation (Sympathetic and Parasympathetic) in Somatization-Nocebo Phenomena. In Proceedings of the Suggestion and Suggestibility Theory in Research, Rome, Italy, 1994; Available online: https://www.meg-stiftung.de/index.php/de/component/phocadownload/category/1-artikel?download=239:16-wickramasekera-2000-4-hyint-245-252&Itemid=216 (accessed on 17 October 2021).
- Aldrich, L.B.; Moattari, A.R.; Vinik, A.I. Distinguishing Features of Idiopathic Flushing and Carcinoid Syndrome. Arch. Intern. Med. 1988, 148, 2614–2618. [Google Scholar] [CrossRef]
- Halperin, J.L.; Cohen, R.A.; Coffman, J.D. Digital Vasodilatation during Mental Stress in Patients with Raynaud’s Disease. Cardiovasc. Res. 1983, 17, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P.; Creager, M.A.; Osmundson, P.J.; Shepherd, J.T. Sex Differences in Control of Cutaneous Blood Flow. Circulation 1990, 82, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.A.; Andridge, R.; Shrout, M.R.; Renna, M.E.; Bennett, J.M.; Jaremka, L.M.; Fagundes, C.P.; Belury, M.A.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Frequent Interpersonal Stress and Inflammatory Reactivity Predict Depressive-Symptom Increases: Two Tests of the Social-Signal-Transduction Theory of Depression. Psychol. Sci. 2022, 33, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Wickramasekera, I.; Wickramasekera, I., II. A Case Study: Electromyographic Correlates in the Hypnotic Recall of a Repressed Memory. J. Trauma Dissociation 1997, 10, 11–20. [Google Scholar]
- McCarthy, R.E. An Investigation of Paradoxical Temperature Increase During Cognitve Stress in an Abused Population. Doctoral Dissertation, Saybrook Graduate School and Research Center, San Francisco, CA, USA, 2003. [Google Scholar]
- House, J.S.; Landis, K.R.; Umberson, D. Social Relationships and Health. Science 1988, 241, 540–545. [Google Scholar] [CrossRef]
- Smith, P.J.; Merwin, R.M. The Role of Exercise in Management of Mental Health Disorders: An Integrative Review. Annu. Rev. Med. 2021, 72, 45–62. [Google Scholar] [CrossRef]
- Holt-Lunstad, J.; Steptoe, A. Social Isolation: An Underappreciated Determinant of Physical Health. Curr. Opin. Psychol. 2021, 43, 232–237. [Google Scholar] [CrossRef]
- Smith, R.; Weihs, K.L.; Alkozei, A.; Killgore, W.D.S.; Lane, R.D. An Embodied Neurocomputational Framework for Organically Integrating Biopsychosocial Processes: An Application to the Role of Social Support in Health and Disease. Psychosom. Med. 2019, 81, 125–145. [Google Scholar] [CrossRef]
- De Pascalis, V.; Chiaradia, C.; Carotenuto, E. The Contribution of Suggestibility and Expectation to Placebo Analgesia Phenomenon in an Experimental Setting. Pain 2002, 96, 393–402. [Google Scholar] [CrossRef]
- De Pascalis, V.; Scacchia, P. Hypnotizability and Placebo Analgesia in Waking and Hypnosis as Modulators of Auditory Startle Responses in Healthy Women: An ERP Study. PLoS ONE 2016, 11, e0159135. [Google Scholar] [CrossRef]
- Owens, J.E.; Menard, M. The Quantification of Placebo Effects Within a General Model of Health Care Outcomes. J. Altern. Complement. Med. 2011, 17, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Lui, F.; Porro, C.A. Hypnotic Susceptibility Modulates Brain Activity Related to Experimental Placebo Analgesia. Pain 2013, 154, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R.D.; Bergmann, S.; Wiech, K.; Terhune, D.B. Direct Verbal Suggestibility as a Predictor of Placebo Hypoalgesia Responsiveness. Psychosom. Med. 2021, 83, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Corsi, N.; Colloca, L. Placebo and Nocebo Effects: The Advantage of Measuring Expectations and Psychological Factors. Front. Psychol. 2017, 8, 308. [Google Scholar] [CrossRef]
- Winter, D.; Braw, Y. Effects of Diagnosis Threat on Cognitive Complaints after COVID-19. Health Psychol. 2023, 42, 335–342. [Google Scholar] [CrossRef]
- Khan, A.Y.; Baade, L.; Ablah, E.; McNerney, V.; Golewale, M.H.; Liow, K. Can Hypnosis Differentiate Epileptic from Nonepileptic Events in the Video/EEG Monitoring Unit? Data from a Pilot Study. Epilepsy Behav. 2009, 15, 314–317. [Google Scholar] [CrossRef]
- Stein, M.V.; Heller, M.; Hughes, N.; Marr, D.; Brake, B.; Chapman, S.; James Rubin, G.; Terhune, D.B. Moderators of Nocebo Effects in Controlled Experiments: A Multi-Level Meta-Analysis. Neurosci. Biobehav. Rev. 2025, 172, 106042. [Google Scholar] [CrossRef]
- Lynn, S.J.; Laurence, J.-R.; Kirsch, I. Hypnosis, Suggestion, and Suggestibility: An Integrative Model. Am. J. Clin. Hypn. 2015, 57, 314–329. [Google Scholar] [CrossRef]
- Tellegen, A. Practicing the Two Disciplines for Relaxation and Enlightenment: Comment on “Role of the Feedback Signal in Electromyograph Biofeedback: The Relevance of Attention” by Qualls and Sheehan. J. Exp. Psychol. Gen. 1981, 110, 217–231. [Google Scholar] [CrossRef]
- Zinn, M.L.; McCain, C.; Zinn, M. Musical Performance Anxiety and the High-Risk Model of Threat Perception. Med. Probl. Perform. Artist. 2000, 15, 65–71. [Google Scholar] [CrossRef]
- Ferdek, M.A.; Adamczyk, A.K.; van Rijn, C.M.; Oosterman, J.M.; Wyczesany, M. Pain Catastrophizing Is Associated with Altered EEG Effective Connectivity during Pain-related Mental Imagery. Acta Neurobiol. Exp. 2019, 79, 53–72. [Google Scholar]
- Quartana, P.J.; Campbell, C.M.; Edwards, R.R. Pain Catastrophizing: A Critical Review. Expert. Rev. Neurother. 2009, 9, 745–758. [Google Scholar] [CrossRef]
- Darnall, B.D.; Colloca, L. Chapter Six—Optimizing Placebo and Minimizing Nocebo to Reduce Pain, Catastrophizing, and Opioid Use: A Review of the Science and an Evidence-Informed Clinical Toolkit. In International Review of Neurobiology; Colloca, L., Ed.; Neurobiology of the Placebo Effect Part II; Academic Press: Cambridge, MA, USA, 2018; Volume 139, pp. 129–157. [Google Scholar]
- Grosso, F.; Barbiani, D.; Cavalera, C.; Volpato, E.; Pagnini, F. Risk Factors Associated with Nocebo Effects: A Review of Reviews. Brain Behav. Immun. Health 2024, 38, 100800. [Google Scholar] [CrossRef]
- Sarter, L.; Heider, J.; Kirchner, L.; Schenkel, S.; Witthöft, M.; Rief, W.; Kleinstäuber, M. Cognitive and Emotional Variables Predicting Treatment Outcome of Cognitive Behavior Therapies for Patients with Medically Unexplained Symptoms: A Meta-Analysis. J. Psychosom. Res. 2021, 146, 110486. [Google Scholar] [CrossRef]
- Van Den Houte, M.; Bogaerts, K.; Van Diest, I.; De Bie, J.; Persoons, P.; Van Oudenhove, L.; Van den Bergh, O. Inducing Somatic Symptoms in Functional Syndrome Patients: Effects of Manipulating State Negative Affect. Psychosom. Med. 2017, 79, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, K.; Van Den Houte, M.; Jongen, D.; Ly, H.G.; Coppens, E.; Schruers, K.; Van Diest, I.; Jan, T.; Van Wambeke, P.; Petre, B.; et al. Brain Mediators of Negative Affect-Induced Physical Symptom Reporting in Patients with Functional Somatic Syndromes. Transl. Psychiatry 2023, 13, 285. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Tyrrell, D.A.J.; Smith, A.P. Psychological Stress and Susceptibility to the Common Cold. N. Engl. J. Med. 1991, 325, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.D. Neural Substrates of Implicit and Explicit Emotional Processes: A Unifying Framework for Psychosomatic Medicine. Psychosom. Med. 2008, 70, 214–231. [Google Scholar] [CrossRef]
- McCrae, R.R.; Costa, P.T. Social Desirability Scales: More Substance than Style. J. Consult. Clin. Psychol. 1983, 51, 882–888. [Google Scholar] [CrossRef]
- Williams, M.M.; Rogers, R.; Sharf, A.J.; Ross, C.A. Faking Good: An Investigation of Social Desirability and Defensiveness in an Inpatient Sample With Personality Disorder Traits. J. Pers. Assess. 2019, 101, 253–263. [Google Scholar] [CrossRef]
- Weinberger, D.A. The Construct Validity of the Repressive Coping Style. In Repression and Dissociation: Implications for Personality Theory, Psychopathology, and Health; The John D. and Catherine T. MacArthur Foundation series on mental health and development; The University of Chicago Press: Chicago, IL, USA, 1995; pp. 337–386. ISBN 978-0-226-76105-3. [Google Scholar]
- Slavich, G.M.; Irwin, M.R. From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef]
- Cohen, S.; Murphy, M.L.M.; Prather, A.A. Ten Surprising Facts About Stressful Life Events and Disease Risk. Annu. Rev. Psychol. 2019, 70, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Muscatell, K.A.; Dedovic, K.; Slavich, G.M.; Jarcho, M.R.; Breen, E.C.; Bower, J.E.; Irwin, M.R.; Eisenberger, N.I. Greater Amygdala Activity and Dorsomedial Prefrontal-Amygdala Coupling Are Associated with Enhanced Inflammatory Responses to Stress. Brain Behav. Immun. 2015, 43, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Nilaweera, D.; Gurvich, C.; Freak-Poli, R.; Woods, R.L.; Owen, A.; McNeil, J.; Nelson, M.; Stocks, N.; Ryan, J. The Association between Adverse Events in Later Life and Mortality in Older Individuals. Compr. Psychoneuroendocrinol. 2023, 16, 100210. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-J.; Aldwin, C.M.; Igarashi, H.; Spiro, A. Do Hassles and Uplifts Trajectories Predict Mortality? Longitudinal Findings from the VA Normative Aging Study. J. Behav. Med. 2016, 39, 408–419. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Bourassa, K.J.; Moffitt, T.E.; Harrington, H.; Houts, R.; Poulton, R.; Ramrakha, S.; Caspi, A. Lower Cardiovascular Reactivity Is Associated With More Childhood Adversity and Poorer Midlife Health: Replicated Findings From the Dunedin and MIDUS Cohorts. Clin. Psychol. Sci. 2021, 9, 961–978. [Google Scholar] [CrossRef]
- Brennan, G.M.; Moffitt, T.E.; Bourassa, K.; Harrington, H.; Hogan, S.; Houts, R.M.; Poulton, R.; Ramrakha, S.; Caspi, A. The Continuity of Adversity: Negative Emotionality Links Early Life Adversity With Adult Stressful Life Events. Clin. Psychol. Sci. 2024, 12, 1111–1126. [Google Scholar] [CrossRef]
- Smith, R.; Kirlic, N.; Stewart, J.L.; Touthang, J.; Kuplicki, R.; McDermott, T.J.; Taylor, S.; Khalsa, S.S.; Paulus, M.P.; Aupperle, R.L. Long-Term Stability of Computational Parameters during Approach-Avoidance Conflict in a Transdiagnostic Psychiatric Patient Sample. Sci. Rep. 2021, 11, 11783. [Google Scholar] [CrossRef]
- Roth, S.; Cohen, L.J. Approach, Avoidance, and Coping with Stress. Am. Psychol. 1986, 41, 813–819. [Google Scholar] [CrossRef]
- Livermore, J.J.A.; Klaassen, F.H.; Bramson, B.; Hulsman, A.M.; Meijer, S.W.; Held, L.; Klumpers, F.; de Voogd, L.D.; Roelofs, K. Approach-Avoidance Decisions Under Threat: The Role of Autonomic Psychophysiological States. Front. Neurosci. 2021, 15, 353. [Google Scholar] [CrossRef] [PubMed]
- Moos, R.H. Coping Responses Inventory: CRI from Adults. Professional Manual; Psychological Assessment Resources: Odessa, TX, USA, 1993. [Google Scholar]
- Krypotos, A.-M.; Effting, M.; Kindt, M.; Beckers, T. Avoidance Learning: A Review of Theoretical Models and Recent Developments. Front. Behav. Neurosci. 2015, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, W. Behavioral Methods for Chronic Pain and Illness; C.V. Mosby, Company: St. Louis, MO, USA, 1976. [Google Scholar]
- Meyer, H.C.; Odriozola, P.; Cohodes, E.M.; Mandell, J.D.; Li, A.; Yang, R.; Hall, B.S.; Haberman, J.T.; Zacharek, S.J.; Liston, C.; et al. Ventral Hippocampus Interacts with Prelimbic Cortex during Inhibition of Threat Response via Learned Safety in Both Mice and Humans. Proc. Natl. Acad. Sci. USA 2019, 116, 26970–26979. [Google Scholar] [CrossRef]
- Watson, D.; Naragon, K. Positive Affectivity: The Disposition to Experience Positive Emotional States. In Oxford Handbook of Positive Psychology, 2nd ed.; Oxford library of psychology; Oxford University Press: New York, NY, USA, 2009; pp. 207–215. ISBN 978-0-19-518724-3. [Google Scholar]
- Geers, A.L.; Close, S.; Caplandies, F.C.; Vogel, C.L.; Murray, A.B.; Pertiwi, Y.; Handley, I.M.; Vase, L. Testing a Positive-Affect Induction to Reduce Verbally Induced Nocebo Hyperalgesia in an Experimental Pain Paradigm. Pain 2019, 160, 2290–2297. [Google Scholar] [CrossRef]
- Geers, A.L.; Faasse, K.; Guevarra, D.A.; Clemens, K.S.; Helfer, S.G.; Colagiuri, B. Affect and Emotions in Placebo and Nocebo Effects: What Do We Know so Far? Social. Personal. Psychol. Compass 2021, 15, e12575. [Google Scholar] [CrossRef]
- Bagby, R.M.; Parker, J.D.; Taylor, G.J. The Twenty-Item Toronto Alexithymia Scale--I. Item Selection and Cross-Validation of the Factor Structure. J. Psychosom. Res. 1994, 38, 23–32. [Google Scholar] [CrossRef]
- Montgomery, G.H.; David, D.; Winkel, G.; Silverstein, J.H.; Bovbjerg, D.H. The Effectiveness of Adjunctive Hypnosis with Surgical Patients: A Meta-Analysis. Anesth. Analg. 2002, 94, 1639–1645. [Google Scholar] [CrossRef]
- Council, J.R.; Kirsch, I.; Hafner, L.P. Expectancy versus Absorption in the Prediction of Hypnotic Responding. J. Personal. Social. Psychol. 1986, 50, 182–189. [Google Scholar] [CrossRef]
- Berntson, G.G.; Thomas Bigger, J., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart Rate Variability: Origins, Methods, and Interpretive Caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Barber, T.X. Hypnosis: A Scientific Approach; Van Nostrand Reinhold: New York, NY, USA, 1969. [Google Scholar]
- Hilgard, E.R. Hypnotic Susceptibility; Hypnotic susceptibility; Harcourt, Brace & World: Oxford, UK, 1965. [Google Scholar]
- Kihlstrom, J.F. The Cognitive Unconscious. Science 1987, 237, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Velten, E. A Laboratory Task for Induction of Mood States. Behav. Res. Ther. 1968, 6, 473–482. [Google Scholar] [CrossRef]
- Klein, K.B.; Spiegel, D. Modulation of Gastric Acid Secretion by Hypnosis. Gastroenterology 1989, 96, 1383–1387. [Google Scholar] [CrossRef]
- De Benedittis, G. Hypnotic Modulation of Autonomic Nervous System (ANS) Activity. Brain Sci. 2024, 14, 249. [Google Scholar] [CrossRef]
- Terhune, D.B.; Hedman, L.R.A. Metacognition of Agency Is Reduced in High Hypnotic Suggestibility. Cognition 2017, 168, 176–181. [Google Scholar] [CrossRef]
- Das, J.P. Conditioning and Hypnosis. J. Exp. Psychol. 1958, 56, 110–113. [Google Scholar] [CrossRef]
- King, D.R.; McDonald, R.D. Hypnotic Susceptibility and Verbal Conditioning. Int. J. Clin. Exp. Hypn. 1976, 24, 29–37. [Google Scholar] [CrossRef]
- Laurence, J. Hypnotisability and Automaticity from a Synergistic Perspective? Montreal, QC, Canada, 2018. [Google Scholar]
- Raz, A.; Shapiro, T.; Fan, J.; Posner, M.I. Hypnotic Suggestion and the Modulation of Stroop Interference. Arch. Gen. Psychiatry 2002, 59, 1155–1161. [Google Scholar] [CrossRef]
- Lifshitz, M.; Cusumano, E.; Raz, A. Hypnosis as Neurophenomenology. Front. Human. Neurosci. 2013, 7, 469. [Google Scholar] [CrossRef]
- Wickramasekera, I. (Ed.) Biofeedback, Behavior Therapy and Hypnosis: Potentiating the Verbal Control of Behavior for Clinicians; Nelson-Hall: Oxford, UK, 1976; pp. xv, 591. [Google Scholar]
- Spanos, N.P.; Perlini, A.H.; Robertson, L.A. Hypnosis, Suggestion, and Placebo in the Reduction of Experimental Pain. J. Abnorm. Psychol. 1989, 98, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Benham, G.; Woody, E.Z.; Wilson, K.S.; Nash, M.R. Expect the Unexpected: Ability, Attitude, and Responsiveness to Hypnosis. J. Personal. Social. Psychol. 2006, 91, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Karoly, P. Motivation and Expectancy Factors in Symptom Perception: A Laboratory Study of the Placebo Effect. Psychosom Med. 1991, 53, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.E.; Crawford, H.J. Neruophysiological and Genetic Determinants of High Hypnotizability. In The Highly Hypnotizable Person: Theoretical, Experimental and Clinical Issues; Heap, M., Brown, R.J., Oakley, D.A., Eds.; Routledge: London, UK, 2004; pp. 133–151. ISBN 978-0-203-48782-2. [Google Scholar]
- McGeown, W.J.; Mazzoni, G.; Venneri, A.; Kirsch, I. Hypnotic Induction Decreases Anterior Default Mode Activity. Conscious. Cogn. 2009, 18, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.; Hilgard, E.R. Failure of the Opiate Antagonist Naloxone to Modify Hypnotic Analgesia. Proc. Natl. Acad. Sci. USA 1975, 72, 2041–2043. [Google Scholar] [CrossRef]
- Stoyva, J.M. Posthypnotically Suggested Dreams and the Sleep Cycle. Arch. General. Psychiatry 1965, 12, 287–294. [Google Scholar] [CrossRef]
- Cordi, M.J.; Rossier, L.; Rasch, B. Hypnotic Suggestions given before Nighttime Sleep Extend Slow-Wave Sleep as Compared to a Control Text in Highly Hypnotizable Subjects. Int. J. Clin. Exp. Hypn. 2020, 68, 105–129. [Google Scholar] [CrossRef]
- Hilgard, E.R.; Tart, C.T. Responsiveness to Suggestions Following Waking and Imagination Instructions and Following Induction of Hypnosis. J. Abnorm. Psychol. 1966, 71, 196–208. [Google Scholar] [CrossRef]
- Kirsch, I.; Braffman, W. Imaginative Suggestibility and Hypnotizability. Curr. Dir. Psychol. Sci. 2001, 10, 57–61. [Google Scholar] [CrossRef]
- Piccione, C.; Hilgard, E.R.; Zimbardo, P.G. On the Degree of Stability of Measured Hypnotizability over a 25-Year Period. J. Pers. Soc. Psychol. 1989, 56, 289–295. [Google Scholar] [CrossRef]
- Morgan, A.H. The Heritability of Hypnotic Susceptibility in Twins. J. Abnorm. Psychol. 1973, 82, 55–61. [Google Scholar] [CrossRef]
- Morgan, A.H.; Hilgard, E.R.; Davert, E.C. The Heritability of Hypnotic Susceptibility of Twins: A Preliminary Report. Behav. Genet. 1970, 1, 213–224. [Google Scholar] [CrossRef]
- Rominger, C.; Weiss, E.M.; Nagl, S.; Niederstätter, H.; Parson, W.; Papousek, I. Carriers of the COMT Met/Met Allele Have Higher Degrees of Hypnotizability, Provided That They Have Good Attentional Control: A Case of Gene-Trait Interaction. Int. J. Clin. Exp. Hypn. 2014, 62, 455–482. [Google Scholar] [CrossRef]
- Nordenstrom, B.K.; Council James, R.; and Meier, B.P. The “Big Five” and Hypnotic Suggestibility. Int. J. Clin. Exp. Hypn. 2002, 50, 276–281. [Google Scholar] [CrossRef]
- Bates, B.L. Individual Differences in Response to Hypnosis. In Handbook of Clinical Hypnosis; American Psychological Association: Washington, DC, USA, 1993; pp. 23–54. ISBN 978-1-55798-440-1. [Google Scholar]
- Frankel, F.H.; Orne, M.T. Hypnotizability and Phobic Behavior. Arch. Gen. Psychiatry 1976, 33, 1259–1261. [Google Scholar] [CrossRef]
- Pettinati, H.M.; Kogan, L.G.; Evans, F.J.; Wade, J.H.; Horne, R.L.; Staats, J.M. Hypnotizability of Psychiatric Inpatients According to Two Different Scales. Am. J. Psychiatry 1990, 147, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, J.; Alldredge, C.T.; Haddenhorst, A. Meta-Analytic Evidence on the Efficacy of Hypnosis for Mental and Somatic Health Issues: A 20-Year Perspective. Front. Psychol. 2024, 14, 1330238. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.; Castanheira, J.d.S.; Boisvert, C.; Rousseaux, F.; Sackur, J.; Raz, A.; Richebé, P.; Ogez, D.; Rainville, P.; Jerbi, K. Aperiodic Activity as a Central Neural Feature of Hypnotic Susceptibility Outside of Hypnosis. bioRxiv 2024. bioRxiv:2023.11.16.567097. [Google Scholar]
- De Pascalis, V. Brain Functional Correlates of Resting Hypnosis and Hypnotizability: A Review. Brain Sci. 2024, 14, 115. [Google Scholar] [CrossRef]
- Graffin, N.F.; Ray, W.J.; Lundy, R. EEG Concomitants of Hypnosis and Hypnotic Susceptibility. J. Abnorm. Psychol. 1995, 104, 123–131. [Google Scholar] [CrossRef]
- Sabourin, M.E.; Cutcomb, S.D.; Crawford, H.J.; Pribram, K. EEG Correlates of Hypnotic Susceptibility and Hypnotic Trance: Spectral Analysis and Coherence. Int. J. Psychophysiol. 1990, 10, 125–142. [Google Scholar] [CrossRef]
- Cortade, D.L.; Markovits, J.; Spiegel, D.; Wang, S.X. Point-of-Care Testing of Enzyme Polymorphisms for Predicting Hypnotizability and Postoperative Pain. J. Mol. Diagn. 2023, 25, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Slade, G.D.; Sanders, A.E.; Ohrbach, R.; Bair, E.; Maixner, W.; Greenspan, J.D.; Fillingim, R.B.; Smith, S.; Diatchenko, L. COMT Diplotype Amplifies Effect of Stress on Risk of Temporomandibular Pain. J. Dent. Res. 2015, 94, 1187–1195. [Google Scholar] [CrossRef]
- Connors, M.H.; Quinto, L.; Deeley, Q.; Halligan, P.W.; Oakley, D.A.; Kanaan, R.A. Hypnosis and Suggestion as Interventions for Functional Neurological Disorder: A Systematic Review. Gen. Hosp. Psychiatry 2024, 86, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Wagaman, M.J. Physiological and Psychological Effects of Various Hypnotic Suggestions with Asthma Patients. Ph.D. Dissertation, The Union Institute, Cincinnati, OH, USA, 2000. [Google Scholar]
- MacLeod, C.M. Hypnosis and the Control of Attention: Where to from Here? Conscious. Cogn. An. Int. J. 2011, 20, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.; Fan, J.; Posner, M.I. Hypnotic Suggestion Reduces Conflict in the Human Brain. Proc. Natl. Acad. Sci. USA 2005, 102, 9978–9983. [Google Scholar] [CrossRef]
- Raz, A.; Kirsch, I.; Pollard, J.; Nitkin-Kaner, Y. Suggestion Reduces the Stroop Effect. Psychol. Sci. 2006, 17, 91–95. [Google Scholar] [CrossRef]
- Dixon, M.; Brunet, A.; Laurence, J.-R. Hypnotizability and Automaticity: Toward a Parallel Distributed Processing Model of Hypnotic Responding. J. Abnorm. Psychol. 1990, 99, 336–343. [Google Scholar] [CrossRef]
- Shiffrin, R.M.; Schneider, W. Controlled and Automatic Human Information Processing: II. Perceptual Learning, Automatic Attending and a General Theory. Psychol. Rev. 1977, 84, 127–190. [Google Scholar] [CrossRef]
- Weitzenhoffer, A.M. When Is an “Instruction” an “Instruction”? Int. J. Clin. Exp. Hypn. 1974, 22, 258–269. [Google Scholar] [CrossRef]
- Bowers, K.S. Do the Stanford Scales Tap the “Classic Suggestion Effect”? Int. J. Clin. Exp. Hypn. 1981, 29, 42–53. [Google Scholar] [CrossRef]
- Balthazard, C.; Woody, E. The Spectral Analysis of Hypnotic Performance with Respect to Absorption. Int. J. Exp. Clin. Hypn. 1992, 40, 21–43. [Google Scholar] [CrossRef]
- Kirsch, I.; Council, J.R. Situational and Personality Correlates of Hypnotic Responsiveness. In Contemporary Hypnosis Research; The Guilford Press: New York, NY, USA, 1992; pp. 267–291. ISBN 978-0-89862-893-7. [Google Scholar]
- Wickramasekera, I. The Placebo Effect and Its Use in Biofeedback Therapy. In Handbook of Mind-Body Medicine for Primary Care; Moss, D., McGrady, A., Davies, T., Wickramasekera, I., Eds.; Sage: Thousand Oaks, CA, USA, 2003; pp. 69–82. [Google Scholar]
- Voudouris, N.J.; Peck, C.L.; Coleman, G. Conditioned Placebo Responses. J. Pers. Soc. Psychol. 1985, 48, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Gruzelier, J. New and Rediscovered Insights about the Nature of Hynotizability: Exceptional Ability and Vulnerability. Contemp. Hypn. Integr. Ther. 2011, 28, 116–135. [Google Scholar]
- Lifshitz, M.; Aubert Bonn, N.; Fischer, A.; Kashem, I.F.; Raz, A. Using Suggestion to Modulate Automatic Processes: From Stroop to McGurk and Beyond. Cortex 2013, 49, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Barnier, A.J.; McConkey, K.M. Absorption, Hypnotizability and Context: Non-Hypnotic Contexts Are Not All the Same. Contemp. Hypn. 1999, 16, 1–8. [Google Scholar] [CrossRef]
- Tellegen, A.; Lykken, D.T.; Bouchard, T.J.; Wilcox, K.J.; Segal, N.L.; Rich, S. Personality Similarity in Twins Reared Apart and Together. J. Pers. Soc. Psychol. 1988, 54, 1031–1039. [Google Scholar] [CrossRef]
- Bräscher, A.-K.; Sütterlin, S.; Scheuren, R.; Van den Bergh, O.; Witthöft, M. Somatic Symptom Perception From a Predictive Processing Perspective: An Empirical Test Using the Thermal Grill Illusion. Psychosom. Med. 2020, 82, 708–714. [Google Scholar] [CrossRef]
- Qualls, P.J.; Sheehan, P.W. Imagery Encouragement, Absorption Capacity, and Relaxation during Electromyograph Biofeedback. J. Personal. Soc. Psychol. 1981, 41, 370–379. [Google Scholar] [CrossRef]
- Zillmer, E.; Wickramasekera, I. Biofeedback and Hypnotizability: Initial Treatment Considerations. Clin. Biofeedback Health 1987, 10, 51057. [Google Scholar]
- Morrow, G.R.; Morrell, C. Behavioral Treatment for the Anticipatory Nausea and Vomiting Induced by Cancer Chemotherapy. N. Engl. J. Med. 1982, 307, 1476–1480. [Google Scholar] [CrossRef]
- Vaitl, D.; Ott, U. Altered States of Consciousness Induced by Psychophysiological Techniques. Mind Matter 2005, 3, 9–30. [Google Scholar]
- Jamieson, G.A. The Modified Tellegen Absorption Scale: A Clearer Window on the Structure and Meaning of Absorption. Aust. J. Clin. Exp. Hypn. 2005, 33, 119–139. [Google Scholar]
- Lifshitz, M.; Sacchet, M.D.; Huntenburg, J.M.; Thiery, T.; Fan, Y.; Gärtner, M.; Grimm, S.; Winnebeck, E.; Fissler, M.; Schroeter, T.A.; et al. Mindfulness-Based Therapy Regulates Brain Connectivity in Major Depression. PPS 2019, 88, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Sifneos, P.E. The Prevalence of “alexithymic” Characteristics in Psychosomatic Patients. Psychother. Psychosom. 1973, 22, 255–262. [Google Scholar] [CrossRef]
- Spiegel, H.; Spiegel, D. Trance and Treatment: Clinical Uses of Hypnosis; American Psychiatric Publishing: Washington, DC, USA, 2008; ISBN 978-1-58562-727-1. [Google Scholar]
- Heap, M.; Brown, R.J.; Oakley, D.A. (Eds.) The Highly Hypnotizable Person: Theoretical, Experimental and Clinical Issues; Routledge: London, UK, 2004; ISBN 978-0-203-48782-2. [Google Scholar]
- Jørgensen, M.M.; Zachariae, R.; Skytthe, A.; Kyvik, K. Genetic and Environmental Factors in Alexithymia: A Population-Based Study of 8785 Danish Twin Pairs. Psychother. Psychosom. 2007, 76, 369–375. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, J.; Servaas, M.N.; Goerlich, K.S.; Bruggeman, R.; Horton, P.; Costafreda, S.G.; Aleman, A. Neural Correlates of Alexithymia: A Meta-Analysis of Emotion Processing Studies. Neurosci. Biobehav. Rev. 2013, 37, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Muratsubaki, T.; Yagihashi, M.; Morishita, J.; Mugikura, S.; Dupont, P.; Takase, K.; Kanazawa, M.; Van Oudenhove, L.; Fukudo, S. Insula Activity to Visceral Stimulation and Endocrine Stress Responses as Associated with Alexithymia in Patients With Irritable Bowel Syndrome. Psychosom. Med. 2020, 82, 29. [Google Scholar] [CrossRef]
- Kano, M.; Fukudo, S. The Alexithymic Brain: The Neural Pathways Linking Alexithymia to Physical Disorders. Biopsychosoc. Med. 2013, 7, 1. [Google Scholar] [CrossRef]
- Preti, A.; Sancassiani, F.; Cadoni, F.; Carta, M.G. Alexithymia Affects Pre-Hospital Delay of Patients with Acute Myocardial Infarction: Meta-Analysis of Existing Studies. Clin. Pract. Epidemiol. Ment. Health 2013, 9, 69–73. [Google Scholar] [CrossRef]
- Terhune, D.B.; Oakley, D.A. Hypnosis and Imagination. In The Cambridge Handbook of the Imagination; Cambridge University Press: New York, NY, USA, 2020; pp. 711–727. ISBN 978-1-108-45342-4. [Google Scholar]
- Kano, M.; Oudenhove, L.V.; Dupont, P.; Wager, T.D.; Fukudo, S. Imaging Brain Mechanisms of Functional Somatic Syndromes: Potential as a Biomarker? Tohoku J. Exp. Med. 2020, 250, 137–152. [Google Scholar] [CrossRef]
- LeDoux, J. The Emotional Brain, Fear, and the Amygdala. Cell Mol. Neurobiol. 2003, 23, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, G.H.; Kirsch, I. Classical Conditioning and the Placebo Effect. Pain 1997, 72, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.B.; Kaptchuk, T.J.; Kirsch, I.; Raicek, J.; Lindstrom, K.M.; Berna, C.; Gollub, R.L.; Ingvar, M.; Kong, J. Nonconscious Activation of Placebo and Nocebo Pain Responses. Proc. Natl. Acad. Sci. USA 2012, 109, 15959–15964. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Adachi, T.; Hakimian, S. Brain Oscillations, Hypnosis, and Hypnotizability. Am. J. Clin. Hypn. 2015, 57, 230–253. [Google Scholar] [CrossRef]
- Jensen, K.B.; Kaptchuk, T.J.; Chen, X.; Kirsch, I.; Ingvar, M.; Gollub, R.L.; Kong, J. A Neural Mechanism for Nonconscious Activation of Conditioned Placebo and Nocebo Responses. Cereb. Cortex 2015, 25, 3903–3910. [Google Scholar] [CrossRef]
- Bąbel, P. Classical Conditioning as a Distinct Mechanism of Placebo Effects. Front. Psychiatry 2019, 10, 449. [Google Scholar] [CrossRef]
- Colloca, L.; Barsky, A.J. Placebo and Nocebo Effects. N. Engl. J. Med. 2020, 382, 554–561. [Google Scholar] [CrossRef]
- Colloca, L. The Nocebo Effect. Annu. Rev. Pharmacol. Toxicol. 2023, 64, 171–190. [Google Scholar] [CrossRef]
- Martins, J.; Czamara, D.; Sauer, S.; Rex-Haffner, M.; Dittrich, K.; Dörr, P.; de Punder, K.; Overfeld, J.; Knop, A.; Dammering, F.; et al. Childhood Adversity Correlates with Stable Changes in DNA Methylation Trajectories in Children and Converges with Epigenetic Signatures of Prenatal Stress. Neurobiol. Stress 2021, 15, 100336. [Google Scholar] [CrossRef]
- Raffington, L. Utilizing Epigenetics to Study the Shared Nature of Development and Biological Aging across the Lifespan. Npj Sci. Learn. 2024, 9, 24. [Google Scholar] [CrossRef]
- Schmidt, L.J.; Belopolsky, A.V.; Theeuwes, J. The Time Course of Attentional Bias to Cues of Threat and Safety. Cogn. Emot. 2017, 31, 845–857. [Google Scholar] [CrossRef]
- Barbiani, D.; Camerone, E.M.; Grosso, F.; Geers, A.L.; Pagnini, F. The Role of Attention in Placebo and Nocebo Effects. Ann. Behav. Med. 2024, 58, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Miller, G.A.; McDavitt, J.R.B.; Spielberg, J.M.; Crocker, L.D.; Infantolino, Z.P.; Towers, D.N.; Warren, S.L.; Heller, W. Interactive Effects of Trait and State Affect on Top-down Control of Attention. Soc. Cogn. Affect. Neurosci. 2015, 10, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. Vagal Tone: A Physiologic Marker of Stress Vulnerability. Pediatrics 1992, 90, 498–504. [Google Scholar] [CrossRef]
- Porges, S.W. The Polyvagal Perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The Polyvagal Theory: New Insights into Adaptive Reactions of the Autonomic Nervous System. Cleve Clin. J. Med. 2009, 76, S86–S90. [Google Scholar] [CrossRef]
- Zajonc, R.B. On the Primacy of Affect. Am. Psychol. 1984, 39, 117–123. [Google Scholar] [CrossRef]
- Cromwell, H.C.; Panksepp, J. Rethinking the Cognitive Revolution from a Neural Perspective: How Overuse/Misuse of the Term ‘Cognition’ and the Neglect of Affective Controls in Behavioral Neuroscience Could Be Delaying Progress in Understanding the BrainMind. Neurosci. Biobehav. Rev. 2011, 35, 2026–2035. [Google Scholar] [CrossRef]
- Folkman, S. Personal Control and Stress and Coping Processes: A Theoretical Analysis. J. Pers. Soc. Psychol. 1984, 46, 839–852. [Google Scholar] [CrossRef]
- Neuhuber, W.L.; Berthoud, H.-R. Functional Anatomy of the Vagus System: How Does the Polyvagal Theory Comply? Biol. Psychol. 2022, 174, 108425. [Google Scholar] [CrossRef]
- Grossman, P. Fundamental Challenges and Likely Refutations of the Five Basic Premises of the Polyvagal Theory. Biol. Psychol. 2023, 180, 108589. [Google Scholar] [CrossRef]
- Harris, R.M.; Porges, S.W.; Carpenter, M.E.; Vincenz, L.M. Hypnotic Susceptibility, Mood State, and Cardiovascular Reactivity. Am. J. Clin. Hypn. 1993, 36, 15–25. [Google Scholar] [CrossRef]
- Diamond, S.G.; Davis, O.C.; Howe, R.D. Heart-Rate Variability as a Quantitative Measure of Hypnotic Depth. Int. J. Clin. Exp. Hypn. 2008, 56, 1–18. [Google Scholar] [CrossRef]
- Stern, D.B.; Spiegel, H.; Nee, J.C. The Hypnotic Induction Profile: Normative Observations, Reliability and Validity. Am. J. Clin. Hypn. 1978, 21, 109–133. [Google Scholar] [CrossRef]
- Mather, M.; Thayer, J. How Heart Rate Variability Affects Emotion Regulation Brain Networks. Curr. Opin. Behav. Sci. 2018, 19, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The Effect of Heart Rate Variability Biofeedback Training on Stress and Anxiety: A Meta-Analysis. Psychol. Med. 2017, 47, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Kolacz, J.; Porges, S.W. Chronic Diffuse Pain and Functional Gastrointestinal Disorders After Traumatic Stress: Pathophysiology Through a Polyvagal Perspective. Front. Med. 2018, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Wickramasekera II, I.E.; Szlyk, J.P. Could Empathy Be a Predictor of Hypnotic Ability? Int. J. Clin. Exp. Hypn. 2003, 51, 390–399. [Google Scholar] [CrossRef]
- Sargent, K.S.; Martinez, E.L.; Reed, A.C.; Guha, A.; Bartholomew, M.E.; Diehl, C.K.; Chang, C.S.; Salama, S.; Popov, T.; Thayer, J.F.; et al. Oscillatory Coupling Between Neural and Cardiac Rhythms. Psychol. Sci. 2024, 35, 517–528. [Google Scholar] [CrossRef]
- Friedman, B.H.; Thayer, J.F. Autonomic Balance Revisited: Panic Anxiety and Heart Rate Variability. J. Psychosom. Res. 1998, 44, 133–151. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A Model of Neurovisceral Integration in Emotion Regulation and Dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Aronoff, G.M. Editorial. Clin. J. Pain 1985, 1, 1–3. Available online: https://journals.lww.com/clinicalpain/citation/1985/01010/editorial.1.aspx (accessed on 23 August 2025). [CrossRef]
- Henningsen, P.; Zipfel, S.; Sattel, H.; Creed, F. Management of Functional Somatic Syndromes and Bodily Distress. Psychother. Psychosom. 2018, 87, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Donnachie, E.; Zipfel, S.; Enck, P. Patients With Somatoform Disorders Are Prone to Expensive and Potentially Harmful Medical Procedures—Results of a Retrospective Cohort Study Over 15 Years. Dtsch. Arztebl. Int. 2021, 118, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Jonas, W.B.; Crawford, C.; Colloca, L.; Kriston, L.; Linde, K.; Moseley, B.; Meissner, K. Are Invasive Procedures Effective for Chronic Pain? A Systematic Review. Pain Med. 2019, 20, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Ashar, Y.K.; Gordon, A.; Schubiner, H.; Uipi, C.; Knight, K.; Anderson, Z.; Carlisle, J.; Polisky, L.; Geuter, S.; Flood, T.F.; et al. Effect of Pain Reprocessing Therapy vs Placebo and Usual Care for Patients With Chronic Back Pain: A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 13–23. [Google Scholar] [CrossRef]
- Sullivan, M.D.; Ballantyne, J.C. Randomised Trial Reveals Opioids Relieve Acute Back Pain No Better than Placebo. Lancet 2023, 402, 267–269. [Google Scholar] [CrossRef]
- Kohn, L.T.; Corrigan, J.M.; Donaldson, M.S. To Err Is Human: Building a Safer Health System; Institute of Medicine (US) Committee on Quality of Health Care in America, Ed.; National Academies Press (US): Washington, DC, USA, 2000; ISBN 978-0-309-06837-6. [Google Scholar]
- Landrigan, C.P.; Parry, G.J.; Bones, C.B.; Hackbarth, A.D.; Goldmann, D.A.; Sharek, P.J. Temporal Trends in Rates of Patient Harm Resulting from Medical Care. N. Engl. J. Med. 2010, 363, 2124–2134. [Google Scholar] [CrossRef]
- Makary, M.A.; Daniel, M. Medical Error—The Third Leading Cause of Death in the US. BMJ 2016, 353, i2139. [Google Scholar] [CrossRef]
- Rodziewicz, T.L.; Houseman, B.; Vaqar, S.; Hipskind, J.E. Medical Error Reduction and Prevention. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of Stress in the Brain. Nat. Neurosci. 2015, 18, 1353–1363. [Google Scholar] [CrossRef]
- Andrasik, F.; Holroyd, K.A. A Test of Specific and Nonspecific Effects in the Biofeedback Treatment of Tension Headache. J. Consult. Clin. Psychol. 1980, 48, 575–586. [Google Scholar] [CrossRef]
- Cohen, H.D.; Graham, C.; Fotopoulos, S.S.; Cook, M.R. A Double-Blind Methodology for Biofeedback Research. Psychophysiology 1977, 14, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Taub, E.; School, P.J. Some Methodological Considerations in Thermal Biofeedback Training. Behav. Res. Methods Instrum. 1978, 10, 617–622. [Google Scholar] [CrossRef]
- Melzack, R.; Perry, C. Self-Regulation of Pain: The Use of Alpha-Feedback and Hypnotic Training for the Control of Chronic Pain. Exp. Neurol. 1975, 46, 452–469. [Google Scholar] [CrossRef]
- Wickramasekera, I. Temperature Feedback for the Control of Migraine. J. Behav. Ther. Exp. Psychiatry 1973, 4, 343–345. [Google Scholar] [CrossRef]
- Wickramasekera, I. The Placebo Effect and Medical Instruments in Biofeedback. J. Clin. Eng. 1977, 2, 227–230. [Google Scholar] [CrossRef]
- Kirsch, I. Response Expectancy as a Determinant of Experience and Behavior. Am. Psychologist. 1985, 40, 1189–1202. [Google Scholar] [CrossRef]
- Colloca, L.; Miller, F.G. How Placebo Responses Are Formed: A Learning Perspective. Philos. Trans. Biol. Sci. 2011, 366, 1859–1869. [Google Scholar] [CrossRef]
- Landry, M.; Lifshitz, M.; Raz, A. Brain Correlates of Hypnosis: A Systematic Review and Meta-Analytic Exploration. Neurosci. Biobehav. Rev. 2017, 81, 75–98. [Google Scholar] [CrossRef]
- Barnes, K.; Wang, R.; Faasse, K. Practitioner Warmth and Empathy Attenuates the Nocebo Effect and Enhances the Placebo Effect. Appl. Psychol. Health Well-Being 2024, 16, 421–441. [Google Scholar] [CrossRef]
- Ellingsen, D.-M.; Isenburg, K.; Jung, C.; Lee, J.; Gerber, J.; Mawla, I.; Sclocco, R.; Grahl, A.; Anzolin, A.; Edwards, R.R.; et al. Brain-to-Brain Mechanisms Underlying Pain Empathy and Social Modulation of Pain in the Patient-Clinician Interaction. Proc. Natl. Acad. Sci. USA 2023, 120, e2212910120. [Google Scholar] [CrossRef]
- Mulej Bratec, S.; Bertram, T.; Starke, G.; Brandl, F.; Xie, X.; Sorg, C. Your Presence Soothes Me: A Neural Process Model of Aversive Emotion Regulation via Social Buffering. Social Cogn. Affect. Neurosci. 2020, 15, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Reddan, M.C.; Young, H.; Falkner, J.; López-Solà, M.; Wager, T.D. Touch and Social Support Influence Interpersonal Synchrony and Pain. Soc. Cogn. Affect. Neurosci. 2020, 15, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Traux, C.B.; Carkhuff, R.R. The Measurement of Interpersonal Sensitivity: The Jacobson Empathy Scale and the Carkhuff-Truax Empathy Scale. 1967. [Google Scholar]
- Wampold, B.E. Healing in a Social Context: The Importance of Clinician and Patient Relationship. Front. Pain Res. 2021, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, U.; Chalamish, Y.; Krieger, I.; Erez, H.B.; Braw, Y.; Lichtenberg, P. Suggestibility as a Predictor of Response to Antidepressants: A Preliminary Prospective Trial. J. Affect. Disord. 2015, 185, 8–11. [Google Scholar] [CrossRef]
- Beecher, H.K. Increased Stress and Effectiveness of Placebos and “Active” Drugs. Science 1960, 132, 91–92. [Google Scholar] [CrossRef]
- Bräscher, A.-K.; Witthöft, M.; Becker, S. The Underestimated Significance of Conditioning in Placebo Hypoalgesia and Nocebo Hyperalgesia. Pain Res. Manag. 2018, 2018, 6841985. [Google Scholar] [CrossRef]
- Cusin, C.; Colloca, L. Treatment-Resistant Depression—Resistant to Placebos as Well? JAMA Netw. Open 2021, 4, e2127952. [Google Scholar] [CrossRef]
- Schmidt, S.; Loef, M.; Ostermann, T.; Walach, H. Treatment Effects in Pharmacological Clinical Randomized Controlled Trials Are Mainly Due to Placebo. J. Clin. Epidemiol. 2024, 179, 111658. [Google Scholar] [CrossRef]
- Tuttle, A.; Tohyama, S.; Ramsay, T.; Kimmelman, J.; Schweinhardt, P.; Bennett, G.; Mogil, J. Increasing Placebo Responses over Time in U.S. Clinical Trials of Neuropathic Pain. Pain 2015, 156, 2616–2626. [Google Scholar] [CrossRef]
- Vase, L.; Petersen, G.L.; Lund, K. Placebo Effects in Idiopathic and Neuropathic Pain Conditions. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 225, pp. 121–136. [Google Scholar] [CrossRef]
- Bowers, M.E.; Yehuda, R. Intergenerational Transmission of Stress in Humans. Neuropsychopharmacology 2016, 41, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Carone, B.R.; Fauquier, L.; Habib, N.; Shea, J.M.; Hart, C.E.; Li, R.; Bock, C.; Li, C.; Gu, H.; Zamore, P.D.; et al. Paternally Induced Transgenerational Environmental Reprogramming of Metabolic Gene Expression in Mammals. Cell 2010, 143, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, E.; Raz, G. Transgenerational Epigenetic Inheritance: Prevalence, Mechanisms, and Implications for the Study of Heredity and Evolution. Q. Rev. Biol. 2009, 84, 131–176. [Google Scholar] [CrossRef]
- Colloca, L. The Placebo Effect in Pain Therapies. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 191–211. [Google Scholar] [CrossRef]
- Truax, C.B.; Carkhuff, R.R. Toward Effective Counseling and Psychotherapy: Training and Practice; Aldine Publishing Co.: Hawthorne, NY, USA, 1967; pp. xiv, 416. [Google Scholar]
- Anderson, S.R.; Gianola, M.; Medina, N.A.; Perry, J.M.; Wager, T.D.; Losin, E.A.R. Doctor Trustworthiness Influences Pain and Its Neural Correlates in Virtual Medical Interactions. Cereb. Cortex 2023, 33, 3421–3436. [Google Scholar] [CrossRef]
- Wickramasekera, I. Desensitization, Re-Sensitization and Desensitization Again: A Preliminary Study. J. Behav. Ther. Exp. Psychiatry 1970, 1, 257–262. [Google Scholar] [CrossRef]
- Wilson, S.J.; Woody, A.; Padin, A.C.; Lin, J.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Loneliness and Telomere Length: Immune and Parasympathetic Function in Associations With Accelerated Aging. Ann. Behav. Med. 2019, 53, 541–550. [Google Scholar] [CrossRef]
- Coan, J.A.; Schaefer, H.S.; Davidson, R.J. Lending a Hand: Social Regulation of the Neural Response to Threat. Psychol. Sci. 2006, 17, 1032–1039. [Google Scholar] [CrossRef]
- Rainville, P.; Streff, A.; Chen, J.-I.; Houzé, B.; Desmarteaux, C.; Piché, M. Hypnotic Automaticity in the Brain at Rest: An Arterial Spin Labelling Study. Int. J. Clin. Exp. Hypn. 2019, 67, 512–542. [Google Scholar] [CrossRef]
- Palsson, O.S.; Kekecs, Z.; De Benedittis, G.; Moss, D.; Elkins, G.R.; Terhune, D.B.; Varga, K.; Shenefelt, P.D.; Whorwell, P.J. Current Practices, Experiences, and Views in Clinical Hypnosis: Findings of an International Survey. Int. J. Clin. Exp. Hypn. 2023, 71, 92–114. [Google Scholar] [CrossRef]
- Palsson, O.S.; Ballou, S. Hypnosis and Cognitive Behavioral Therapies for the Management of Gastrointestinal Disorders. Curr. Gastroenterol. Rep. 2020, 22, 31. [Google Scholar] [CrossRef]
- Vase, L.; Petersen, G.L.; Riley, J.L.; Price, D.D. Factors Contributing to Large Analgesic Effects in Placebo Mechanism Studies Conducted between 2002 and 2007. Pain 2009, 145, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Klosterhalfen, S. Placebo Responses and Placebo Effects in Functional Gastrointestinal Disorders. Front. Psychiatry 2020, 11, 797. [Google Scholar] [CrossRef]
- Pagnini, F.; Barbiani, D.; Grosso, F.; Cavalera, C.; Volpato, E.; Minazzi, G.A.; Poletti, V.; Riva, G.; Phillips, D. Enacting the Mind/Body Connection: The Role of Self-Induced Placebo Mechanisms. Humanit. Soc. Sci. Commun. 2024, 11, 977. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wickramasekera, I. The High-Risk Model of Threat Perception Modulates Learning of Placebo and Nocebo Effects and Functional Somatic Disorders. Brain Sci. 2025, 15, 955. https://doi.org/10.3390/brainsci15090955
Wickramasekera I. The High-Risk Model of Threat Perception Modulates Learning of Placebo and Nocebo Effects and Functional Somatic Disorders. Brain Sciences. 2025; 15(9):955. https://doi.org/10.3390/brainsci15090955
Chicago/Turabian StyleWickramasekera, Ian. 2025. "The High-Risk Model of Threat Perception Modulates Learning of Placebo and Nocebo Effects and Functional Somatic Disorders" Brain Sciences 15, no. 9: 955. https://doi.org/10.3390/brainsci15090955
APA StyleWickramasekera, I. (2025). The High-Risk Model of Threat Perception Modulates Learning of Placebo and Nocebo Effects and Functional Somatic Disorders. Brain Sciences, 15(9), 955. https://doi.org/10.3390/brainsci15090955




