CT-Based Quantitative Analysis of Ossification Centres in the C7 Vertebra of Human Fetuses
Abstract
1. Introduction
2. Materials and Methods
2.1. Examined Sample
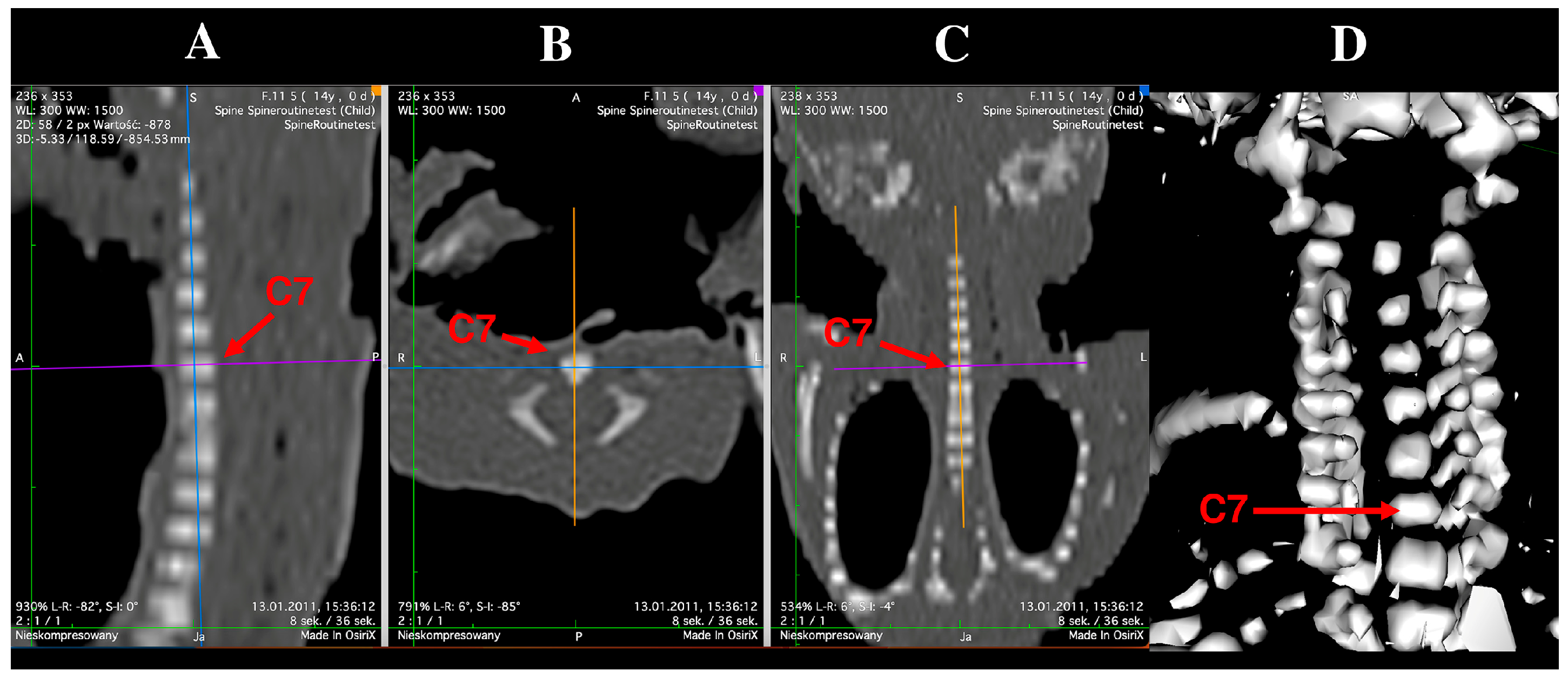
2.2. Morphometric Measurements and Assessment of Ossification Centers
2.3. Statistical Analysis
3. Results
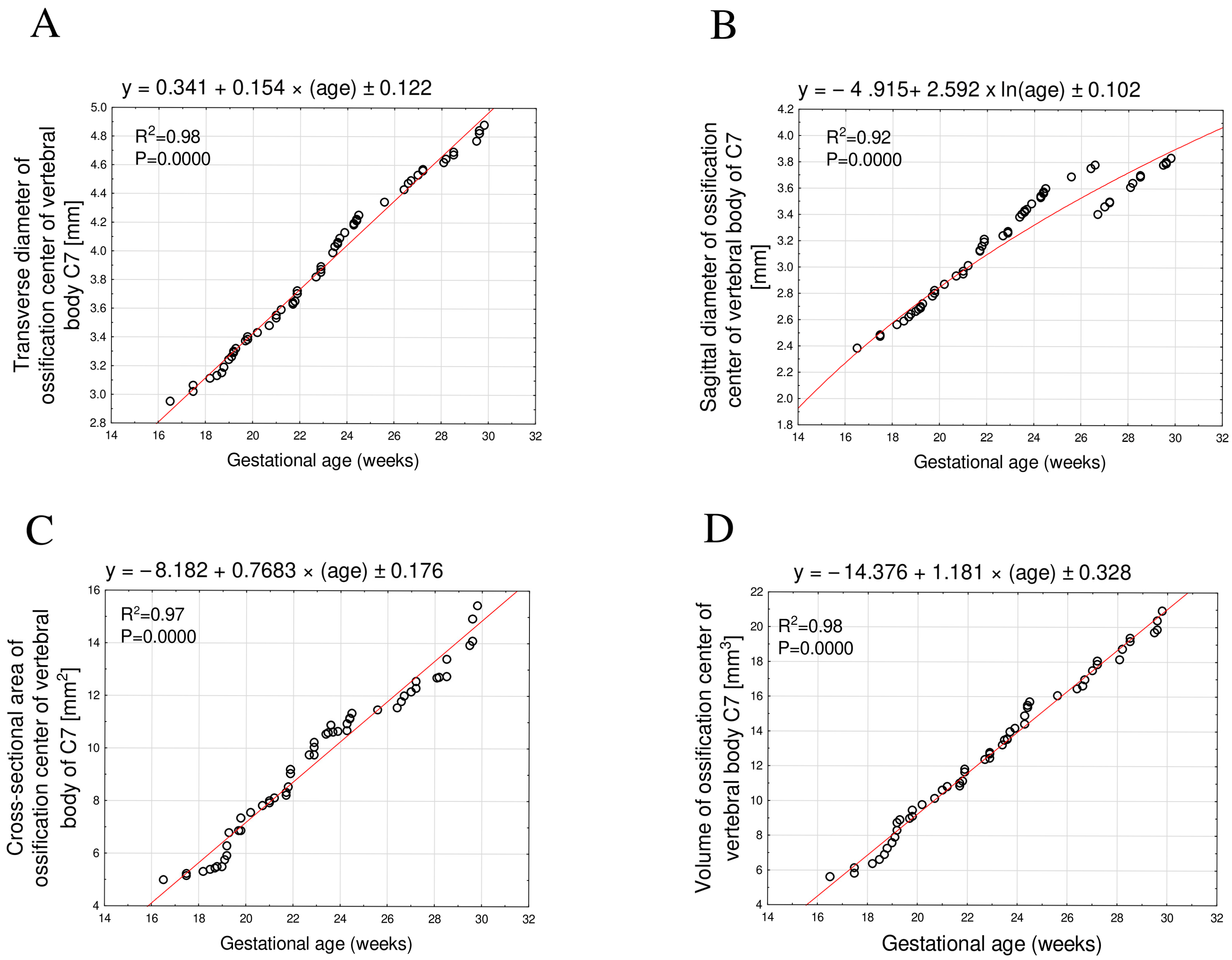
3.1. Morphometric Parameters of the Ossification Center of the C7 Vertebral Body
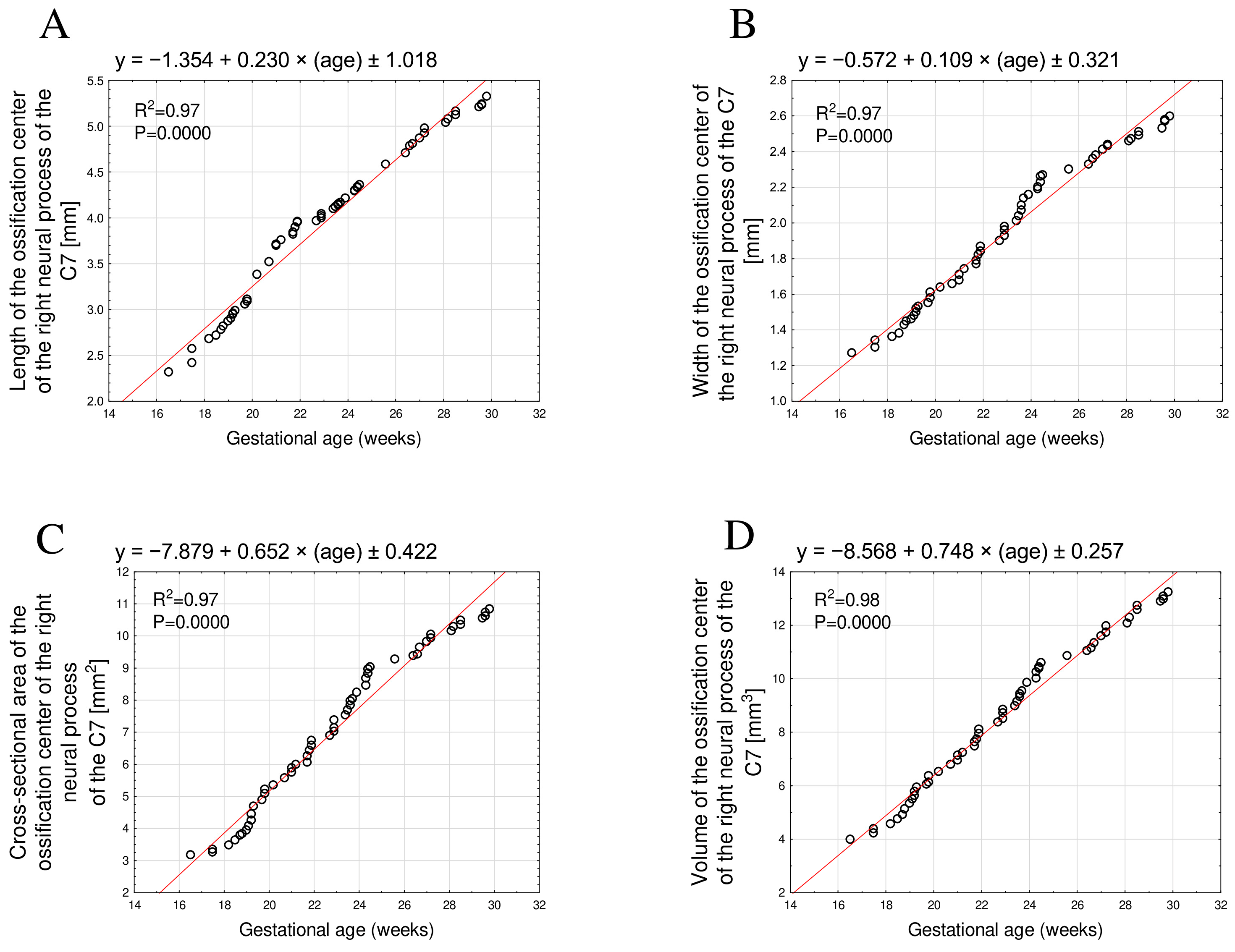
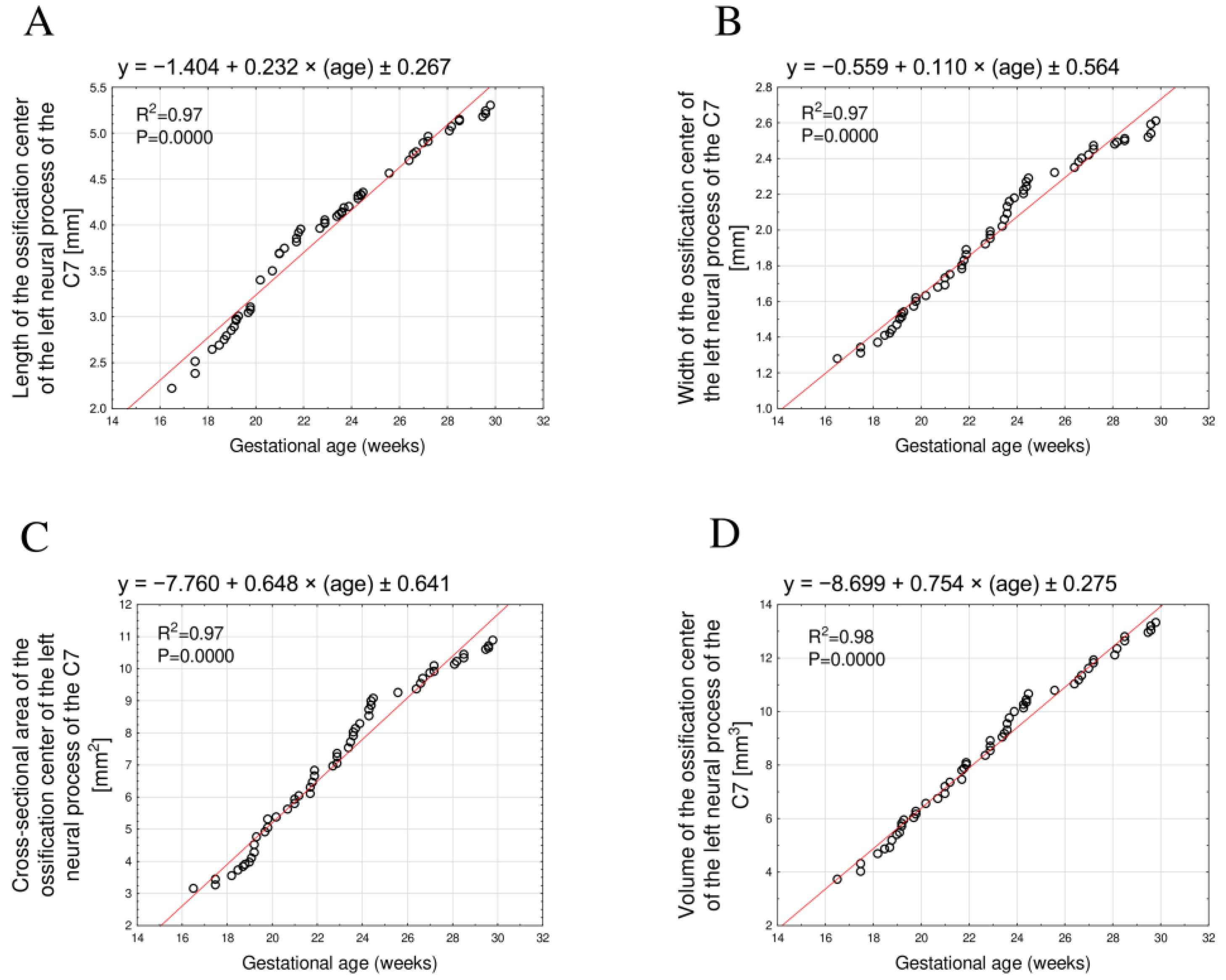
3.2. Morphometric Parameters of the Right and Left C7 Neural Process Ossification Centers
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Draga, M.; Scaal, M. Building a vertebra: Development of the amniote sclerotome. J. Morphol. 2024, 285, e21665. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Grzonkowska, M.; Bogacz, K.; Żytkowski, A.; Szkultecka-Dębek, M.; Kułakowski, M.; Janiak, M.; Rogalska, A.; Baumgart, M. Digital Image Analysis of Vertebral Body S1 and Its Ossification Center in the Human Fetus. Brain Sci. 2025, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Nemec, U.; Nemec, S.F.; Brugger, P.C.; Bettelheim, D.; Rotmensch, S.; Graham, J.M.; Rimoin, D.L.; Prayer, D. MR imaging of the fetal musculoskeletal system. Prenat. Diagn. 2012, 32, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Rufener, S.L.; Ibrahim, M.; Parmar, H.A. Imaging of congenital spine and spinal cord malformations. Neuroimaging Clin. N. Am. 2011, 21, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Lalit, M.; Piplani, S.; Piplani, N. Exploring the Anatomical and Clinical Perspectives of the Vertebra Prominens (C7). J. Hum. Anat. 2024, 8, 000207. [Google Scholar]
- Shin, S.; Yoon, D.-M.; Yoon, K.B. Identification of the correct cervical level by palpation of spinous processes. Anesth. Analg. 2011, 112, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Raveendranath, V.; Kavitha, T.; Umamageswari, A. Morphometry of the uncinate process, vertebral body, and lamina of the C3–7 vertebrae relevant to cervical spine surgery. Neurospine 2019, 16, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Tolis, T.; Sammer, A.; Piagkou, M.; Natis, K.; Emfietzis, P.K.; Karageorgos, F.; Tsakotos, G.; Triantafyllou, G.; Feigl, G. Variability in the projection level of the vertebra prominens: A cadaveric study. Anat. Cell Biol. 2023, 57, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Ezra, D.; Hershkovitz, I.; Salame, K.; Alperovitch-Najenson, K.; Sloon, V. Osteophytes in the Cervical Vertebral Bodies (C3-C7)—Demographical Perspectives. Anat. Rec. 2019, 302, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Bazaldúa, C.J.J.; González, L.A.; Gómez, S.A.; Villarreal, S.E.E.; Velázquez, G.S.E.; Sánchez, U.A.; Elizondo-Omaña, R.E.; Guzmán, L.S. Morphometric Study of Cervical Vertebrae C3-C7 in a Population from Northeastern Mexico. Int. J. Morphol. 2011, 29, 325–330. [Google Scholar] [CrossRef]
- Baumgart, M.; Szpinda, M.; Szpinda, A. New anatomical data on the growing C4 vertebra and its three ossification centers in human fetuses. Surg. Radiol. Anat. 2013, 35, 191–203. [Google Scholar] [CrossRef][Green Version]
- Gopinathan, N.R.; Viswanathan, V.K.; Crawford, A.H. Cervical Spine Evaluation in Pediatric Trauma: A Review and an Update of Current Concepts. Indian J. Orthop. 2018, 52, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Chano, T.; Masumoto, K.; Ishizawa, M.; Morimoto, S.; Hukuda, S.; Okabe, H.; Kato, H.; Fujino, S. Analysis of the presence of osteocalcin, S-100 protein, and proliferating cell nuclear antigen in cells of various types of osteosarcomas. Eur. J. Histochem. 1996, 40, 189–198. [Google Scholar]
- Duarte, W.R.; Shibata, T.; Takenaga, K.; Takahashi, E.; Kubota, K.; Ohya, K.; Ishikawa, I.; Yamauchi, M.; Kasugai, S. S100A4: A novel negative regulator of mineralization and osteoblast differentiation. J. Bone Miner. Res. 2003, 18, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, M.; Wiśniewski, M.; Grzonkowska, M.; Małkowski, B.; Badura, M.; Dąbrowska, M.; Szpinda, M. Digital image analysis of ossification centers in the axial dens and body in the human fetus. Surg. Radiol. Anat. 2016, 38, 1195–1203. [Google Scholar] [CrossRef]
- Baumgart, M.; Wiśniewski, M.; Grzonkowska, M.; Małkowski, B.; Badura, M.; Dąbrowska, M.; Szpinda, M. Morphometric study of the neural ossification centers of the atlas and axis in the human fetus. Surg. Radiol. Anat. 2016, 38, 1205–1215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szpinda, M.; Baumgart, M.; Szpinda, A.; Woźniak, A.; Mila-Kierzenkowska, C. Cross-sectional study of the neural ossification centers of vertebrae C1–S5 in the human fetus. Surg. Radiol. Anat. 2013, 35, 701–711. [Google Scholar] [CrossRef]
- Szpinda, M.; Baumgart, M.; Szpinda, A.; Woźniak, A.; Małkowski, B.; Wiśniewski, M.; Mila-Kierzenkowska, C.; Króliczewski, D. Cross-sectional study of the ossification center of the C1–S5 vertebral bodies. Surg. Radiol. Anat. 2013, 35, 395–402. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Victoria, T.; Epelman, M.; Coleman, B.G.; Horii, S.C.; Oliver, E.R.; Mahboubi, S.; Khalek, N.; Kasperski, S.; Edgar, J.C.; Jaramillo, D. Low-dose fetal CT in the prenatal evaluation of skeletal dysplasias and other severe skeletal abnormalities. Am. J. Roentgenol. 2013, 200, 989–1000. [Google Scholar] [CrossRef] [PubMed]

| Gestational Age | Crown–Rump Length (mm) | Number of Fetuses | Sex | ||||
|---|---|---|---|---|---|---|---|
| Weeks (Hbd-Life) | Mean | SD | Min. | Max. | ♂ | ♀ | |
| 17 | 115.00 | - | 115.00 | 115.00 | 1 | 0 | 1 |
| 18 | 133.33 | 5.77 | 130.00 | 140.00 | 3 | 1 | 2 |
| 19 | 149.50 | 3.82 | 143.00 | 154.00 | 8 | 3 | 5 |
| 20 | 161.00 | 2.71 | 159.00 | 165.00 | 4 | 2 | 2 |
| 21 | 174.75 | 2.87 | 171.00 | 178.00 | 4 | 3 | 1 |
| 22 | 185.00 | 1.41 | 183.00 | 186.00 | 4 | 1 | 3 |
| 23 | 197.60 | 2.61 | 195.00 | 202.00 | 5 | 2 | 3 |
| 24 | 208.67 | 3.81 | 204.00 | 213.00 | 9 | 5 | 4 |
| 25 | 214.00 | - | 214.00 | 214.00 | 1 | 0 | 1 |
| 26 | 229.00 | 5.66 | 225.00 | 233.00 | 2 | 1 | 1 |
| 27 | 237.50 | 3.33 | 233.00 | 241.00 | 6 | 6 | 0 |
| 28 | 249.50 | 0.71 | 249.00 | 250.00 | 2 | 0 | 2 |
| 29 | 253.00 | 0.00 | 253.00 | 253.00 | 2 | 0 | 2 |
| 30 | 263.25 | 1.26 | 262.00 | 265.00 | 4 | 3 | 1 |
| Total | 55 | 27 | 28 | ||||
| Parameter of the Body Ossification Center of C7 Vertebra | ICC |
|---|---|
| Transverse diameter | 0.998 * |
| Sagittal diameter | 0.995 * |
| Cross-sectional area | 0.996 * |
| Volume | 0.997 * |
| Parameter of the Right Ossification Center of the Neural Process of C7 | |
| Length | 0.996 * |
| Width | 0.997 * |
| Cross-sectional area | 0.994 * |
| Volume | 0.998 * |
| Parameter of the Left Ossification Center of the Neural Process of C7 | |
| Length | 0.997 * |
| Width | 0.996 * |
| Cross-sectional area | 0.997 * |
| Volume | 0.997 * |
| GA (Weeks) | N | Ossification Center of the Vertebral Body C7 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Transverse Diameter (mm) | Sagittal Diameter (mm) | Cross-Sectional Area (mm2) | Volume (mm3) | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 17 | 1 | 2.95 | 2.38 | 4.99 | 5.60 | ||||
| 18 | 3 | 3.06 | 0.05 | 2.50 | 0.05 | 5.22 | 0.07 | 6.10 | 0.27 |
| 19 | 8 | 3.24 | 0.07 | 2.66 | 0.04 | 5.81 | 0.49 | 7.76 | 0.83 |
| 20 | 4 | 3.40 | 0.03 | 2.82 | 0.04 | 7.15 | 0.35 | 9.31 | 0.36 |
| 21 | 4 | 3.54 | 0.05 | 2.97 | 0.03 | 7.95 | 0.13 | 10.53 | 0.29 |
| 22 | 5 | 3.67 | 0.04 | 3.16 | 0.04 | 8.65 | 0.44 | 11.30 | 0.41 |
| 23 | 5 | 3.88 | 0.06 | 3.28 | 0.06 | 10.05 | 0.34 | 12.70 | 0.33 |
| 24 | 9 | 4.13 | 0.07 | 3.49 | 0.07 | 10.82 | 0.21 | 14.31 | 0.77 |
| 25 | 1 | 4.25 | 3.60 | 11.32 | 15.67 | ||||
| 26 | 2 | 4.39 | 0.06 | 3.72 | 0.04 | 11.50 | 0.04 | 16.24 | 0.30 |
| 27 | 5 | 4.52 | 0.04 | 3.53 | 0.15 | 12.14 | 0.29 | 17.38 | 0.61 |
| 28 | 2 | 4.63 | 0.01 | 3.63 | 0.02 | 12.68 | 0.03 | 18.44 | 0.43 |
| 29 | 2 | 4.68 | 0.01 | 3.70 | 0.01 | 13.05 | 0.46 | 19.27 | 0.16 |
| 30 | 4 | 4.83 | 0.05 | 3.80 | 0.02 | 14.58 | 0.71 | 20.20 | 0.57 |
| GA (Weeks) | N | Right Ossification Center of the Neural Process of C7 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Length (mm) | Width (mm) | Cross-Sectional Area (mm2) | Volume (mm3) | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 17 | 1 | 2.32 | 1.27 | 3.17 | 3.99 | ||||
| 18 | 3 | 2.56 | 0.13 | 1.33 | 0.03 | 3.37 | 0.11 | 4.40 | 0.17 |
| 19 | 8 | 2.87 | 0.09 | 1.47 | 0.05 | 4.08 | 0.36 | 5.37 | 0.42 |
| 20 | 4 | 3.16 | 0.15 | 1.60 | 0.04 | 5.13 | 0.20 | 6.27 | 0.21 |
| 21 | 4 | 3.67 | 0.11 | 1.70 | 0.04 | 5.79 | 0.19 | 7.02 | 0.20 |
| 22 | 5 | 3.89 | 0.06 | 1.82 | 0.04 | 6.41 | 0.27 | 7.78 | 0.26 |
| 23 | 5 | 4.03 | 0.05 | 1.96 | 0.04 | 7.19 | 0.26 | 8.68 | 0.25 |
| 24 | 9 | 4.23 | 0.09 | 2.15 | 0.07 | 8.30 | 0.46 | 9.81 | 0.48 |
| 25 | 1 | 4.36 | 2.27 | 9.03 | 10.60 | ||||
| 26 | 2 | 4.65 | 0.09 | 2.32 | 0.02 | 9.33 | 0.09 | 10.95 | 0.12 |
| 27 | 5 | 4.87 | 0.08 | 2.40 | 0.03 | 9.78 | 0.24 | 11.55 | 0.32 |
| 28 | 2 | 5.06 | 0.03 | 2.47 | 0.01 | 10.22 | 0.09 | 12.19 | 0.16 |
| 29 | 2 | 5.14 | 0.03 | 2.50 | 0.01 | 10.42 | 0.09 | 12.65 | 0.10 |
| 30 | 4 | 5.25 | 0.05 | 2.57 | 0.03 | 10.69 | 0.13 | 13.04 | 0.15 |
| GA (Weeks) | N | Left Ossification Center of the Neural Process of C7 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Length (mm) | Width (mm) | Cross-Sectional Area (mm2) | Volume (mm3) | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 17 | 1 | 2.22 | 1.28 | 3.15 | 3.73 | ||||
| 18 | 3 | 2.51 | 0.13 | 1.34 | 0.03 | 3.41 | 0.14 | 4.33 | 0.33 |
| 19 | 8 | 2.86 | 0.11 | 1.48 | 0.05 | 4.13 | 0.36 | 5.41 | 0.40 |
| 20 | 4 | 3.15 | 0.17 | 1.61 | 0.03 | 5.16 | 0.21 | 6.25 | 0.22 |
| 21 | 4 | 3.65 | 0.11 | 1.71 | 0.03 | 5.84 | 0.18 | 7.05 | 0.27 |
| 22 | 5 | 3.89 | 0.06 | 1.83 | 0.04 | 6.47 | 0.28 | 7.84 | 0.25 |
| 23 | 5 | 4.03 | 0.05 | 1.97 | 0.04 | 7.23 | 0.24 | 8.70 | 0.28 |
| 24 | 9 | 4.22 | 0.09 | 2.17 | 0.07 | 8.35 | 0.45 | 9.88 | 0.46 |
| 25 | 1 | 4.35 | 2.29 | 9.07 | 10.64 | ||||
| 26 | 2 | 4.63 | 0.10 | 2.34 | 0.02 | 9.29 | 0.08 | 10.90 | 0.16 |
| 27 | 5 | 4.86 | 0.08 | 2.42 | 0.04 | 9.82 | 0.21 | 11.57 | 0.31 |
| 28 | 2 | 5.05 | 0.04 | 2.49 | 0.01 | 10.17 | 0.07 | 12.22 | 0.18 |
| 29 | 2 | 5.14 | 0.01 | 2.51 | 0.01 | 10.38 | 0.09 | 12.70 | 0.11 |
| 30 | 4 | 5.23 | 0.05 | 2.57 | 0.04 | 10.70 | 0.12 | 12.70 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzonkowska, M.; Kułakowski, M.; Elster, K.; Hankiewicz, B.; Janiak, M.; Rogalska, A.; Świtońska, M.; Żytkowski, A.; Baumgart, M. CT-Based Quantitative Analysis of Ossification Centres in the C7 Vertebra of Human Fetuses. Brain Sci. 2025, 15, 1018. https://doi.org/10.3390/brainsci15091018
Grzonkowska M, Kułakowski M, Elster K, Hankiewicz B, Janiak M, Rogalska A, Świtońska M, Żytkowski A, Baumgart M. CT-Based Quantitative Analysis of Ossification Centres in the C7 Vertebra of Human Fetuses. Brain Sciences. 2025; 15(9):1018. https://doi.org/10.3390/brainsci15091018
Chicago/Turabian StyleGrzonkowska, Magdalena, Michał Kułakowski, Karol Elster, Bartłomiej Hankiewicz, Michał Janiak, Agnieszka Rogalska, Milena Świtońska, Andrzej Żytkowski, and Mariusz Baumgart. 2025. "CT-Based Quantitative Analysis of Ossification Centres in the C7 Vertebra of Human Fetuses" Brain Sciences 15, no. 9: 1018. https://doi.org/10.3390/brainsci15091018
APA StyleGrzonkowska, M., Kułakowski, M., Elster, K., Hankiewicz, B., Janiak, M., Rogalska, A., Świtońska, M., Żytkowski, A., & Baumgart, M. (2025). CT-Based Quantitative Analysis of Ossification Centres in the C7 Vertebra of Human Fetuses. Brain Sciences, 15(9), 1018. https://doi.org/10.3390/brainsci15091018








