Potential Predictors of Mortality in Adults with Severe Traumatic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion Criteria
- Age ≥ 18 years;
- Admission to ICU due to sTBI (GCS ≤ 8);
- Surgical treatment for intracranial hematoma with decompressive craniotomy;
- Availability of complete laboratory results within 12 h after admission.
2.3. Exclusion Criteria
2.4. Pre-Injury Use of Anticoagulant Therapy
2.5. Prehospital and ICU Management
2.6. Data Collection
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Study Population
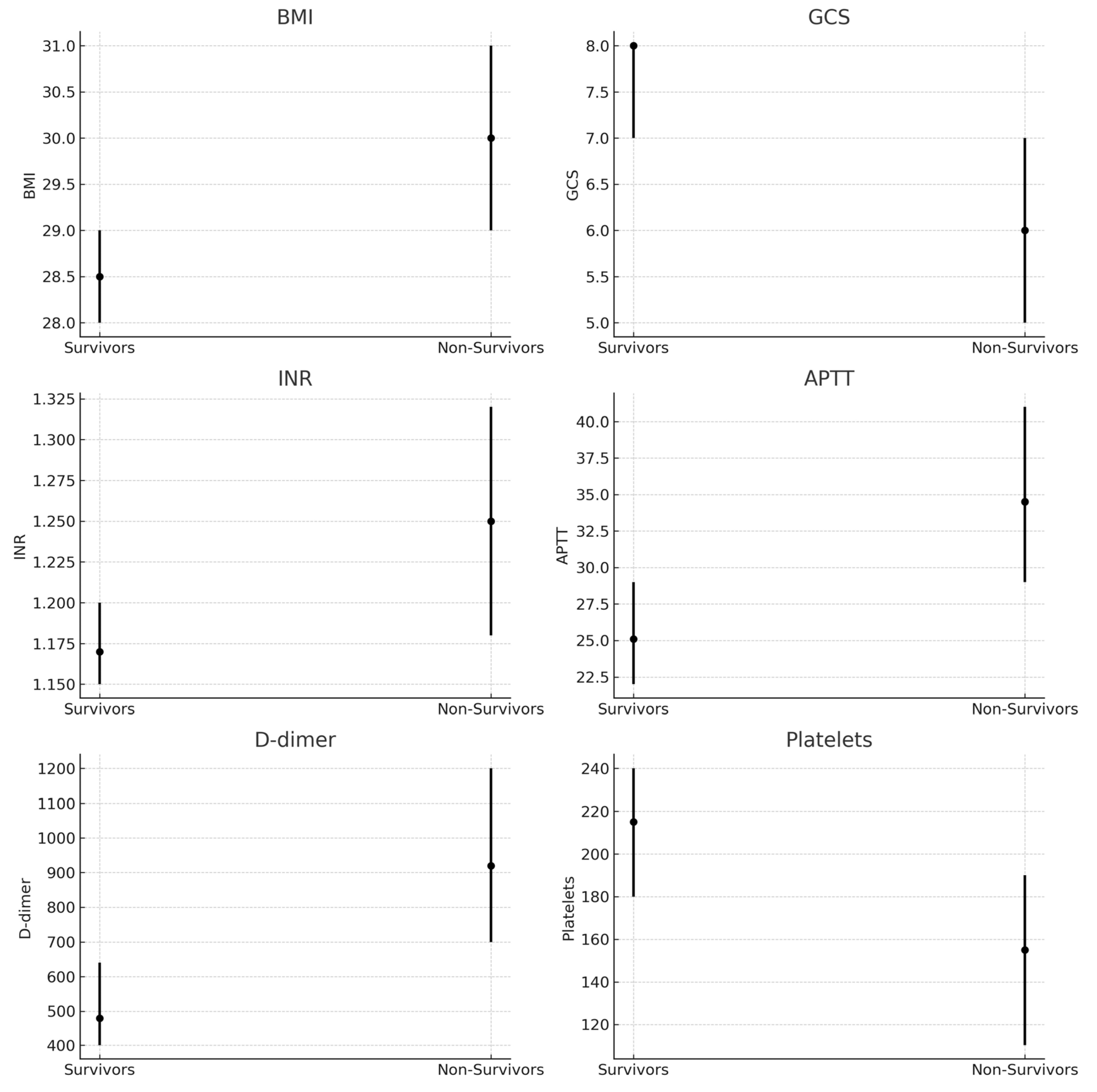
3.2. Survivors vs. Non-Survivors
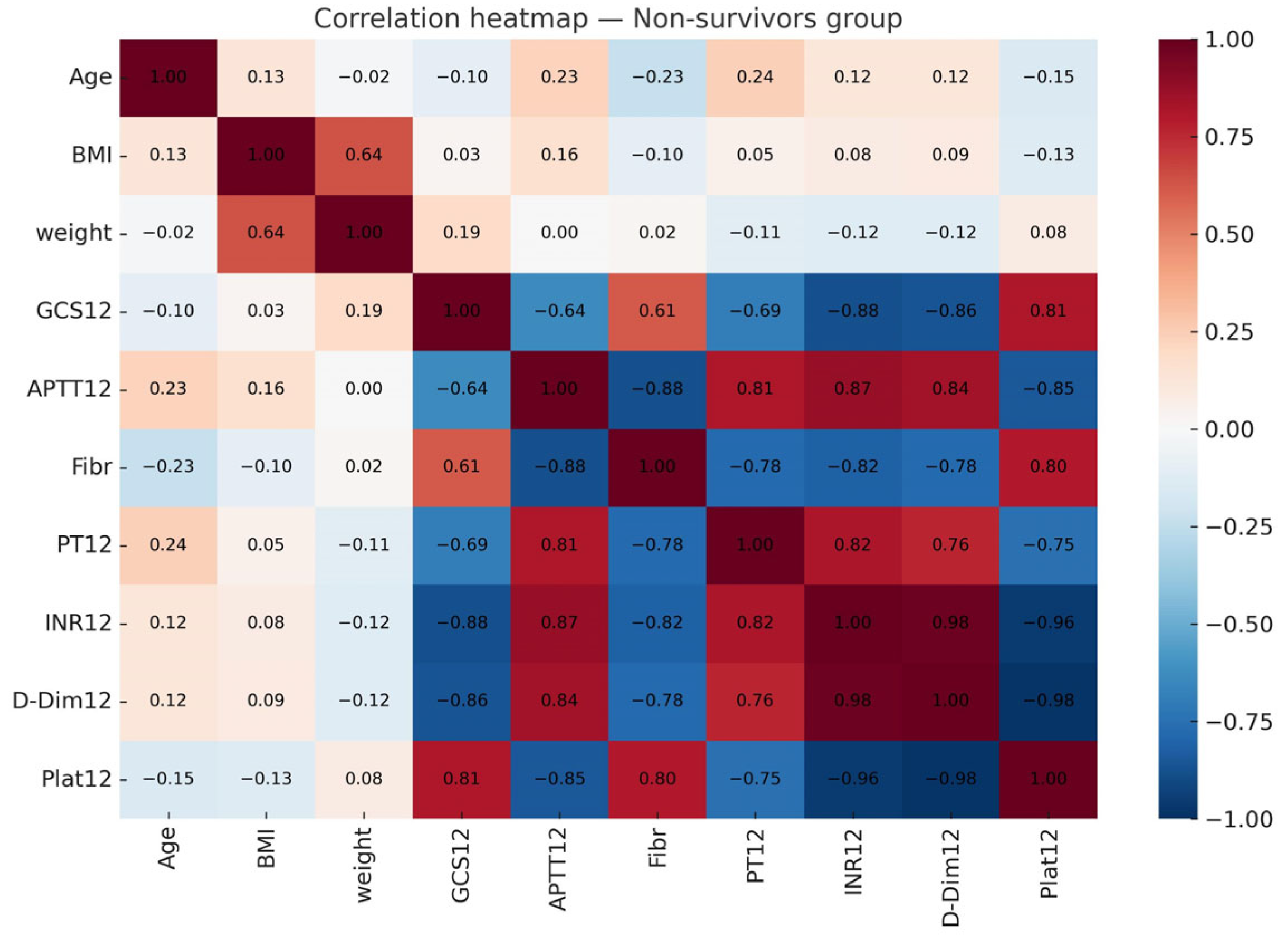
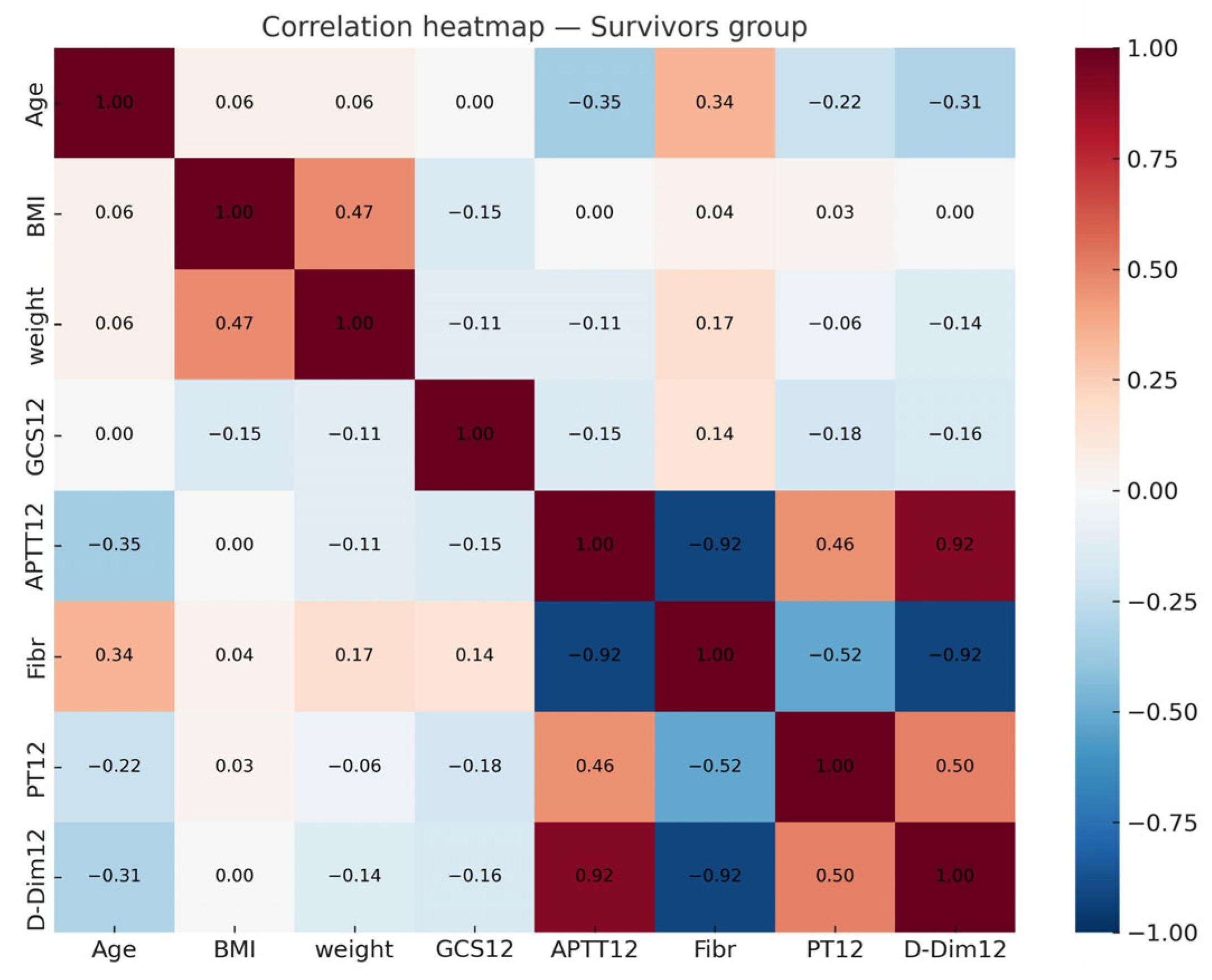
3.3. Multicollinearity and Regression
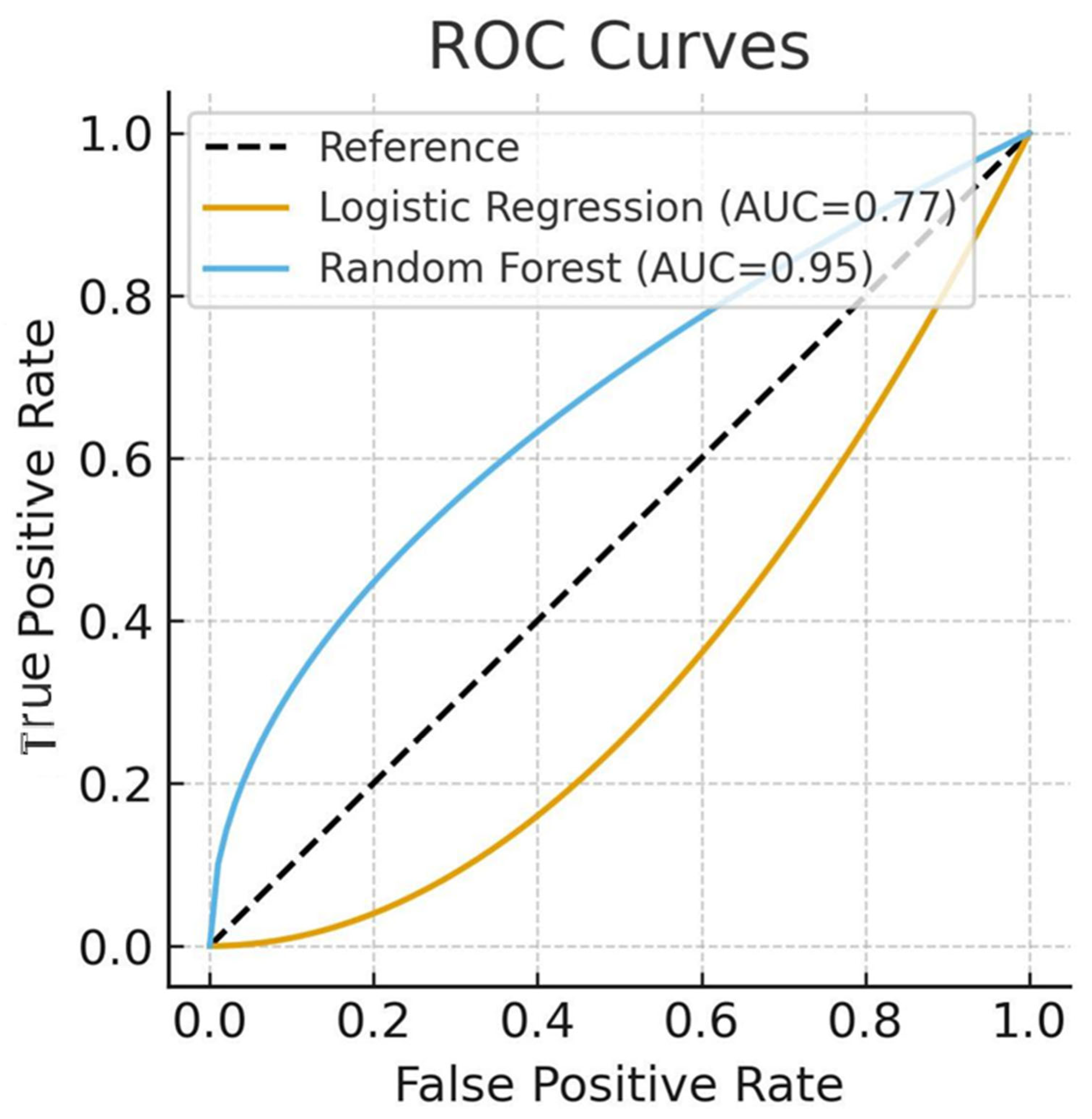
3.4. Model Performance
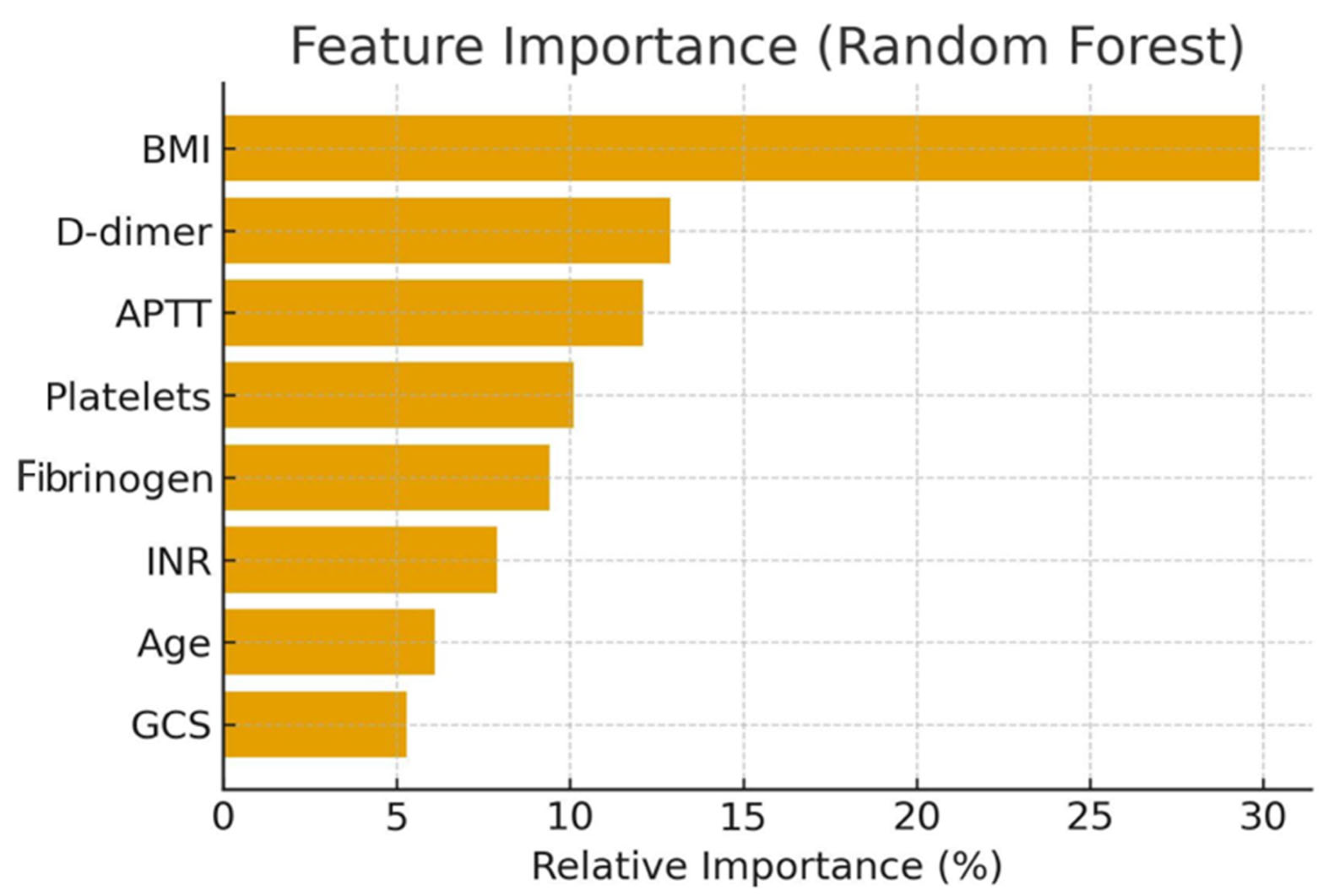
3.5. Feature Importance
- BMI (29.9%);
- D-dimer (12.9%);
- APTT (12.1%);
- Platelet count, fibrinogen, INR, age, and GCS.
4. Discussion
4.1. Key Predictors of Mortality
4.2. Methodological Considerations
4.3. Comparison with Previous Studies
4.4. Limitations
4.4.1. Clinical Implications
4.4.2. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Kulesza, B.; Litak, J.; Grochowski, C. Urazowe uszkodzenie mózgu = Traumatic brain injury. J. Educ. Health Sport 2016, 6, 215–221. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Manley, G.T. Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. Lancet Neurol. 2022, 21, 1004–1060. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- McCredie, V.A.; Chavarría, J.; Baker, A.J. How do we identify the crashing traumatic brain injury patient—The intensivist’s view. Curr. Opin. Crit. Care 2021, 27, 320–327. [Google Scholar] [CrossRef]
- Wiles, M.D.; Braganza, M.; Edwards, H. Management of traumatic brain injury in the non-neurosurgical intensive care unit: A narrative review of current evidence. Anaesthesia 2023, 78, 510–520. [Google Scholar] [CrossRef]
- Riemann, L.; Mikolic, A.; Maas, A.I.R. CENTER-TBIInvestigators Participants Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI). J. Neurotrauma 2023, 40, 1243–1254. [Google Scholar] [CrossRef]
- Nelson, L.D.; Temkin, N.R.; Barber, J. Functional Recovery, Symptoms, and Quality of Life 1 to 5 Years After Traumatic Brain Injury: Findings From the TRACK-TBI Study. JAMA Netw. Open 2023, 6, e233660. [Google Scholar] [CrossRef]
- Roman, G.; Hrdy, O.; Vrbica, K. Brain Tissue Oxygen Levels as a Perspective Therapeutic Target in Traumatic Brain Injury: Retrospective Cohort Study. J. Crit. Care Med. 2023, 9, 12–19. [Google Scholar] [CrossRef]
- Lisi, I.; Moro, F.; Mazzone, E. Exploiting blood-based biomarkers to align preclinical models with human traumatic brain injury. Brain 2024, 147, awae350. [Google Scholar] [CrossRef]
- Amare, A.T.; Tesfaye, T. Survival status and predictors of mortality among traumatic brain injury patients in an Ethiopian hospital: A retrospective cohort study. Afr. J. Emerg. Med. 2021, 11, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Cucher, D.; Harmon, L.; Myer, B. Critical traumatic brain injury is associated with worse coagulopathy. J. Trauma Acute Care Surg. 2021, 91, 331–335. [Google Scholar] [CrossRef]
- You, C.Y.; Lu, S.W.; Fu, Y.Q. Relationship between admission coagulopathy and prognosis in children with traumatic brain injury: A retrospective study. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 67. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Qu, Z.; Liu, R. Traumatic brain injury: Advances in coagulopathy. Biomed. Rep. 2024, 21, 156. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Sandersjöö, A.; Thelin, E.P.; Maegele, M. Time Course of Hemostatic Disruptions After Traumatic Brain Injury: A Systematic Review. Neurocrit. Care 2021, 34, 635–656. [Google Scholar] [CrossRef]
- Picetti, E.; Catena, F.; Abu-Zidan, F. Early management of isolated severe traumatic brain injury patients in a hospital without neurosurgical capabilities: WSES consensus recommendations. World J. Emerg. Surg. 2023, 18, 5. [Google Scholar] [CrossRef]
- Filiberto, D.M.; Byerly, S.; Lenart, E. Body Mass Index and Pharmacologic Venous Thromboembolism Prophylaxis in Traumatic Brain Injury. J. Surg. Res. 2023, 291, 245–249. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for Managing Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef]
- Yang, R.; Zubair, M.; Moosavi, L. Prothrombin Time. In StatPearls. Treasure Island (FL); StatPearls Publishing: Petersburg, PL, USA, 2025. [Google Scholar] [PubMed]
- Bauça, J.M.; Ajzner, E.; Cadamuro, J. An international study on activated partial thromboplastin time prolongation. Clin. Chim. Acta 2022, 535, 167–173. [Google Scholar] [CrossRef]
- Dorgalaleh, A.; Favaloro, E.; Bahraini, M. Standardization of Prothrombin Time/International Normalized Ratio (PT/INR). Int. J. Lab. Hematol. 2021, 43, 21–28. [Google Scholar] [CrossRef]
- Wauthier, L.; Favresse, J.; Hardy, M. D-dimer testing: A narrative review. Adv. Clin. Chem. 2023, 114, 151–223. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Galwankar, S.; Konar, S. Obesity as a predictor of outcome following traumatic brain injury: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2022, 217, 107260. [Google Scholar] [CrossRef] [PubMed]
- Czorlich, P.; Dreimann, M.; Emami, P. Body Mass Index >35 as Independent Predictor of Mortality in Severe Traumatic Brain Injury. World Neurosurg. 2017, 107, 515–521. [Google Scholar] [CrossRef]
- Aminmansour, B.; Sameri, S.; Shafiei, M. The impact of body mass index changes on traumatic brain injury patients’ outcomes during hospitalization. Chin. J. Traumatol. 2024, 27, 323–327. [Google Scholar] [CrossRef]
- Yuan, Q.; Yu, J.; Wu, X. Prognostic value of coagulation tests for in-hospital mortality in patients with traumatic brain injury. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 3. [Google Scholar] [CrossRef]
- Anderson, E.; Morera, A.; Kour, S. Traumatic injury compromises nucleocytoplasmic transport and leads to TDP−43 pathology. eLife 2021, 10, e67587. [Google Scholar] [CrossRef]
- Albert, V.; Arulselvi, S.; Agrawal, D. Early posttraumatic changes in coagulation and fibrinolysis in isolated severe traumatic brain injury and its influence on immediate outcome. Hematol. Oncol. Stem Cell Ther. 2019, 12, 32–43. [Google Scholar] [CrossRef]
- Böhm, J.; Schaeben, V.; Schäfer, N. Extended Coagulation Profiling in Isolated Traumatic Brain Injury: A CENTER-TBI Analysis. Neurocritical Care 2022, 36, 927–941. [Google Scholar] [CrossRef]
- Mathur, R.; Suarez, J.I. Coagulopathy in Isolated Traumatic Brain Injury: Myth or Reality? Extracranial complications after TBI. Neurocritical Care 2023, 38, 429–438. [Google Scholar] [CrossRef]
- Nakae, R.; Murai, Y.; Morita, A. Coagulopathy and Traumatic Brain Injury: Overview of New Diagnostic and Therapeutic Strategies. Neurol. Med. Chir. 2022, 62, 261–269. [Google Scholar] [CrossRef]
- Langness, S.; Ward, E.; Halbach, J. Plasma D-dimer safely reduces unnecessary CT scans in pediatric head trauma. J. Pediatr. Surg. 2018, 53, 752–757. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Dong, J.F. Coagulopathy induced by traumatic brain injury: Systemic manifestation of a localized injury. Blood 2018, 131, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; Liu, Y. Plasma D-dimer levels as a biomarker for in-hospital complications and long-term mortality in patients with traumatic brain injury. Front. Mol. Neurosci. 2023, 16, 1276726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Liu, X.; Lu, X. Biomaterials and tissue engineering in traumatic brain injury: Novel perspectives on promoting neural regeneration. Neural Regen. Res. 2023, 19, 2157–2174. [Google Scholar] [CrossRef] [PubMed]
- Folkerson, L.; Sloan, D.; Davis, E. Coagulopathy as a predictor of mortality after penetrating traumatic brain injury. Am. J. Emerg. Med. 2018, 36, 38–42. [Google Scholar] [CrossRef]
- Gupta, V.; Liras, I.; Allukian, M. Injury Severity, Arrival Physiology, Coagulopathy, and Outcomes Among the Youngest Trauma Patients. J. Surg. Res. 2021, 264, 236–241. [Google Scholar] [CrossRef]
- Ma, C.; Wu, X.; Shen, X.; Yang, Y. Sex differences in traumatic brain injury: A multi-dimensional exploration in genes, hormones, cells, individuals, and society. Chin. Neurosurg. J. 2019, 5, 24. [Google Scholar] [CrossRef]
- World Medical Association. WMA Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Participants. 2025. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki/ (accessed on 16 September 2025).
| Variable | Survivors (n = 200) | Non-Survivors (n = 101) | p-Value |
|---|---|---|---|
| Age, years | 44 (37–52) | 48 (40–56) | 0.031 |
| BMI, kg/m2 | 28.5 (28–29) | 30.0 (29–31) | <0.001 |
| GCS at admission | 8 (7–8) | 6 (5–7) | <0.001 |
| INR | 1.17 (1.15–1.20) | 1.25 (1.18–1.32) | <0.001 |
| APTT, sec | 25.1 (22–29) | 34.5 (29–41) | <0.001 |
| D-dimer, ng/ml | 480 (400–640) | 920 (700–1200) | <0.001 |
| Platelets, ×109/L | 215 (180–240) | 155 (110–190) | <0.001 |
| Metric | Logistic Regression | Random Forest |
|---|---|---|
| AUC | 0.77 | 0.95 |
| Accuracy | 70.1% | 90.2% |
| Precision (death class) | 0.56 | 0.88 |
| Recall (death class) | 0.40 | 0.84 |
| F1-score (death class) | 0.46 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marta, R.; Svitlana, Y.; Konstiantyn, K.; Maryna, M.; Andriy, D.; Oleksandr, O. Potential Predictors of Mortality in Adults with Severe Traumatic Brain Injury. Brain Sci. 2025, 15, 1014. https://doi.org/10.3390/brainsci15091014
Marta R, Svitlana Y, Konstiantyn K, Maryna M, Andriy D, Oleksandr O. Potential Predictors of Mortality in Adults with Severe Traumatic Brain Injury. Brain Sciences. 2025; 15(9):1014. https://doi.org/10.3390/brainsci15091014
Chicago/Turabian StyleMarta, Rachel, Yaroslavska Svitlana, Kreniov Konstiantyn, Mamonowa Maryna, Dobrorodniy Andriy, and Oliynyk Oleksandr. 2025. "Potential Predictors of Mortality in Adults with Severe Traumatic Brain Injury" Brain Sciences 15, no. 9: 1014. https://doi.org/10.3390/brainsci15091014
APA StyleMarta, R., Svitlana, Y., Konstiantyn, K., Maryna, M., Andriy, D., & Oleksandr, O. (2025). Potential Predictors of Mortality in Adults with Severe Traumatic Brain Injury. Brain Sciences, 15(9), 1014. https://doi.org/10.3390/brainsci15091014







