Primary Cilia and Cardiovascular Risk Factors in Alzheimer’s Disease
Abstract
1. Introduction
2. Primary Cilia and Ciliary Receptors

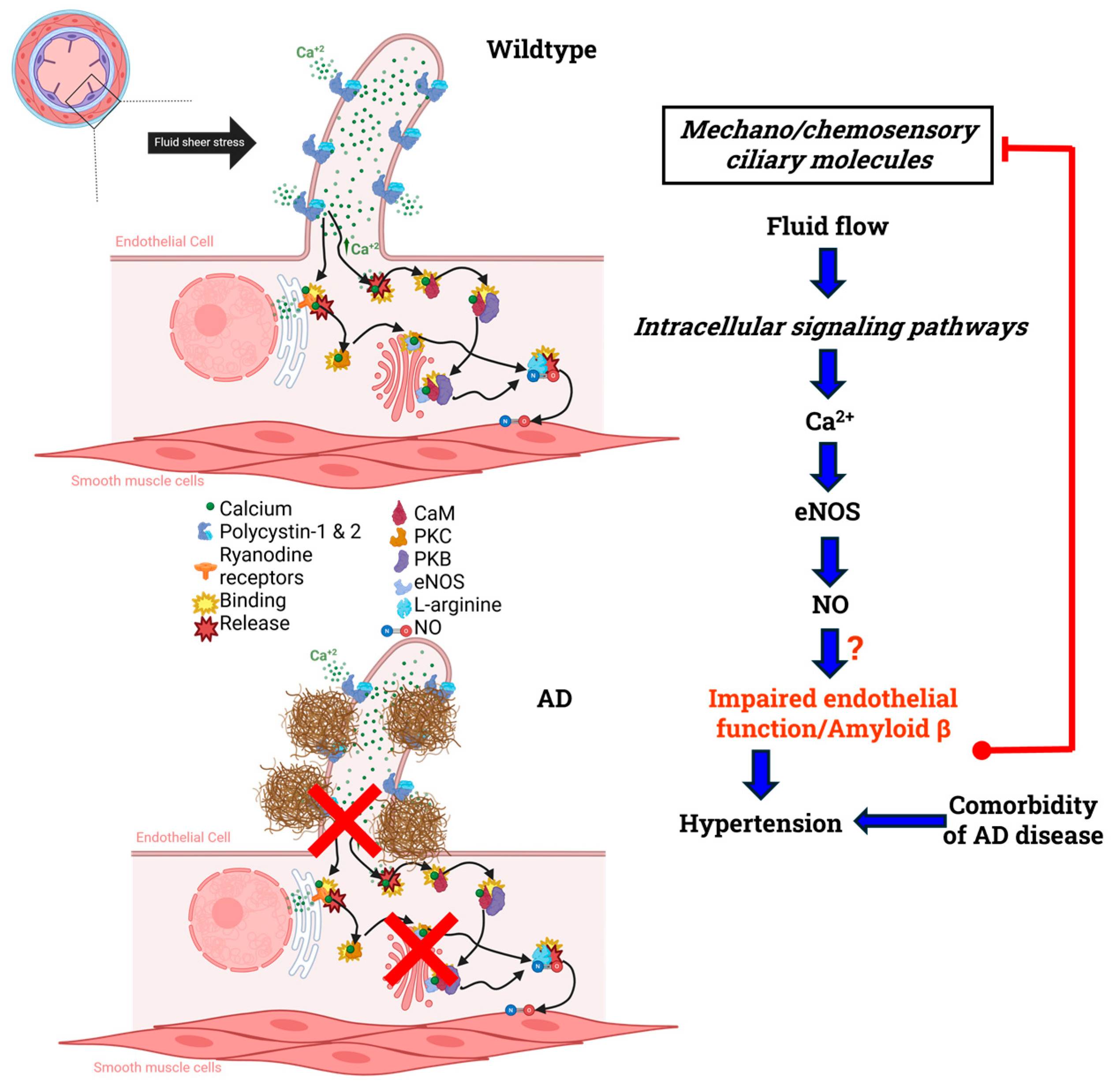
3. Nitric Oxide and Alzheimer’s Disease
4. Cardiovascular Risk Factors
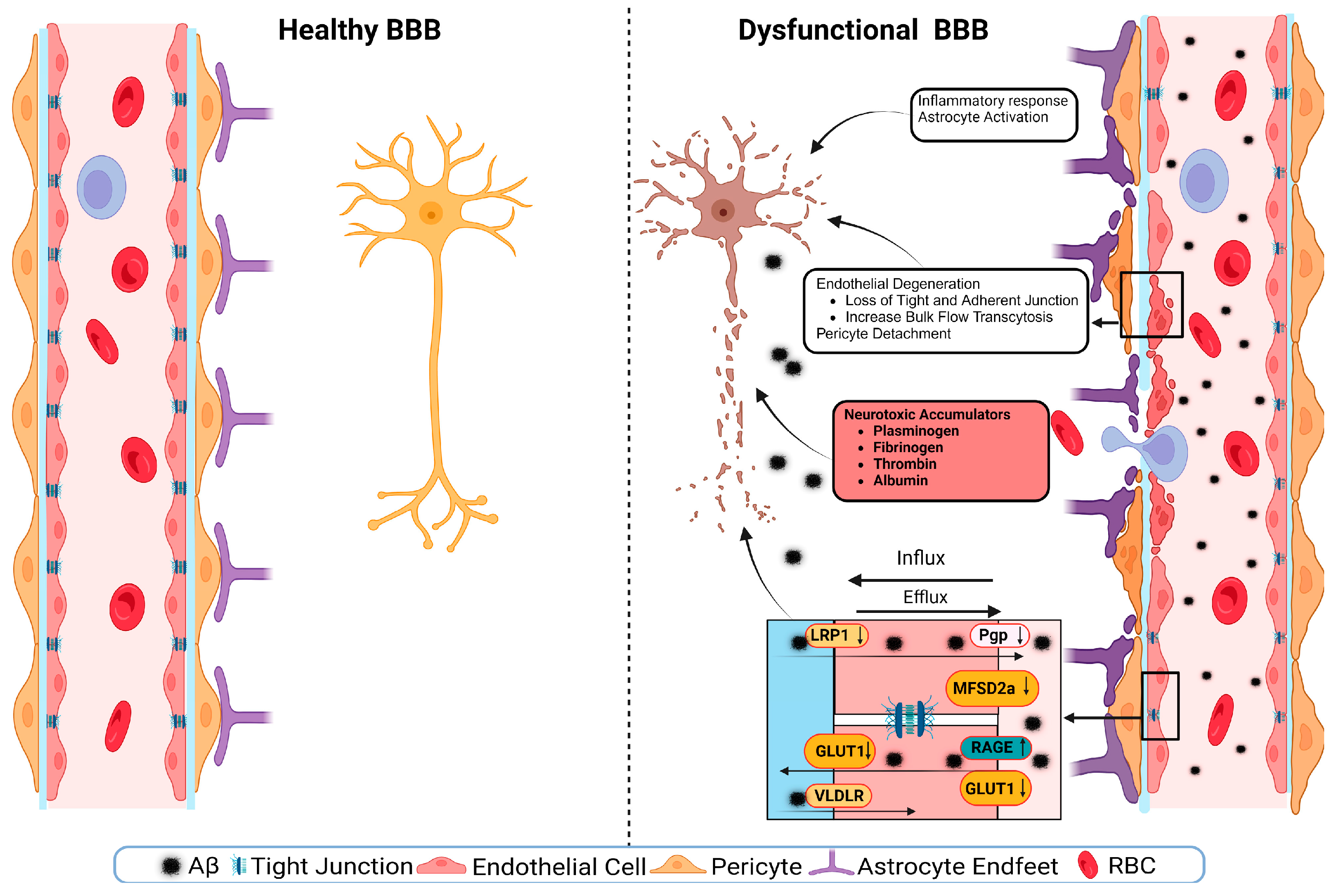
5. Small-Scale Vascular Manifestations
6. Large-Scale Vascular Manifestations
7. Cilia as Potential Therapeutic Target
8. Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| NO | Nitric oxide |
| Aβ | Amyloid beta |
| APP | Amyloid precursor protein |
| MPRAGE | Magnetization Prepared Rapid Gradient Echo |
| NLRP3 | nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 |
| BACE-1 | Beta-Site Amyloid Precursor Protein Cleaving Enzyme 1 |
| Ca2+ | Calcium |
| Shh | Sonic Hedgehog |
| CaM | Calmodulin |
| PKC | Protein kinase C |
| PKB | Protein kinase B |
| eNOS | Endothelial nitric oxide synthase |
| Hh | Hedgehog pathway |
| GPCRs | G-protein-coupled receptors |
| SSTR3 | Somatostatin Receptor 3 |
| 5-HT6 | 5-hydroxytryptamine |
| MR | Muscarinic acetylcholine receptor |
| AChEI | Acetylcholinesterase inhibitors |
| ACh | Acetylcholine |
| M1R | Muscarinic receptor 1 |
| M3R | Muscarinic receptor 3 |
| p-eNOS | Phosphorylated endothelial nitric oxide synthase |
| CNS | Central nervous system |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| NOS | Nitric oxide synthase |
| cGMP | Cyclic guanosine monophosphate |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| nNOS | Neuronal nitric oxide synthase |
| iNOS | Inducible nitric oxide synthase |
| NMDA | N-methyl-D-aspartate |
| CBF | Cerebral blood flow |
| IGBP-2 | Insulin-like growth factor binding protein-2 |
| IGF-1R | Insulin-like growth 1 factor |
| BBB | Blood–brain barrier |
| CSF | Cerebrospinal fluid |
| PET | Positron emission tomography |
| CAA | Cerebral amyloid angiopathy |
| LRP2 | Low-density lipoprotein receptor-related protein 2 |
| GLUT1 | Glucose Transporter Type 1 |
| VLDLR | very-low-density-lipoprotein receptor |
| Pgp | Permeability Glycoprotein |
| MFSD2a | Major facilitator superfamily domain-containing protein 2 |
| RAGE | Receptor for advanced glycation end products |
| RBC | Red blood cell |
| ABC | ATP-binding cassette |
| AQP-4 | Aquaporin-4 |
| LRP1 | Low-density lipoprotein receptor-related protein 1 |
| sLRP1 | Soluble low-density lipoprotein receptor-related protein 1 |
| TRPM2 | Transient Receptor Potential Cation Channel, Subfamily M, Member 2 |
| NVU | Neurovascular unit |
| APOE4 | Apolipoprotein E4 |
| MRI | Magnetic resonance imaging |
| GBCA | Gadolinium-based contrast agents |
| NC | Normal cognition |
| MCI | Mild cognitive impairment |
| ANGPT-2 | Angiopoietin-2 |
References
- Oishi, E.; Ohara, T.; Sakata, S.; Fukuhara, M.; Hata, J.; Yoshida, D.; Shibata, M.; Ohtsubo, T.; Kitazono, T.; Kiyohara, Y.; et al. Day-to-Day Blood Pressure Variability and Risk of Dementia in a General Japanese Elderly Population. Circulation 2017, 136, 516–525. [Google Scholar] [CrossRef]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.S.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef]
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-β oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Morris, J.K.; Honea, R.A.; Vidoni, E.D.; Swerdlow, R.H.; Burns, J.M. Is Alzheimer’s disease a systemic disease? Biochim. Biophys. Acta 2014, 1842, 1340–1349. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, J.; Huang, S.; Wang, X.-Y.; Chen, X.; Liu, G.-H.; Ye, K.; Song, W.; Masters, C.L.; Wang, J.; et al. Antiageing strategy for neurodegenerative diseases: From mechanisms to clinical advances. Signal Transduct. Target. Ther. 2025, 10, 76. [Google Scholar] [CrossRef]
- Kelser, B.M.; Teichner, E.M.; Subtirelu, R.C.; Hoss, K.N. A review of proposed mechanisms for neurodegenerative disease. Front. Aging Neurosci. 2024, 16, 1370580. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef]
- Kamila, P.; Kar, K.; Chowdhury, S.; Chakraborty, P.; Dutta, R.S.S.; Singh, S.A.; Prajapati, B.G. Effect of neuroinflammation on the progression of Alzheimer’s disease and its significant ramifications for novel anti-inflammatory treatments. IBRO Neurosci. Rep. 2025, 18, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-M.; Zhang, Y.-R.; Huang, Y.-Y.; Dong, Q.; Tan, L.; Yu, J.-T. The role of the immune system in Alzheimer’s disease. Ageing Res. Rev. 2021, 70, 101409. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Savonenko, A.V.; King, J.F.; Fangmark Tucker, S.M.; Rudow, G.L.; Xu, G.; Borchelt, D.R.; Troncoso, J.C. Amyloid precursor protein increases cortical neuron size in transgenic mice. Neurobiol. Aging 2009, 30, 1238–1244. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Cai, F.; Zhang, M.; Wu, Y.; Zhang, J.; Song, W. BACE1 Cleavage Site Selection Critical for Amyloidogenesis and Alzheimer’s Pathogenesis. J. Neurosci. 2017, 37, 6915–6925. [Google Scholar] [CrossRef]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef]
- Puthiyedth, N.; Riveros, C.; Berretta, R.; Moscato, P. Identification of Differentially Expressed Genes through Integrated Study of Alzheimer’s Disease Affected Brain Regions. PLoS ONE 2016, 11, e0152342. [Google Scholar] [CrossRef]
- Convit, A.; de Asis, J.; de Leon, M.J.; Tarshish, C.Y.; De Santi, S.; Rusinek, H. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer’s disease. Neurobiol. Aging 2000, 21, 19–26. [Google Scholar] [CrossRef]
- Ownby, R.L.; Crocco, E.; Acevedo, A.; John, V.; Loewenstein, D. Depression and risk for Alzheimer disease: Systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry 2006, 63, 530–538. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, J.T.; Wang, H.F.; Meng, X.F.; Tan, C.C.; Wang, J.; Wang, C.; Tan, L. Association between stroke and Alzheimer’s disease: Systematic review and meta-analysis. J. Alzheimers Dis. 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Fu, P.; Gao, M.; Yung, K.K.L. Association of Intestinal Disorders with Parkinson’s Disease and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. ACS Chem. Neurosci. 2020, 11, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Noble, J.; Tang, M.X.; Schupf, N.; Mayeux, R.; Luchsinger, J.A. Type 2 diabetes and late-onset Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2011, 31, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Parashar, A.; Jha, D.; Mehta, V.; Chauhan, B.; Ghosh, P.; Deb, P.K.; Jaiswal, M.; Prajapati, S.K. Sonic hedgehog signalling pathway contributes in age-related disorders and Alzheimer’s disease. Ageing Res. Rev. 2024, 96, 102271. [Google Scholar] [CrossRef] [PubMed]
- Abou Alaiwi, W.A.; Lo, S.T.; Nauli, S.M. Primary Cilia: Highly Sophisticated Biological Sensors. Sensors 2009, 9, 7003–7020. [Google Scholar] [CrossRef]
- Venkatesh, D. Primary cilia. J. Oral Maxillofac. Pathol. 2017, 21, 8–10. [Google Scholar] [CrossRef]
- Lee, J.E.; Gleeson, J.G. Cilia in the nervous system: Linking cilia function and neurodevelopmental disorders. Curr. Opin. Neurol. 2011, 24, 98–105. [Google Scholar] [CrossRef]
- Narita, K.; Takeda, S. Cilia in the choroid plexus: Their roles in hydrocephalus and beyond. Front. Cell. Neurosci. 2015, 9, 39. [Google Scholar] [CrossRef]
- Takeuchi, H. Olfactory cilia, regulation and control of olfaction. Physiol. Rep. 2024, 12, e70057. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Z.; Peng, H.; Ward, S.M.; Hennig, G.W.; Zheng, H.; Yan, W. Oviductal motile cilia are essential for oocyte pickup but dispensable for sperm and embryo transport. Proc. Natl. Acad. Sci. USA 2021, 118, e2102940118. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, S.-N.; Baek, J.; Yoon, J.-H.; Lee, H. Mechanical compression enhances ciliary beating through cytoskeleton remodeling in human nasal epithelial cells. Acta Biomater. 2021, 128, 346–356. [Google Scholar] [CrossRef]
- Tong, C.K.; Han, Y.-G.; Shah, J.K.; Obernier, K.; Guinto, C.D.; Alvarez-Buylla, A. Primary cilia are required in a unique subpopulation of neural progenitors. Proc. Natl. Acad. Sci. USA 2014, 111, 12438–12443. [Google Scholar] [CrossRef] [PubMed]
- Sterpka, A.; Chen, X. Neuronal and astrocytic primary cilia in the mature brain. Pharmacol. Res. 2018, 137, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, Q.; Acharya, S.; Zheng, X.; Huynh, V.; Whitmore, B.; Yimit, A.; Malhotra, M.; Chatterji, S.; Rosin, N.; et al. Primary cilia signaling in astrocytes mediates development and regional-specific functional specification. Nat. Neurosci. 2024, 27, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Chinipardaz, Z.; Liu, M.; Graves, D.T.; Yang, S. Role of Primary Cilia in Bone and Cartilage. J. Dent. Res. 2022, 101, 253–260. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Sun, Y. Primary cilia in hard tissue development and diseases. Front. Med. 2021, 15, 657–678. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, M.; Cao, X.; Yang, S. Ciliary IFT80 regulates dental pulp stem cells differentiation by FGF/FGFR1 and Hh/BMP2 signaling. Int. J. Biol. Sci. 2019, 15, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Luu, V.Z.; Chowdhury, B.; Al-Omran, M.; Hess, D.A.; Verma, S. Role of endothelial primary cilia as fluid mechanosensors on vascular health. Atherosclerosis 2018, 275, 196–204. [Google Scholar] [CrossRef]
- Schwartz, E.A.; Leonard, M.L.; Bizios, R.; Bowser, S.S. Analysis and modeling of the primary cilium bending response to fluid shear. Am. J. Physiol. Ren. Physiol. 1997, 272, F132–F138. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Q.; Peng, Y.; Geng, F.; Shao, X.; Zhou, H.; Cao, Y.; Zhang, R. Primary cilia mediate Klf2-dependant Notch activation in regenerating heart. Protein Cell 2020, 11, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.J.; Du, H.; Wu, J.; Jansen, D.A.; Jordan, K.L.; Xu, N.; Sieck, G.C.; Qian, Q. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press. Res. 2008, 31, 171–184. [Google Scholar] [CrossRef]
- McGrath, J.; Roy, P.; Perrin, B.J. Stereocilia morphogenesis and maintenance through regulation of actin stability. Semin. Cell Amp. Dev. Biol. 2017, 65, 88–95. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J. The Kinocilia of Cochlear Hair Cells: Structures, Functions, and Diseases. Front. Cell Dev. Biol. 2021, 9, 715037. [Google Scholar] [CrossRef]
- Masyuk, A.I.; Gradilone, S.A.; Banales, J.M.; Huang, B.Q.; Masyuk, T.V.; Lee, S.O.; Splinter, P.L.; Stroope, A.J.; Larusso, N.F. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G725–G734. [Google Scholar] [CrossRef]
- Rhlich, P.L. The sensory cilium of retinal rods is analogous to the transitional zone of motile cilia. Cell Tissue Res. 1975, 161, 421–430. [Google Scholar] [CrossRef]
- Liu, X.; Pacwa, A.; Bresciani, G.; Swierczynska, M.; Dorecka, M.; Smedowski, A. Retinal primary cilia and their dysfunction in retinal neurodegenerative diseases: Beyond ciliopathies. Mol. Med. 2024, 30, 109. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, H.M.; Valente, E.M. Motile and non-motile cilia in human pathology: From function to phenotypes. J. Pathol. 2017, 241, 294–309. [Google Scholar] [CrossRef]
- Saternos, H.; Ley, S.; Aboualaiwi, W. Primary Cilia and Calcium Signaling Interactions. Int. J. Mol. Sci. 2020, 21, 7109. [Google Scholar] [CrossRef]
- Bangs, F.; Anderson, K.V. Primary Cilia and Mammalian Hedgehog Signaling. Cold Spring Harb. Perspect. Biol. 2017, 9, a028175. [Google Scholar] [CrossRef]
- Choudhry, Z.; Rikani, A.A.; Choudhry, A.M.; Tariq, S.; Zakaria, F.; Asghar, M.W.; Sarfraz, M.K.; Haider, K.; Shafiq, A.A.; Mobassarah, N.J. Sonic hedgehog signalling pathway: A complex network. Ann. Neurosci. 2014, 21, 28–31. [Google Scholar] [CrossRef]
- Guo, A.; Wang, H.; Zhang, Y.; Huang, H. Changes of the Primary Cilia in Alzheimer’s Disease Pathogenesis. Eur. J. Neurosci. 2025, 61, e70125. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kohbuchi, S.; Koganezawa, N.; Sekino, Y.; Shirao, T.; Saido, T.C.; Saito, T.; Saito, Y. Impairment of ciliary dynamics in an APP knock-in mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2022, 610, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Vorobyeva, A.G.; Saunders, A.J. Amyloid-β interrupts canonical Sonic hedgehog signaling by distorting primary cilia structure. Cilia 2018, 7, 5. [Google Scholar] [CrossRef]
- Saternos, H.C.; AbouAlaiwi, W.A. Implications of Dysfunction of Mechanosensory Cilia in Polycystic Kidney Disease. In Polycystic Kidney Disease; Li, X., Ed.; Codon Publications: Brisbane, QLD, Australia, 2015; pp. 397–421. [Google Scholar]
- Hierck, B.P.; Van Der Heiden, K.; Alkemade, F.E.; Van De Pas, S.; Van Thienen, J.V.; Groenendijk, B.C.W.; Bax, W.H.; Van Der Laarse, A.; Deruiter, M.C.; Horrevoets, A.J.G.; et al. Primary cilia sensitize endothelial cells for fluid shear stress. Dev. Dyn. 2008, 237, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Saternos, H.C.; Forero, K.V.; Meqdad, M.A.; Buqaileh, R.; Sunderman, C.L.; Gallagher, G.; Messer, W.S.; Mohieldin, A.M.; Mucci, C.A.; Kumariya, S.; et al. Muscarinic acetylcholine receptor 3 localized to primary endothelial cilia regulates blood pressure and cognition. Sci. Rep. 2025, 15, 3745. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Cho, S.J.; Descant, K.; Li, P.H.; Shapson-Coe, A.; Januszewski, M.; Berger, D.R.; Meyer, C.; Casingal, C.; Huda, A.; et al. Mapping of neuronal and glial primary cilia contactome and connectome in the human cerebral cortex. Neuron 2024, 112, 41–55.e43. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Li, X.; Sun, Z.; Yu, F.; Pashang, A.; Kulasiri, D.; Li, H.W.; Chen, H.; Hou, H.; et al. The Primary Cilia are Associated with the Axon Initial Segment in Neurons. Adv. Sci. 2025, 12, e2407405. [Google Scholar] [CrossRef]
- Sunderman, C.; Forero, K.; Buqaileh, R.; Aboualaiwi, W. Role of primary cerebrovascular cilia in the pathogenesis of blood pressure and Alzheimer’s disease manifestations. Alzheimer’s Dement. 2025, 20, e089289. [Google Scholar] [CrossRef]
- Katusic, Z.S.; D’Uscio, L.V.; He, T. Emerging Roles of Endothelial Nitric Oxide in Preservation of Cognitive Health. Stroke 2023, 54, 686–696. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Latorraca, N.R.; Venkatakrishnan, A.J.; Dror, R.O. GPCR Dynamics: Structures in Motion. Chem. Rev. 2017, 117, 139–155. [Google Scholar] [CrossRef]
- Valente, E.M.; Rosti, R.O.; Gibbs, E.; Gleeson, J.G. Primary cilia in neurodevelopmental disorders. Nat. Rev. Neurol. 2014, 10, 27–36. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Dwomoh, L.; Tejeda, G.S.; Tobin, A.B. Targeting the M1 muscarinic acetylcholine receptor in Alzheimer’s disease. Neuronal Signal. 2022, 6, NS20210004. [Google Scholar] [CrossRef] [PubMed]
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, Y.; Zhang, C.; Zhao, Y.; Bu, G.; Xu, H.; Zhang, Y.W. M1 muscarinic acetylcholine receptor in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 295–307. [Google Scholar] [CrossRef]
- Poulin, B.; Butcher, A.; Mcwilliams, P.; Bourgognon, J.-M.; Pawlak, R.; Kong, K.C.; Bottrill, A.; Mistry, S.; Wess, J.; Rosethorne, E.M.; et al. The M 3 -muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc. Natl. Acad. Sci. USA 2010, 107, 9440–9445. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Ushio-Fukai, M.; Ash, D.; Nagarkoti, S.; Belin de Chantemèle, E.J.; Fulton, D.J.R.; Fukai, T. Interplay Between Reactive Oxygen/Reactive Nitrogen Species and Metabolism in Vascular Biology and Disease. Antioxid. Redox Signal 2021, 34, 1319–1354. [Google Scholar] [CrossRef]
- Tropea, M.R.; Gulisano, W.; Vacanti, V.; Arancio, O.; Puzzo, D.; Palmeri, A. Nitric oxide/cGMP/CREB pathway and amyloid-beta crosstalk: From physiology to Alzheimer’s disease. Free Radic. Biol. Med. 2022, 193, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Garcia, X.; Stein, F. Nitric Oxide. Semin. Pediatr. Infect. Dis. 2006, 17, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Saternos, H.C.; AbouAlaiwi, W.A. Signaling interplay between primary cilia and nitric oxide: A mini review. Nitric Oxide 2018, 80, 108–112. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Azargoonjahromi, A. Dual role of nitric oxide in Alzheimer’s disease. Nitric Oxide 2023, 134-135, 23–37. [Google Scholar] [CrossRef]
- Tenopoulou, M.; Doulias, P.T. Endothelial nitric oxide synthase-derived nitric oxide in the regulation of metabolism. F1000Res 2020, 9, 1190. [Google Scholar] [CrossRef]
- Förstermann, U.; Kleinert, H. Nitric oxide synthase: Expression and expressional control of the three isoforms. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1995, 352, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Hockley, A.; Berger, J.I.; Smith, P.A.; Palmer, A.R.; Wallace, M.N. Nitric oxide regulates the firing rate of neuronal subtypes in the guinea pig ventral cochlear nucleus. Eur. J. Neurosci. 2020, 51, 963–983. [Google Scholar] [CrossRef]
- Li, H.; Poulos, T.L. Structure–function studies on nitric oxide synthases. J. Inorg. Biochem. 2005, 99, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Mango, D.; Saidi, A.; Cisale, G.Y.; Feligioni, M.; Corbo, M.; Nisticò, R. Targeting Synaptic Plasticity in Experimental Models of Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 778. [Google Scholar] [CrossRef]
- Lüth, H.-J.; Holzer, M.; Gärtner, U.; Staufenbiel, M.; Arendt, T. Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer’s disease, in APP23 transgenic mice and after experimental brain lesion in rat: Evidence for an induction by amyloid pathology. Brain Res. 2001, 913, 57–67. [Google Scholar] [CrossRef]
- Elman-Shina, K.; Efrati, S. Ischemia as a common trigger for Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1012779. [Google Scholar] [CrossRef]
- De La Monte, S.M.; Wands, J.R. Alzheimer’s Disease is Type 3 Diabetes—Evidence Reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef]
- Kroner, Z. The relationship between Alzheimer’s disease and diabetes: Type 3 diabetes? Altern. Med. Rev. 2009, 14, 373–379. [Google Scholar]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef]
- Fontbonne, A.; Berr, C.; Ducimetière, P.; Alpérovitch, A. Changes in Cognitive Abilities Over a 4-Year Period Are Unfavorably Affected in Elderly Diabetic Subjects: Results of the Epidemiology of Vascular Aging Study. Diabetes Care 2001, 24, 366–370. [Google Scholar] [CrossRef]
- Maciejewska, K.; Czarnecka, K.; Szymański, P. A review of the mechanisms underlying selected comorbidities in Alzheimer’s disease. Pharmacol. Rep. 2021, 73, 1565–1581. [Google Scholar] [CrossRef]
- Ungvari, Z.; Toth, P.; Tarantini, S.; Prodan, C.I.; Sorond, F.; Merkely, B.; Csiszar, A. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat. Rev. Nephrol. 2021, 17, 639–654. [Google Scholar] [CrossRef]
- Abdulrahman, H.; Van Dalen, J.W.; Den Brok, M.; Latimer, C.S.; Larson, E.B.; Richard, E. Hypertension and Alzheimer’s disease pathology at autopsy: A systematic review. Alzheimer’s Dement. 2022, 18, 2308–2326. [Google Scholar] [CrossRef] [PubMed]
- Sible, I.J.; Yew, B.; Jang, J.Y.; Alitin, J.P.M.; Li, Y.; Gaubert, A.; Nguyen, A.; Dutt, S.; Blanken, A.E.; Ho, J.K.; et al. Blood pressure variability and plasma Alzheimer’s disease biomarkers in older adults. Sci. Rep. 2022, 12, 17197. [Google Scholar] [CrossRef] [PubMed]
- Heus, R.A.A.d.; Rikkert, M.G.M.O.; Tully, P.J.; Lawlor, B.A.; Claassen, J.A.H.R. Blood Pressure Variability and Progression of Clinical Alzheimer Disease. Hypertension 2019, 74, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Petrovitch, H.; White, L.; Izmirilian, G.; Ross, G.W.; Havlik, R.J.; Wr, M.; Nelson, J.; Davis, D.G.; Hardman, J.; Foley, D.; et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: The HAAS. Neurobiol. Aging-Neurobiol. Aging 2000, 21, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Osset-Malla, M.; Martínez-Velasco, A.; Sánchez-Benavides, G.; Buongiorno, M.; de la Sierra, A.; Shekari, M.; Minguillon, C.; Kollmorgen, G.; Quijano-Rubio, C.; Zetterberg, H.; et al. Blood pressure and Alzheimer’s disease biomarkers in cognitively unimpaired adults: A multicenter study. J. Prev. Alzheimer’s Dis. 2025; 100304, in press. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Iadecola, C. Alzheimer’s Disease and Vascular Aging. J. Am. Coll. Cardiol. 2020, 75, 942–951. [Google Scholar] [CrossRef]
- Dede, D.S.; Yavuz, B.; Yavuz, B.B.; Cankurtaran, M.; Halil, M.; Ulger, Z.; Cankurtaran, E.S.; Aytemir, K.; Kabakci, G.; Ariogul, S. Assessment of endothelial function in Alzheimer’s disease: Is Alzheimer’s disease a vascular disease? J. Am. Geriatr. Soc. 2007, 55, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, R.J.; Soiza, R.L. Evidence of endothelial dysfunction in the development of Alzheimer’s disease: Is Alzheimer’s a vascular disorder? Am. J. Cardiovasc. Dis. 2013, 3, 197–226. [Google Scholar] [PubMed]
- Bracko, O.; Cruz Hernández, J.C.; Park, L.; Nishimura, N.; Schaffer, C.B. Causes and consequences of baseline cerebral blood flow reductions in Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 2021, 41, 1501–1516. [Google Scholar] [CrossRef]
- Korte, N.; Nortley, R.; Attwell, D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020, 140, 793–810. [Google Scholar] [CrossRef]
- Szidonya, L.; Nickerson, J.P. Cerebral Amyloid Angiopathy. Radiol. Clin. North. Am. 2023, 61, 551–562. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; Van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease — one peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.A.; Katusic, Z.S. Partial loss of endothelial nitric oxide leads to increased cerebrovascular beta amyloid. J. Cereb. Blood Flow. Metab. 2020, 40, 392–403. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Leurgans, S.E.; Wang, Z.; Wilson, R.S.; Bennett, D.A.; Schneider, J.A. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann. Neurol. 2011, 69, 320–327. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Odum, J.; Shunnarah, J.G.; Austin, N.; Kaddoumi, A. Blood-Brain Barrier Breakdown in Alzheimer’s Disease: Mechanisms and Targeted Strategies. Int. J. Mol. Sci. 2023, 24, 16288. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Han, J.; Wei, W.; Chen, F. Blood-brain barrier dysfunction and Alzheimer’s disease: Associations, pathogenic mechanisms, and therapeutic potential. Front. Aging Neurosci. 2023, 15, 1258640. [Google Scholar] [CrossRef]
- Nehra, G.; Bauer, B.; Hartz, A.M.S. Blood-brain barrier leakage in Alzheimer’s disease: From discovery to clinical relevance. Pharmacol. Ther. 2022, 234, 108119. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.; Nelson, A.R.; Sagare, A.P.; Zlokovic, B.V. Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: The role, regulation and restoration of LRP1. Front. Aging Neurosci. 2015, 7, 136. [Google Scholar] [CrossRef]
- Shibata, M.; Yamada, S.; Kumar, S.R.; Calero, M.; Bading, J.; Frangione, B.; Holtzman, D.M.; Miller, C.A.; Strickland, D.K.; Ghiso, J.; et al. Clearance of Alzheimer’s amyloid-β1-40 peptide from brain by LDL receptor–related protein-1 at the blood-brain barrier. J. Clin. Investig. 2000, 106, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Sagare, A.; Deane, R.; Bell, R.D.; Johnson, B.; Hamm, K.; Pendu, R.; Marky, A.; Lenting, P.J.; Wu, Z.; Zarcone, T.; et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat. Med. 2007, 13, 1029–1031. [Google Scholar] [CrossRef]
- Donahue, J.E.; Flaherty, S.L.; Johanson, C.E.; Duncan, J.A.; Silverberg, G.D.; Miller, M.C.; Tavares, R.; Yang, W.; Wu, Q.; Sabo, E.; et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer’s disease. Acta Neuropathol. 2006, 112, 405–415. [Google Scholar] [CrossRef]
- Niwa, K.; Carlson, G.A.; Iadecola, C. Exogenous Aβ1–40 Reproduces Cerebrovascular Alterations Resulting from Amyloid Precursor Protein Overexpression in Mice. J. Cereb. Blood Flow. Metab. 2000, 20, 1659–1668. [Google Scholar] [CrossRef]
- Park, L.; Wang, G.; Moore, J.; Girouard, H.; Zhou, P.; Anrather, J.; Iadecola, C. The key role of transient receptor potential melastatin-2 channels in amyloid-β-induced neurovascular dysfunction. Nat. Commun. 2014, 5, 5318. [Google Scholar] [CrossRef] [PubMed]
- Sunderman, C. NO signaling in healthy vs. Alzheimer’s disease primary cilia. BioRender 2025. [Google Scholar]
- Liao, F.F.; Lin, G.; Chen, X.; Chen, L.; Zheng, W.; Raghow, R.; Zhou, F.M.; Shih, A.Y.; Tan, X.L. Endothelial Nitric Oxide Synthase-Deficient Mice: A Model of Spontaneous Cerebral Small-Vessel Disease. Am. J. Pathol. 2021, 191, 1932–1945. [Google Scholar] [CrossRef]
- An, L.; Shen, Y.; Chopp, M.; Zacharek, A.; Venkat, P.; Chen, Z.; Li, W.; Qian, Y.; Landschoot-Ward, J.; Chen, J. Deficiency of Endothelial Nitric Oxide Synthase (eNOS) Exacerbates Brain Damage and Cognitive Deficit in A Mouse Model of Vascular Dementia. Aging Dis. 2021, 12, 732–746. [Google Scholar] [CrossRef]
- Liu, C.; Chen, X.; Yang, S.; Wang, X.; Sun, P.; Wang, J.; Zhu, G. Insight into cerebral microvessel endothelial regulation of cognitive impairment: A systematic review of the causes and consequences. Exp. Neurol. 2025, 385, 115116. [Google Scholar] [CrossRef]
- Hopper, R.A.; Garthwaite, J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J. Neurosci. 2006, 26, 11513–11521. [Google Scholar] [CrossRef]
- Zhu, J.; Song, W.; Li, L.; Fan, X. Endothelial nitric oxide synthase: A potential therapeutic target for cerebrovascular diseases. Mol. Brain 2016, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M.R.; Rege, S.V.; Ma, Q.; Zhao, Z.; Miller, C.A.; Winkler, E.A.; Zlokovic, B.V. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 2016, 36, 216–227. [Google Scholar] [CrossRef]
- French, S.R.; Meyer, B.P.; Arias, J.C.; Levendovzsky, S.R.; Weinkauf, C.C. Biomarkers of blood–brain barrier and neurovascular unit integrity in human cognitive impairment and dementia. Alzheimer’s Dement. 2025, 21, e70104. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Zuo, L.; Hu, W.; Jiang, T. Increase of blood-brain barrier leakage is related to cognitive decline in vascular mild cognitive impairment. BMC Neurol. 2021, 21, 159. [Google Scholar] [CrossRef]
- Preis, L.; Villringer, K.; Brosseron, F.; Düzel, E.; Jessen, F.; Petzold, G.C.; Ramirez, A.; Spottke, A.; Fiebach, J.B.; Peters, O. Assessing blood-brain barrier dysfunction and its association with Alzheimer’s pathology, cognitive impairment and neuroinflammation. Alzheimer’s Res. Ther. 2024, 16, 172. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Van Hulle, C.; Ince, S.; Okonkwo, O.C.; Bendlin, B.B.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Love, S.; Blennow, K.; Zetterberg, H.; et al. Elevated CSF angiopoietin-2 correlates with blood-brain barrier leakiness and markers of neuronal injury in early Alzheimer’s disease. Transl. Psychiatry 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef]
- Tsartsalis, S.; Sleven, H.; Fancy, N.; Wessely, F.; Smith, A.M.; Willumsen, N.; Cheung, T.K.D.; Rokicki, M.J.; Chau, V.; Ifie, E.; et al. A single nuclear transcriptomic characterisation of mechanisms responsible for impaired angiogenesis and blood-brain barrier function in Alzheimer’s disease. Nat. Commun. 2024, 15, 2243. [Google Scholar] [CrossRef]
- Michinaga, S.; Hishinuma, S.; Koyama, Y. Roles of astrocytic sonic hedgehog production and its signal for regulation of the blood-brain barrier permeability. Vitam. Horm. 2024, 126, 97–111. [Google Scholar] [CrossRef]
- Yeo, S.; Jang, J.; Jung, H.J.; Lee, H.; Choe, Y. Primary cilia-mediated regulation of microglial secretion in Alzheimer’s disease. Front. Mol. Biosci. 2023, 10, 1250335. [Google Scholar] [CrossRef]
- Austin, S.A.; Santhanam, A.V.; Hinton, D.J.; Choi, D.S.; Katusic, Z.S. Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J. Neurochem. 2013, 127, 691–700. [Google Scholar] [CrossRef]
- Reiter, J.F.; Leroux, M.R. Genes and molecular pathways underpinning ciliopathies. Nat. Rev. Mol. Cell Biol. 2017, 18, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Otsu, W.; Miyadera, K.; Nishimura, Y. Recent advances in the understanding of cilia mechanisms and their applications as therapeutic targets. Front. Mol. Biosci. 2023, 10, 1232188. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Kutchy, N.A.; Chen, L.; Meigs, D.D.; Hu, G. Primary cilia and ciliary signaling pathways in aging and age-related brain disorders. Neurobiol. Dis. 2022, 163, 105607. [Google Scholar] [CrossRef]
- Gholkar, A.A.; Gimeno, T.V.; Edgemon, J.E.; Sim, M.S.; Torres, J.Z. MI-181 Modulates Cilia Length and Restores Cilia Length in Cells with Defective Shortened Cilia. ACS Chem. Biol. 2024, 19, 1733–1742. [Google Scholar] [CrossRef]
- Khan, N.A.; Willemarck, N.; Talebi, A.; Marchand, A.; Binda, M.M.; Dehairs, J.; Rueda-Rincon, N.; Daniels, V.W.; Bagadi, M.; Thimiri Govinda Raj, D.B.; et al. Identification of drugs that restore primary cilium expression in cancer cells. Oncotarget 2016, 7, 9975–9992. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.E.; Jang, S.; Kim, J.B.; Hyung, H.; Park, N.Y.; Kim, Y.H.; Kim, S.H.; Kim, S.H.; Ha, J.M.; Oh, G.S.; et al. Enhanced primary ciliogenesis via mitochondrial oxidative stress activates AKT to prevent neurotoxicity in HSPA9/mortalin-depleted SH-SY5Y cells. Mol. Brain 2023, 16, 41. [Google Scholar] [CrossRef]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Jack, C.R.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef]
- Jasodanand, V.H.; Kowshik, S.S.; Puducheri, S.; Romano, M.F.; Xu, L.; Au, R.; Kolachalama, V.B. AI-driven fusion of multimodal data for Alzheimer’s disease biomarker assessment. Nat. Commun. 2025, 16, 7407. [Google Scholar] [CrossRef]
- Strooper, B.D.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Salloway, S.P.; Sevingy, J.; Budur, K.; Pederson, J.T.; DeMattos, R.B.; Von Rosenstiel, P.; Paez, A.; Evans, R.; Weber, C.J.; Hendrix, J.A.; et al. Advancing combination therapy for Alzheimer’s disease. Alzheimers Dement 2020, 6, e12073. [Google Scholar] [CrossRef]
- Cummings, J.L.; Osse, A.M.L.; Kinney, J.W.; Cammann, D.; Chen, J. Alzheimer’s Disease: Combination Therapies and Clinical Trials for Combination Therapy Development. CNS Drugs 2024, 38, 613–624. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef]
- Luo, Q.; Yang, J.; Yang, M.; Wang, Y.; Liu, Y.; Liu, J.; Kalvakolanu, D.V.; Cong, X.; Zhang, J.; Zhang, L.; et al. Utilization of nanotechnology to surmount the blood-brain barrier in disorders of the central nervous system. Mater. Today Bio. 2025, 31, 101457. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Verma, R.; Kumar, K.; Chawla, R. Role of pro-inflammatory cytokines in Alzheimer’s disease and neuroprotective effects of pegylated self-assembled nanoscaffolds. Curr. Res. Pharmacol. Drug Discov. 2023, 4, 100149. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Nigam, K.; Srivastava, S.; Tyagi, A.; Dang, S. Memantine nanoemulsion: A new approach to treat Alzheimer’s disease. J. Microencapsul. 2020, 37, 355–365. [Google Scholar] [CrossRef] [PubMed]

| Cell Type | Function | |
|---|---|---|
| Motile | Ependymal cells | Propel cerebrospinal fluid [23] |
| Choroid plexus epithelium | Regulate cerebral spinal fluid production [24] | |
| Tracheal epithelial cells | Remove foreign substances from the body [25] | |
| Oviductal epithelium | Facilitate transport of oocyte, gamete, and embryo [26] | |
| Nasal epithelial cell | Mucociliary clearance [27] | |
| Nonmotile | Neural stem cells | Regulation of Neural stem cells, especially in the ventral region of the ventricular–subventricular zone [28] |
| Neurons | Regulate cognitive function, metabolism, and mood state [29] | |
| Astrocytes | Regulates astrocyte morphology and intracellular signaling balance [30] | |
| Osteoblasts | Participate in osteoblast alignment, differentiation, and polarization, as well as bone formation [31] | |
| Osteocytes | Act as mechanical sensors and bend in response to pulses of extracellular fluid that are generated during running and walking [32] | |
| Chondrocytes | Mechanotransduction [31] | |
| Dental pulp stem cells | IFT80 ciliary protein helps regulate Dental pulp stem cells differentiation [33] | |
| Vascular endothelial cells | Calcium-dependent mechanosensors that sense blood flow [34] | |
| Renal tubule epithelial cells | Mediate the mechanosensation of extracellular urine flow [35] | |
| Endocardial cells | Mediate upregulation of the KLF2 gene expression and succeeding activation of the Notch signal pathway [36] | |
| Vascular smooth muscle cells | Mediate extracellular matrix-protein sensing and fluid-flow-induced mechanosensing [37] | |
| Olfactory epithelial cells | Odorants bind to olfactory receptors to start the olfaction cascade [25] | |
| Stereocilia | Mechanoelectrical transduction- converting physical force to an electrical signal [38] | |
| Kinocilia | Mediate hair cell morphogenesis and planar cell polarity [39] | |
| Cholangiocytes | Mechanosensory, chemosensory, and osmosensory functions to regulate cholangiocyte proliferation [40] | |
| Retinal photoreceptor cells | Connects inner and outer segments- the cellular nucleus to the photopigment [41] | |
| Retinal ganglion cells | Facilitate regenerative responses to Insulin-like Growth Factor-1 [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunderman, C.L.; Forero, K.V.; Alorjani, Q.; Buqaileh, R.; Gallagher, G.M.; Ventresca, S.M.; Messer, W.S., Jr.; AbouAlaiwi, W.A. Primary Cilia and Cardiovascular Risk Factors in Alzheimer’s Disease. Brain Sci. 2025, 15, 1004. https://doi.org/10.3390/brainsci15091004
Sunderman CL, Forero KV, Alorjani Q, Buqaileh R, Gallagher GM, Ventresca SM, Messer WS Jr., AbouAlaiwi WA. Primary Cilia and Cardiovascular Risk Factors in Alzheimer’s Disease. Brain Sciences. 2025; 15(9):1004. https://doi.org/10.3390/brainsci15091004
Chicago/Turabian StyleSunderman, Clare L., Kathleen V. Forero, Qasim Alorjani, Raghad Buqaileh, Gillian M. Gallagher, Sestina M. Ventresca, William S. Messer, Jr., and Wissam A. AbouAlaiwi. 2025. "Primary Cilia and Cardiovascular Risk Factors in Alzheimer’s Disease" Brain Sciences 15, no. 9: 1004. https://doi.org/10.3390/brainsci15091004
APA StyleSunderman, C. L., Forero, K. V., Alorjani, Q., Buqaileh, R., Gallagher, G. M., Ventresca, S. M., Messer, W. S., Jr., & AbouAlaiwi, W. A. (2025). Primary Cilia and Cardiovascular Risk Factors in Alzheimer’s Disease. Brain Sciences, 15(9), 1004. https://doi.org/10.3390/brainsci15091004






