Effect of Sound Amplification on Central Auditory Plasticity: Endbulb of Held as a Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Auditory Brainstem Responses
2.3. Experimental Treatment/Amplification
2.4. Cochlear Surgery and Auditory Nerve Injection
2.5. Tissue Preparation
2.6. Confocal Image Acquisition and Analysis
2.7. Statistical Analyses
3. Results
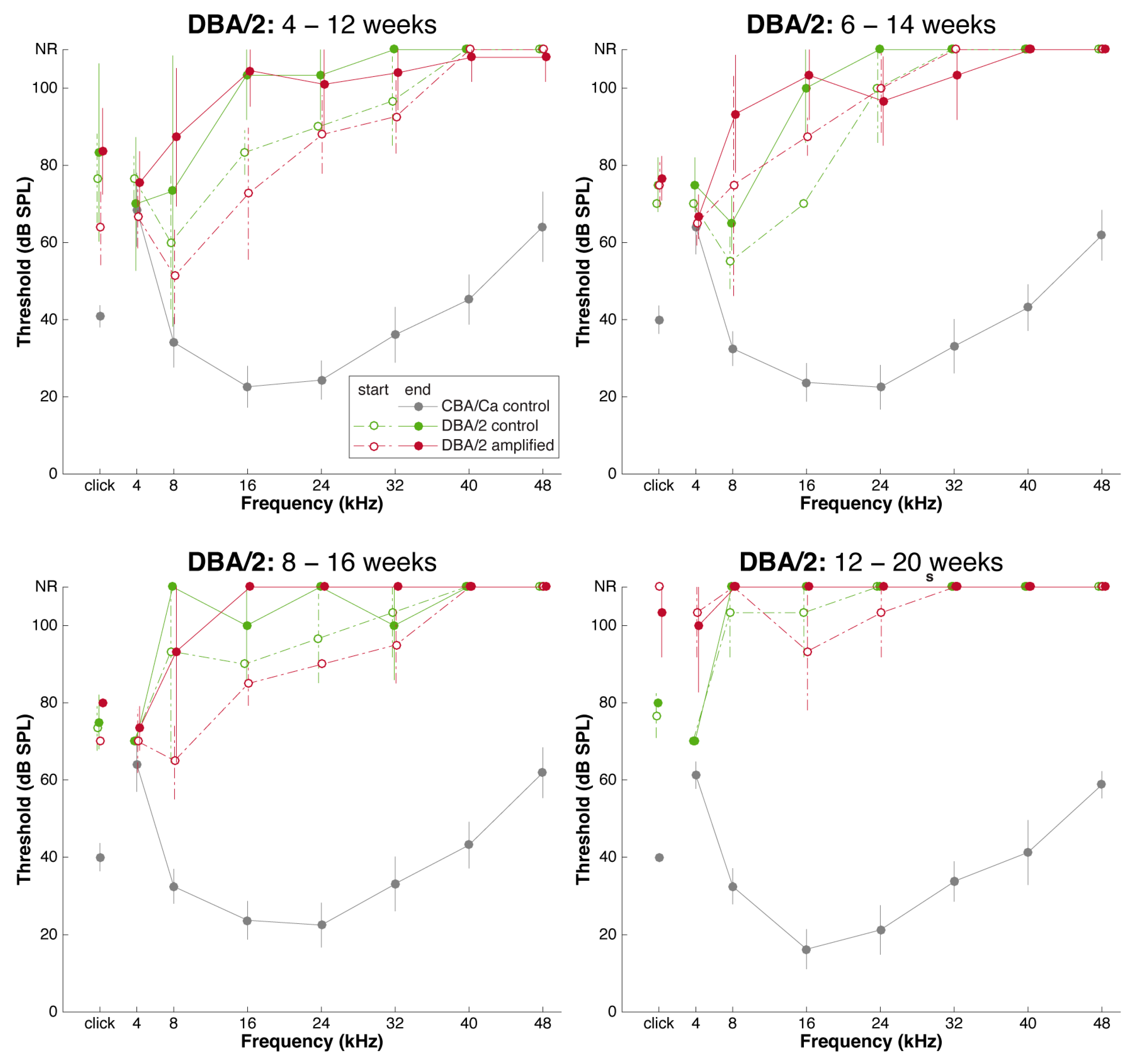
3.1. Sound Amplification and Threshold Change: DBA/2 Mice
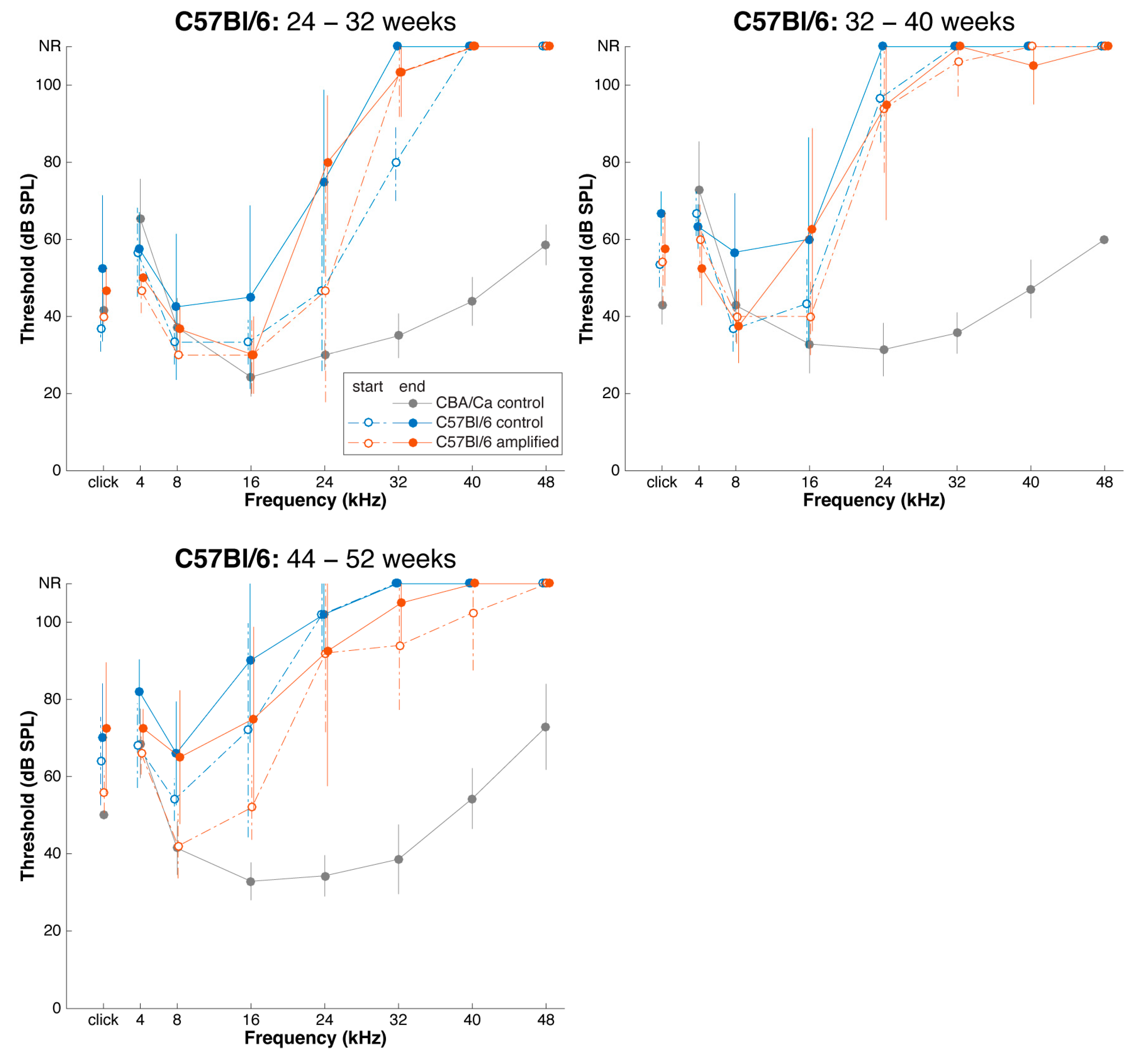
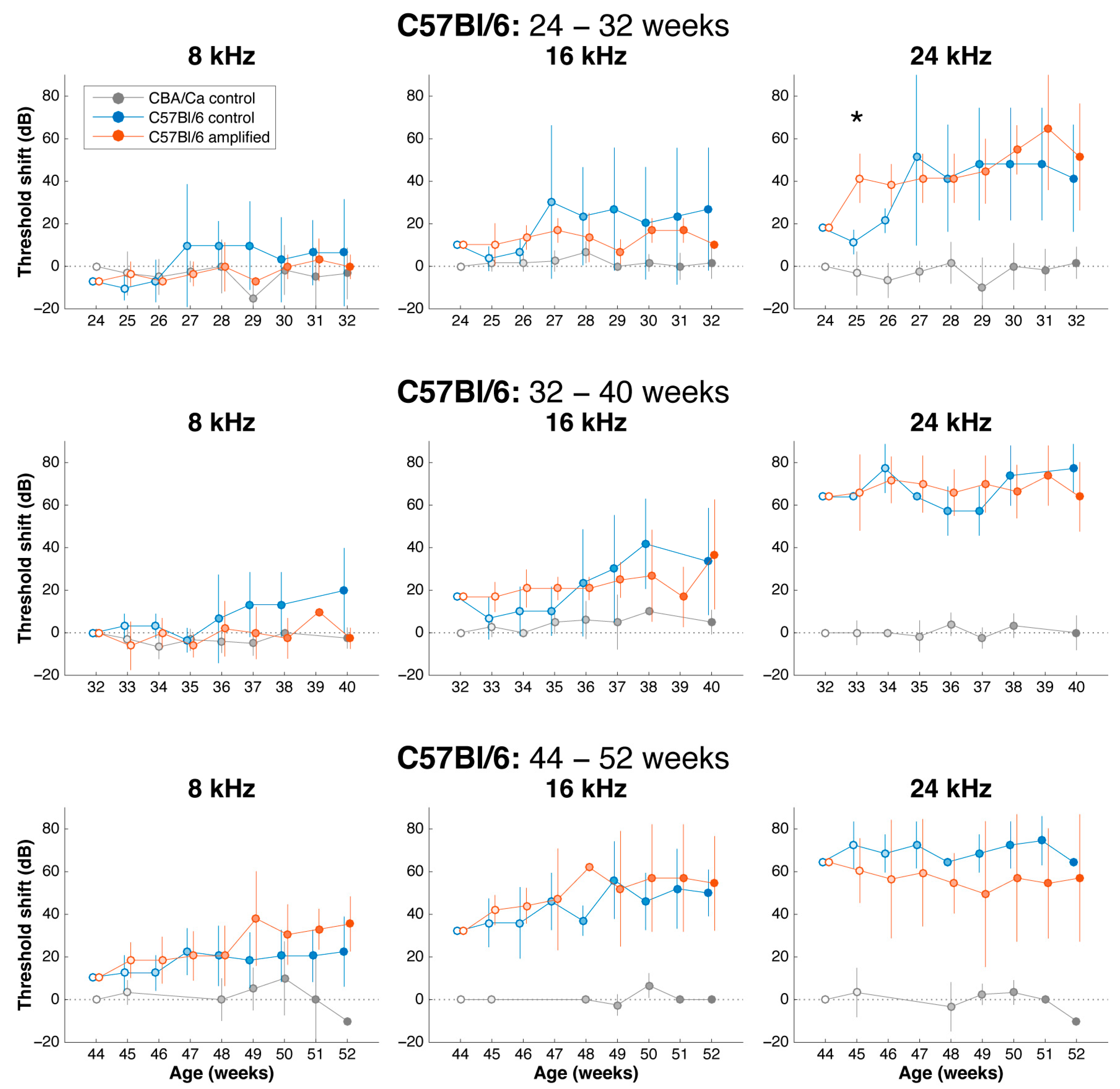
3.2. Sound Amplification and Threshold Change: C57Bl/6 Mice
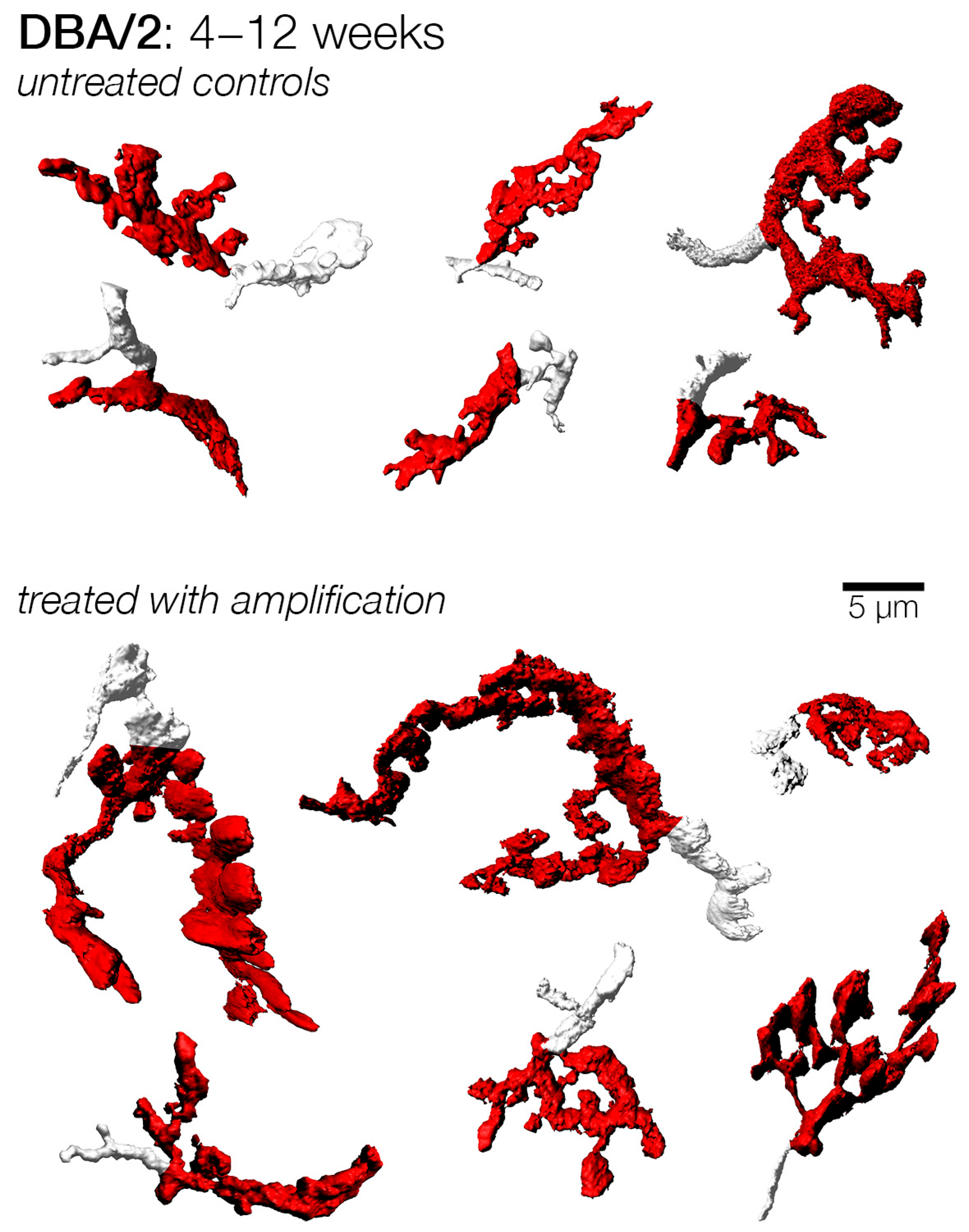
3.3. Effects of Sound Amplification on Endbulbs of Held: DBA/2 Mice
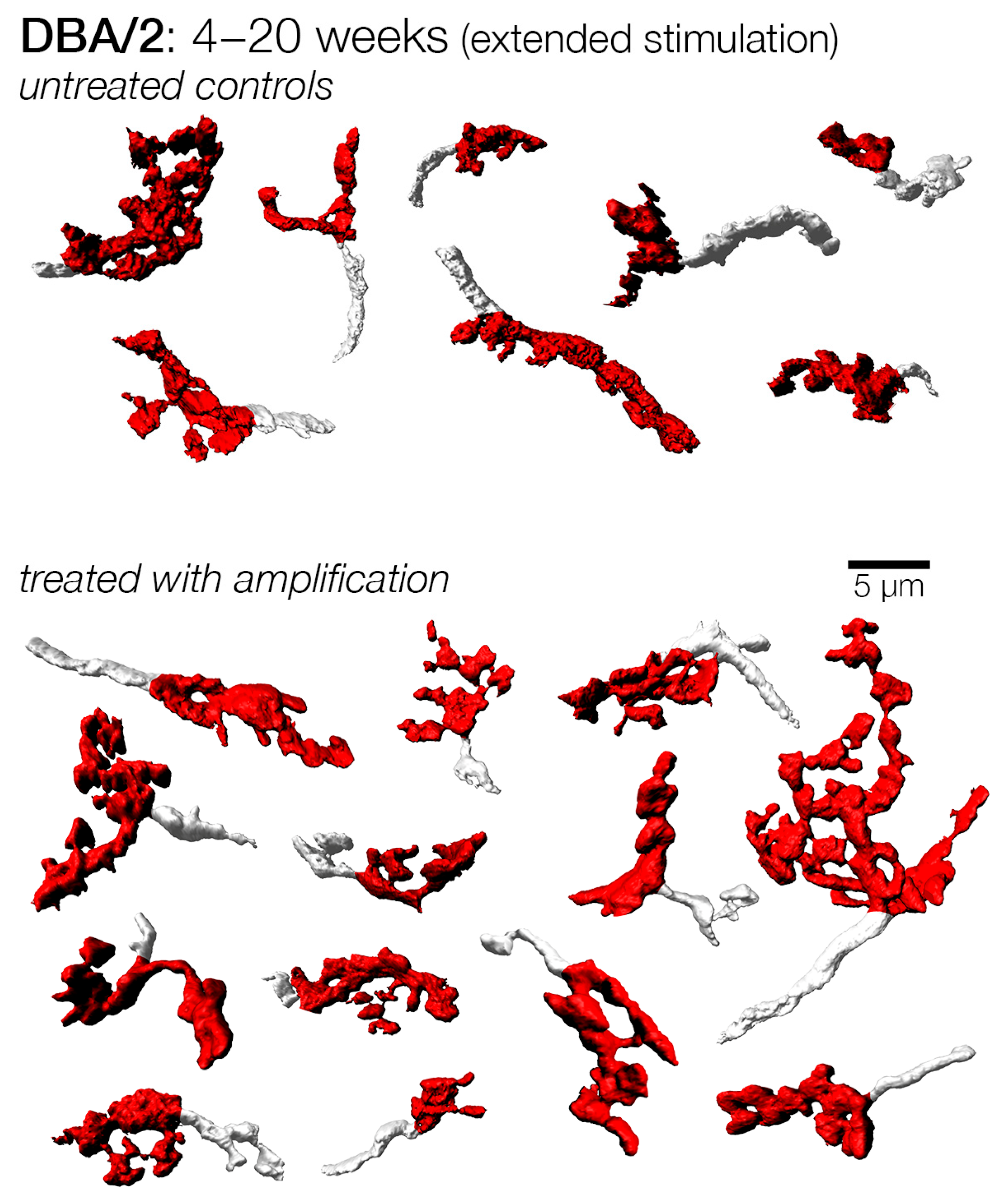
3.4. Endbulbs of Held with Extended Duration of Amplification: DBA/2 Mice
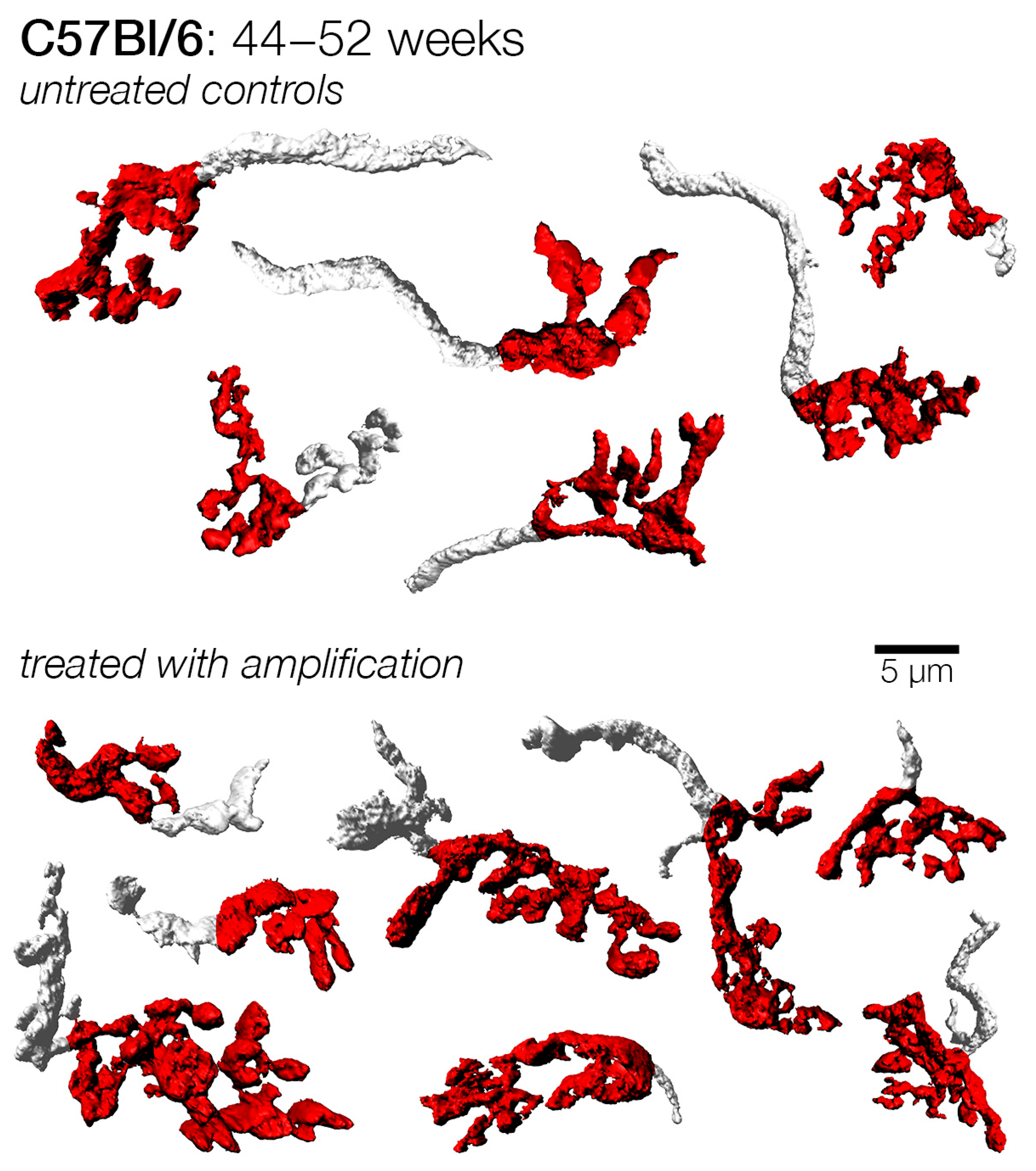
3.5. Effects of Sound Amplification on Endbulbs of Held: C57Bl/6 Mice
4. Discussion
4.1. Threshold Change
4.2. Amplification and Endbulb Changes
4.3. Early Versus Late Treatment and Critical Period
4.4. Impact of Hearing Loss and Ageing on Endbulb Morphology
4.5. Optimal Time to Fit Hearing Aids
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrmann, B.; Butler, B.E. Hearing loss and brain plasticity: The hyperactivity phenomenon. Brain Struct. Funct. 2021, 226, 2019–2039. [Google Scholar] [CrossRef] [PubMed]
- Stotler, W.A. An experimental study of the cells and connections of the superior olivary complex of the cat. J. Comp. Neurol. 1953, 98, 401–431. [Google Scholar] [CrossRef]
- Van der Loos, H.; Woolsey, T.A. Somatosensory cortex: Structural alterations following early injury to sense organs. Science 1973, 179, 395–398. [Google Scholar] [CrossRef] [PubMed]
- West, C.D.; Harrison, J.M. Transneuronal cell atrophy i the congenitally deaf white cat. J. Comp. Neurol. 1973, 151, 377–398. [Google Scholar] [CrossRef]
- Wiesel, T.N.; Hubel, D.H.; Lam, D.M. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974, 79, 273–279. [Google Scholar] [CrossRef]
- LeVay, S.; Hubel, D.H.; Wiesel, T.N. The pattern of ocular dominance columns in macaque visual cortex revealed by a reduced silver stain. J. Comp. Neurol. 1975, 159, 559–576. [Google Scholar] [CrossRef]
- Meisami, E.; Safari, A. Quantitative study of the effects of early unilateral olfactory deprivation on the number and distribution of mitral and tufted cells of glomeruli in the rat olfactory bulb. Brain Res. 1981, 221, 81–107. [Google Scholar] [CrossRef]
- Nordeen, K.W.; Killackey, H.P.; Kitzes, L.M. Ascending projections to the inferior colliculus following unilateral cochlear ablation in the neonatal gerbil, Meriones unguiculatus. J. Comp. Neurol. 1983, 214, 144–153. [Google Scholar] [CrossRef]
- Parks, T.N. Afferent influences on the development of the brain stem auditory nuclei of the chicken: Otocyst ablation. J. Comp. Neurol. 1979, 183, 665–677. [Google Scholar] [CrossRef]
- Trune, D.R. Influence of neonatal cochlear removal on the development of mouse cochlear nucleus: I. Number, size, and density of its neurons. J. Comp. Neurol. 1982, 209, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Willott, J.F. Measurement of the auditory brainstem response (ABR) to study auditory sensitivity in mice. Curr. Protoc. Neurosci. 2006, 34, 8.21B.1–8.21B.12. [Google Scholar] [CrossRef] [PubMed]
- Rigters, S.C.; Bos, D.; Metselaar, M.; Roshchupkin, G.V.; de Jong, R.J.B.; Ikram, M.A.; Vernooij, M.W.; Goedegebure, A. Hearing Impairment Is Associated with Smaller Brain Volume in Aging. Front. Aging Neurosci. 2017, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Ryugo, D.K.; Pongstaporn, T.; Huchton, D.M.; Niparko, J.K. Ultrastructural analysis of primary endings in deaf white cats: Morphologic alterations in endbulbs of Held. J. Comp. Neurol. 1997, 385, 230–244. [Google Scholar] [CrossRef]
- Kaltenbach, J.A.; Zhang, J. Intense sound-induced plasticity in the dorsal cochlear nucleus of rats: Evidence for cholinergic receptor upregulation. Hear. Res. 2007, 226, 232–243. [Google Scholar] [CrossRef]
- Lauer, A.M.; Fuchs, P.A.; Ryugo, D.K.; Francis, H.W. Efferent synapses return to inner hair cells in the aging cochlea. Neurobiol. Aging 2012, 33, 2892–2902. [Google Scholar] [CrossRef]
- Doherty, K.A.; Desjardins, J.L. The benefit of amplification on auditory working memory function in middle-aged and young-older hearing impaired adults. Front. Psychol. 2015, 6, 721. [Google Scholar] [CrossRef]
- Klinke, R.; Kral, A.; Heid, S.; Tillein, J.; Hartmann, R. Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. Science 1999, 285, 1729–1733. [Google Scholar] [CrossRef]
- Noreña, A.J.; Eggermont, J.J. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J. Neurosci. 2005, 25, 699–705. [Google Scholar] [CrossRef]
- Ryugo, D.K.; Kretzmer, E.A.; Niparko, J.K. Restoration of auditory nerve synapses in cats by cochlear implants. Science 2005, 310, 1490–1492. [Google Scholar] [CrossRef] [PubMed]
- Waltzman, S.B.; Roland, J.T. Cochlear Implants, 3rd ed.; Thieme: New York, NY, USA, 2014. [Google Scholar]
- Tirko, N.N.; Ryugo, D.K. Synaptic plasticity in the medial superior olive of hearing, deaf, and cochlear-implanted cats. J. Comp. Neurol. 2012, 520, 2202–2217. [Google Scholar] [CrossRef]
- Kral, A.; Sharma, A. Crossmodal plasticity in hearing loss. Trends Neurosci. 2023, 46, 377–393. [Google Scholar] [CrossRef]
- Turner, J.G.; Willott, J.F. Exposure to an augmented acoustic environment alters auditory function in hearing-impaired DBA/2J mice. Hear. Res. 1998, 118, 101–113. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, F.; Hu, H.; Sun, X.; Kilgard, M.P.; Merzenich, M.M.; Zhou, X. Environmental acoustic enrichment promotes recovery from developmentally degraded auditory cortical processing. J. Neurosci. 2014, 34, 5406–5415. [Google Scholar] [CrossRef]
- Hutchison, P.; Maeda, H.; Formby, C.; Small, B.J.; Eddins, D.A.; Eddins, A.C. Acoustic deprivation modulates central gain in human auditory brainstem and cortex. Hear. Res. 2023, 428, 108683. [Google Scholar] [CrossRef]
- Willott, J.F.; Turner, J.G. Prolonged exposure to an augmented acoustic environment ameliorates age-related auditory changes in C57BL/6J and DBA/2J mice. Hear. Res. 1999, 135, 78–88. [Google Scholar] [CrossRef]
- Tanaka, C.; Bielefeld, E.C.; Chen, G.-D.; Li, M.; Henderson, D. Ameliorative effects of an augmented acoustic environment on age-related hearing loss in middle-aged Fischer 344/NHsd rats. Laryngoscope 2009, 119, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.B. Sound amplification negates central effects of a neonatal conductive hearing loss. Hear. Res. 1988, 32, 193–195. [Google Scholar] [CrossRef]
- Billings, C.J.; Tremblay, K.L.; Souza, P.E.; Binns, M.A. Effects of hearing aid amplification and stimulus intensity on cortical auditory evoked potentials. Audiol. Neurootol. 2007, 12, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Karawani, H.; Jenkins, K.A.; Anderson, S. Neural and behavioral changes after the use of hearing aids. Clin. Neurophysiol. 2018, 129, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Ryugo, D.K.; Parks, T.N. Innervation of the cochlear nucleus in birds and mammals. Brain Res. Bull. 2003, 60, 435–456. [Google Scholar] [CrossRef]
- Lorente de Nó, R. The Primary Acoustic Nuclei; Raven Press: New York, NY, USA, 1981. [Google Scholar]
- Fekete, D.M.; Rouiller, E.M.; Liberman, M.C.; Ryugo, D.K. The central projections of intracellularly labeled auditory nerve fibers in cats. J. Comp. Neurol. 1984, 229, 432–450. [Google Scholar] [CrossRef]
- Rouiller, E.M.; Cronin-Schreiber, R.; Fekete, D.M.; Ryugo, D.K. The central projections of intracellularly labeled auditory nerve fibers in cats: An analysis of terminal morphology. J. Comp. Neurol. 1986, 249, 261–278. [Google Scholar] [CrossRef]
- Manis, P.B.; Marx, S.O. Outward currents in isolated ventral cochlear nucleus neurons. J. Neurosci. 1991, 11, 2865–2880. [Google Scholar] [CrossRef] [PubMed]
- Kuenzel, T.; Borst, J.G.; van der Heijden, M. Factors controlling the input-output relationship of spherical bushy cells in the gerbil cochlear nucleus. J. Neurosci. 2011, 31, 4260–4273. [Google Scholar] [CrossRef]
- Pfeiffer, R.R. Classification of response patterns of spike discharges for units in the cochlear nucleus: Tone-burst stimulation. Exp. Brain Res. 1966, 1, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.H.; Joris, P.X.; Yin, T.C. Projections of physiologically characterized spherical bushy cell axons from the cochlear nucleus of the cat: Evidence for delay lines to the medial superior olive. J. Comp. Neurol. 1993, 331, 245–260. [Google Scholar] [CrossRef]
- Joris, P.; Yin, T.C. A matter of time: Internal delays in binaural processing. Trends Neurosci. 2007, 30, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Ryugo, D.K.; Sento, S. Synaptic connections of the auditory nerve in cats: Relationship between endbulbs of held and spherical bushy cells. J. Comp. Neurol. 1991, 305, 35–48. [Google Scholar] [CrossRef]
- Limb, C.J.; Ryugo, D.K. Development of primary axosomatic endings in the anteroventral cochlear nucleus of mice. J. Assoc. Res. Otolaryngol. 2000, 1, 103–119. [Google Scholar] [CrossRef]
- Wang, Y.; Manis, P.B. Synaptic transmission at the cochlear nucleus endbulb synapse during age-related hearing loss in mice. J. Neurophysiol. 2005, 94, 1814–1824. [Google Scholar] [CrossRef]
- Ngodup, T.; Goetz, J.A.; McGuire, B.C.; Sun, W.; Lauer, A.M.; Xu-Friedman, M.A. Activity-dependent, homeostatic regulation of neurotransmitter release from auditory nerve fibers. Proc. Natl. Acad. Sci. USA 2015, 112, 6479–6484. [Google Scholar] [CrossRef]
- Hintze, A.; Gültas, M.; Semmelhack, E.A.; Wichmann, C. Ultrastructural maturation of the endbulb of Held active zones comparing wild-type and otoferlin-deficient mice. iScience 2021, 24, 102282. [Google Scholar] [CrossRef]
- Sento, S.; Ryugo, D.K. Endbulbs of held and spherical bushy cells in cats: Morphological correlates with physiological properties. J. Comp. Neurol. 1989, 280, 553–562. [Google Scholar] [CrossRef]
- Ryugo, D.K.; Rouiller, E.M. Central projections of intracellularly labeled auditory nerve fibers in cats: Morphometric correlations with physiological properties. J. Comp. Neurol. 1988, 271, 130–142. [Google Scholar] [CrossRef]
- Lee, D.J.; Cahill, H.B.; Ryugo, D.K. Effects of congenital deafness in the cochlear nuclei of Shaker-2 mice: An ultrastructural analysis of synapse morphology in the endbulbs of Held. J. Neurocytol. 2003, 32, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Connelly, C.J.; Ryugo, D.K.; Muniak, M.A. The effect of progressive hearing loss on the morphology of endbulbs of Held and bushy cells. Hear. Res. 2016, 343, 14–33. [Google Scholar] [CrossRef]
- Xie, R.; Manis, P.B. Synaptic transmission at the endbulb of Held deteriorates during age-related hearing loss. J. Physiol. 2017, 595, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, C.; Lin, S.; Wang, Y.; Seicol, B.J.; Robert, W.; Ariss, R.W.; Xie, R. Biased auditory nerve central synaptopathy is associated with age-related hearing loss. J. Physiol. 2021, 599, 1833–1854. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wang, M.; Zhang, C. Mechanisms of age-related hearing loss at the auditory nerve central synapses and postsynaptic neurons in the cochlear nucleus. Hear. Res. 2024, 442, 108935. [Google Scholar] [CrossRef]
- Zhang, L.I.; Bao, S.; Merzenich, M.M. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat. Neurosci. 2001, 4, 1123–1130. [Google Scholar] [CrossRef]
- Sun, Y.; An, P.; Cai, Y.; Yang, W.; Fang, Y.; Liu, H.; Zhang, G.; Shan, Y.; Wang, J.; Zhang, Y.; et al. Environmental enrichment reverses noise induced impairments in learning and memory associated with the hippocampus in female rats. Sci. Rep. 2025, 15, 11509. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L.; Banai, K.; Karni, A.; Attias, J. Hearing Aid-Induced Plasticity in the Auditory System of Older Adults: Evidence From Speech Perception. J. Speech Lang. Hear. Res. 2015, 58, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Ryugo, D. Auditory neuroplasticity, hearing loss and cochlear implants. Cell Tissue Res. 2015, 361, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Persic, D.; Thomas, M.E.; Pelekanos, V.; Ryugo, D.K.; Takesian, A.E.; Krumbholz, K.; Pyott, S.J. Regulation of auditory plasticity during critical periods and following hearing loss. Hear. Res. 2020, 397, 107976. [Google Scholar] [CrossRef]
- Chang, E.F.; Merzenich, M.M. Environmental noise retards auditory cortical development. Science 2003, 300, 498–502. [Google Scholar] [CrossRef]
- Bogaerts, S.; Clements, J.D.; Sullivan, J.M.; Oleskevich, S. Automated threshold detection for auditory brainstem responses: Comparison with visual estimation in a stem cell transplantation study. BMC Neurosci. 2009, 10, 104. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Hammond, L.A.; Kanjhan, R.; Bellingham, M.C.; Noakes, P.G. A method for the three-dimensional reconstruction of Neurobiotin-filled neurons and the location of their synaptic inputs. Front. Neural Circuits 2013, 7, 153. [Google Scholar] [CrossRef]
- Ronneberger, O.; Baddeley, D.; Scheipl, F.; Verveer, P.J.; Burkhardt, H.; Cremer, C.; Fahrmeir, L.; Cremer, T.; Joffe, B. Spatial quantitative analysis of fluorescently labeled nuclear structures: Problems, methods, pitfalls. Chromosome Res. 2008, 16, 523–562. [Google Scholar] [CrossRef]
- DeHoff, R.T. Stereological Uses of the Area Tangent Count. In Geometrical Probability and Biological Structures: Buffon’s 200th Anniversary; Miles, R.E., Serra, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1978; pp. 99–113. [Google Scholar]
- Zheng, Q.Y.; Johnson, K.R.; Erway, L.C. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999, 130, 94–107. [Google Scholar] [CrossRef]
- Johnson, K.R.; Zheng, Q.Y.; Erway, L.C. A major gene affecting age-related hearing loss is common to at least ten inbred strains of mice. Genomics 2000, 70, 171–180. [Google Scholar] [CrossRef]
- Dallos, P.; Harris, D. Properties of auditory nerve responses in absence of outer hair cells. J. Neurophysiol. 1978, 41, 365–383. [Google Scholar] [CrossRef]
- Liberman, M.C.; Dodds, L.W. Single neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear. Res. 1984, 16, 55–74. [Google Scholar] [CrossRef]
- Erway, L.C.; Willott, J.F.; Archer, J.R.; Harrison, D.E. Genetics of age related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear. Res. 1993, 65, 125–132. [Google Scholar] [CrossRef]
- Wiesel, T.N.; Hubel, D.H. Effects of Visual Deprivation on Morphology and Physiology of Cells in the Cats Lateral Geniculate Body. J. Neurophysiol. 1963, 26, 978–993. [Google Scholar] [CrossRef]
- Katz, L.C.; Shatz, C.J. Synaptic activity and the construction of cortical circuits. Science 1996, 274, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Cellerino, A.; Siciliano, R.; Domenici, L.; Maffei, L. Parvalbumin immunoreactivity: A reliable marker for the effects of monocular deprivation in the rat visual cortex. Neuroscience 1992, 51, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Ryugo, D.K.; Wu, M.M.; Pongstaporn, T. Activity-related features of synapse morphology: A study of endbulbs of held. J. Comp. Neurol. 1996, 365, 141–158. [Google Scholar] [CrossRef]
- Henneman, E. Relation between size of neurons and their susceptibility to discharge. Science 1957, 126, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Lewin, G.R. An ultrastructural size principle. Neuroscience 1994, 58, 441–446. [Google Scholar] [CrossRef]
- Russell, F.A.; Moore, D.R. Afferent Reorganization within the Superior Olivary Complex of the Gerbil—Development and Induction by Neonatal, Unilateral Cochlear Removal. J. Comp. Neurol. 1995, 352, 607–625. [Google Scholar] [CrossRef]
- Rajan, R.; Irvine, D.R. Severe and extensive neonatal hearing loss in cats results in auditory cortex plasticity that differentiates into two regions. Eur. J. Neurosci. 2010, 31, 1999–2013. [Google Scholar] [CrossRef] [PubMed]
- Syka, J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol. Rev. 2002, 82, 601–636. [Google Scholar] [CrossRef]
- Hudspeth, A.J. Making an effort to listen: Mechanical amplification in the ear. Neuron 2008, 59, 530–545. [Google Scholar] [CrossRef]
- Zhou, X.; Merzenich, M.M. Enduring effects of early structured noise exposure on temporal modulation in the primary auditory cortex. Proc. Natl. Acad. Sci. USA 2008, 105, 4423–4428. [Google Scholar] [CrossRef]
- Francis, H.W.; Niparko, J.K. Cochlear implantation update. Pediatr. Clin. N. Am. 2003, 50, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Muniak, M.A.; Ayeni, F.E.; Ryugo, D.K. Hidden hearing loss and endbulbs of Held: Evidence for central pathology before detection of ABR threshold increases. Hear. Res. 2018, 364, 104–117. [Google Scholar] [CrossRef]
- Korkmaz, M.H.; Bayir, O.; Er, S.; Isik, E.; Saylam, G.; Tatar, E.C.; Ozdek, A. Satisfaction and compliance of adult patients using hearing aid and evaluation of factors affecting them. Eur. Arch. Otorhinolaryngol. 2016, 273, 3723–3732. [Google Scholar] [CrossRef]
- Kochkin, S. MarkeTrak V: “Why my hearing aids are in the drawer”: The consumers’ perspective. Hear. J. 2000, 53, 34–41. [Google Scholar] [CrossRef]
- Schaette, R.; McAlpine, D. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J. Neurosci. 2011, 31, 13452–13457. [Google Scholar] [CrossRef]
- Sergeyenko, Y.; Lall, K.; Liberman, M.C.; Kujawa, S.G. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J. Neurosci. 2013, 33, 13686–13694. [Google Scholar] [CrossRef] [PubMed]






| Age @ Enrollment (Weeks) | Age @ Termination (Weeks) | N | Surface Area (µm2) | Volume (µm3) | SA/Vol Ratio | Shape Factor | |
|---|---|---|---|---|---|---|---|
| Control | 4 | 12 | 75 | 187.0 ± 127.0 | 40.8 ± 25.9 | 4.61 ± 1.11 | 5.78 ± 2.74 |
| 6 | 14 | 44 | 169.7 ± 119.3 | 40.7 ± 24.6 | 4.07 ± 0.74 | 4.84 ± 2.32 | |
| 8 | 16 | 25 | 137.5 ± 48.6 | 35.1 ± 12.1 | 3.94 ± 0.61 | 4.31 ± 1.13 | |
| 12 | 20 | 23 | 118.9 ± 74.8 | 32.6 ± 21.4 | 3.78 ± 0.54 | 3.65 ± 1.14 | |
| 4 | 20 | 63 | 115.0 ± 54.4 | 29.0 ± 14.7 | 4.07 ± 0.77 | 4.00 ± 1.17 | |
| -- | 52 * | 50 | 99.8 ± 45.5 | 26.5 ± 12.8 | 3.86 ± 0.62 | 3.55 ± 0.97 | |
| Amplified | 4 | 12 | 33 | 250.9 ± 157.3 | 50.4 ± 27.6 | 5.20 ± 1.51 | 7.46 ± 3.29 |
| 6 | 14 | 168 | 199.8 ± 131.6 | 47.3 ± 31.2 | 4.41 ± 1.14 | 5.62 ± 2.47 | |
| 7 | 16 | 71 | 210.8 ± 131.1 | 48.1 ± 24.6 | 4.32 ± 1.07 | 5.88 ± 3.20 | |
| 12 | 20 | 61 | 111.2 ± 77.6 | 31.1 ± 23.5 | 3.76 ± 0.59 | 3.53 ± 1.04 | |
| 4 | 20 | 69 | 139.9 ± 63.4 | 34.1 ± 15.1 | 4.15 ± 0.61 | 4.53 ± 1.23 |
| Age @ Enrollment (Weeks) | Age @ Termination (Weeks) | N | Surface Area (µm2) | Volume (µm3) | SA/Vol Ratio | Shape Factor | |
|---|---|---|---|---|---|---|---|
| Control | 24 | 32 | 61 | 161.5 ± 73.2 | 46.6 ± 20.5 | 3.56 ± 0.82 | 4.20 ± 1.74 |
| 32 | 40 | 39 | 146.5 ± 78.5 | 39.2 ± 21.6 | 3.85 ± 0.74 | 4.22 ± 1.35 | |
| 44 | 52 | 69 | 139.2 ± 77.0 | 36.2 ± 19.7 | 3.92 ± 0.59 | 4.21 ± 1.33 | |
| Amplified | 24 | 32 | 38 | 177.9 ± 85.3 | 49.9 ± 25.9 | 3.69 ± 0.79 | 4.55 ± 1.76 |
| 32 | 40 | 70 | 183.1 ± 89.9 | 49.8 ± 25.0 | 3.78 ± 0.71 | 4.68 ± 1.42 | |
| 44 | 52 | 115 | 157.9 ± 76.9 | 44.2 ± 21.3 | 3.64 ± 0.65 | 4.22 ± 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayeni, F.E.; Muniak, M.A.; Ryugo, D.K. Effect of Sound Amplification on Central Auditory Plasticity: Endbulb of Held as a Substrate. Brain Sci. 2025, 15, 888. https://doi.org/10.3390/brainsci15080888
Ayeni FE, Muniak MA, Ryugo DK. Effect of Sound Amplification on Central Auditory Plasticity: Endbulb of Held as a Substrate. Brain Sciences. 2025; 15(8):888. https://doi.org/10.3390/brainsci15080888
Chicago/Turabian StyleAyeni, Femi E., Michael A. Muniak, and David K. Ryugo. 2025. "Effect of Sound Amplification on Central Auditory Plasticity: Endbulb of Held as a Substrate" Brain Sciences 15, no. 8: 888. https://doi.org/10.3390/brainsci15080888
APA StyleAyeni, F. E., Muniak, M. A., & Ryugo, D. K. (2025). Effect of Sound Amplification on Central Auditory Plasticity: Endbulb of Held as a Substrate. Brain Sciences, 15(8), 888. https://doi.org/10.3390/brainsci15080888






