Exploring Neural Evidence of Attention in Classroom Environments: A Scoping Review
Abstract
1. Introduction
1.1. Classroom Attention and Its Measurements
1.2. Investigating Neural Activities in Classroom Environments
1.3. Rationale for the Review
1.4. The Current Study
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Literature Search
2.3. Literature Selection
2.4. Data Coding and Extraction
3. Results
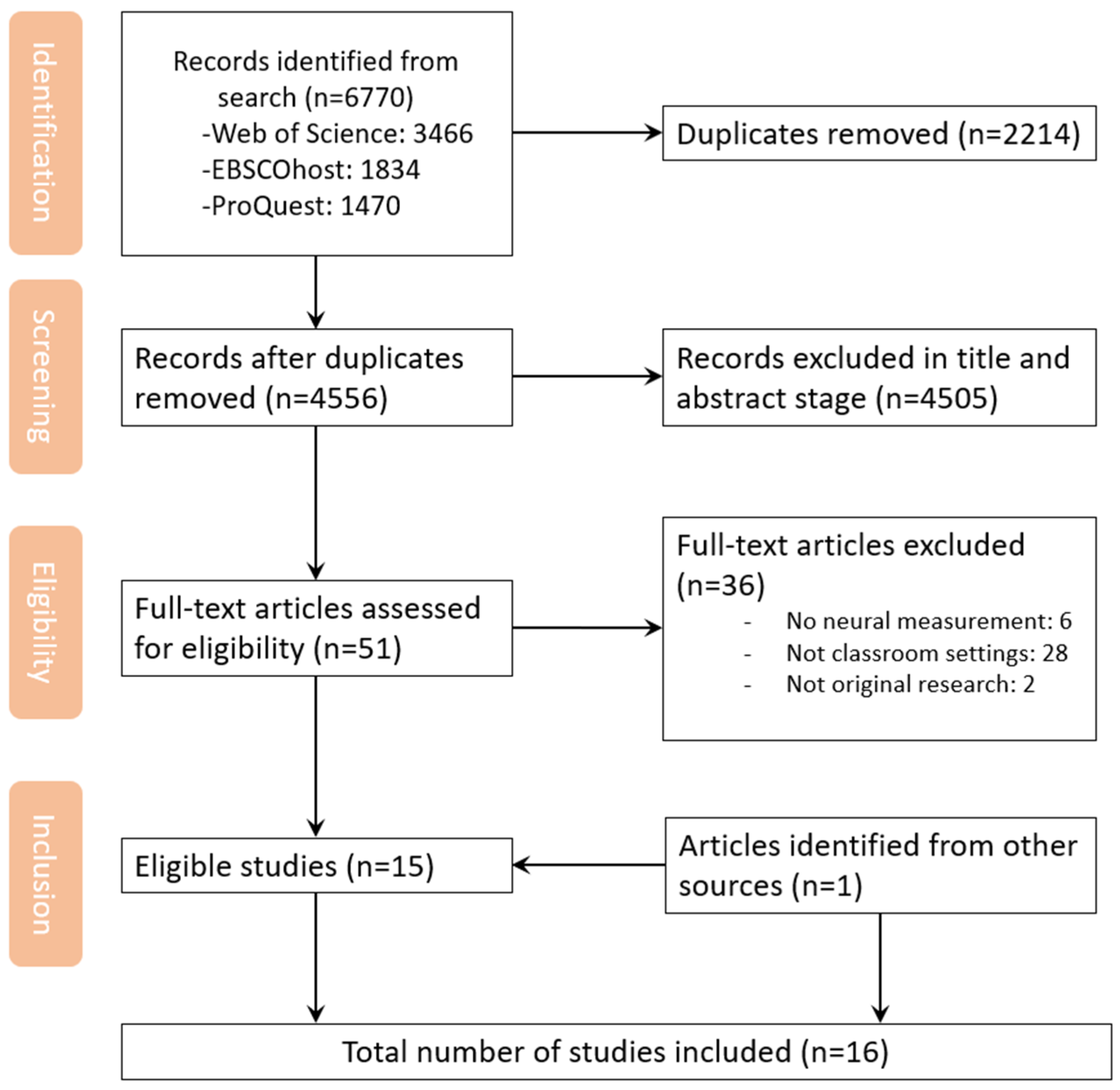
3.1. Search Results
3.2. Description of Included Studies
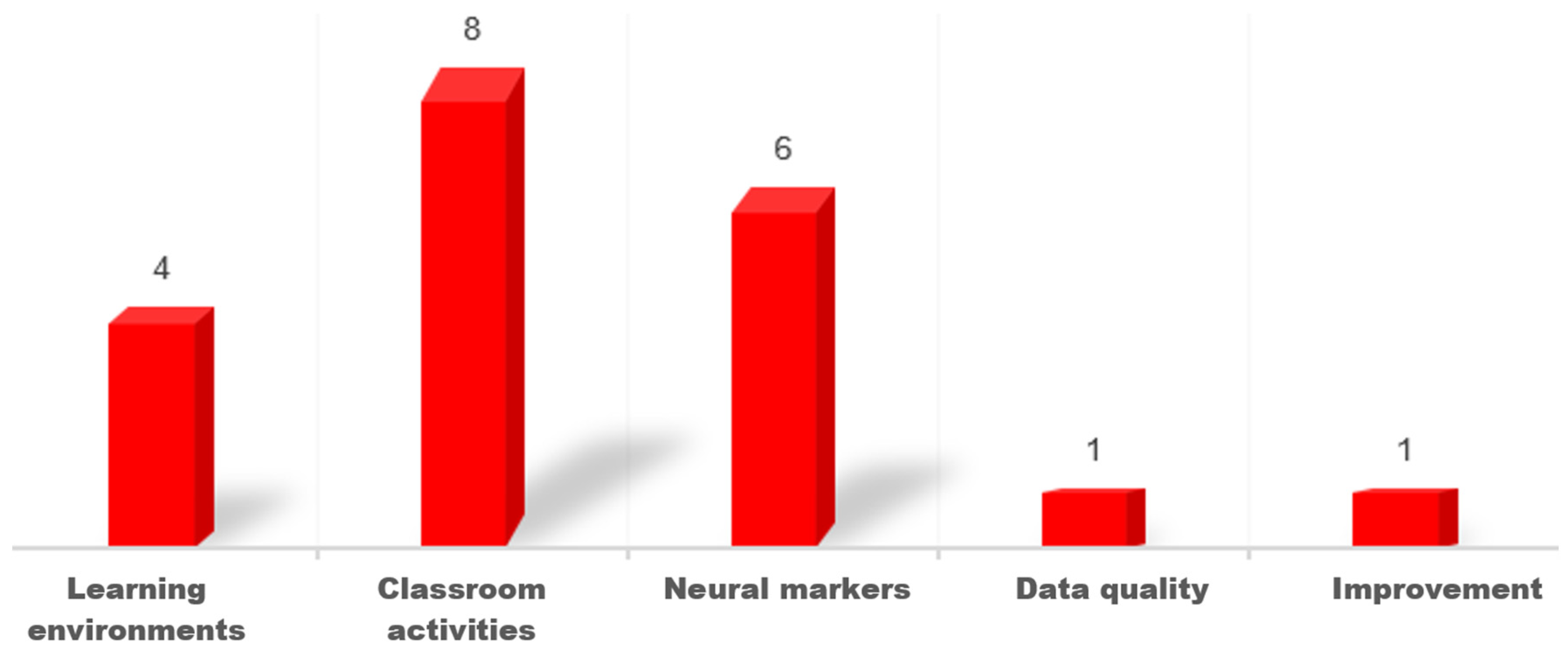
3.3. What Objectives and Outcomes Are Reported in the Included Studies?
3.3.1. Examining Neural Markers of Students’ Attention in Classroom Environments
3.3.2. Comparison of Different Learning Environments on Neural Attention Levels
3.3.3. Comparison of Different Classroom Activities on Student Attention
3.3.4. Data Quality Examination in Classroom Environments
3.3.5. Using Biofeedback Technique to Improve Student Attention
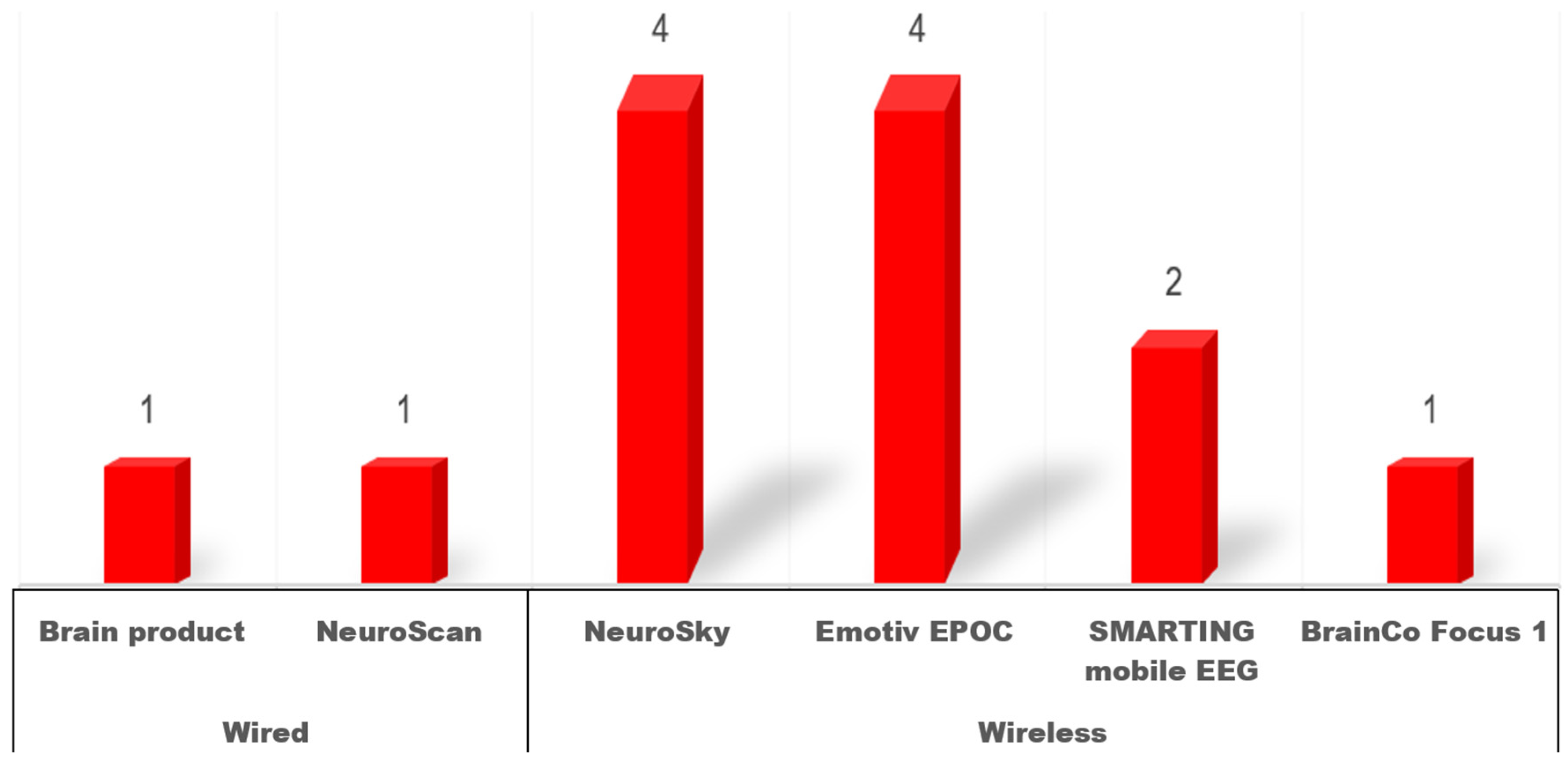
3.4. What Techniques and Devices Are Used in the Included Studies?
3.5. What Neural and Behavioral Measurements of Attention Are Reported in the Included Studies?
3.6. What Differences in Neural Activity Are Observed Across Various Classroom Activities in the Included Studies?
3.7. What Are the Educational Levels, Disciplinary Domains, Durations, and Sample Sizes of the Included Studies?
4. Discussion
4.1. Limitations Regarding Neural Measurements of Attention
4.2. Contradictory Findings
4.3. Lack of Verification
4.4. Methodological Limitations and EEG Device Heterogeneity
4.5. Limited Sample Sizes and Techniques
4.6. Future Directions
Funding
Acknowledgments
Conflicts of Interest
References
- Duncan, G.J.; Dowsett, C.J.; Claessens, A.; Magnuson, K.; Huston, A.C.; Klebanov, P.; Pagani, L.S.; Feinstein, L.; Engel, M.; Brooks-Gunn, J.; et al. School Readiness and Later Achievement. Dev. Psychol. 2007, 43, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.; Bavelier, D. The Role of Selective Attention on Academic Foundations: A Cognitive Neuroscience Perspective. Dev. Cogn. Neurosci. 2012, 2, S30–S48. [Google Scholar] [CrossRef]
- Doyle, W. Recent Research on Classroom Management. J. Teach. Educ. 1985, 36, 31–35. [Google Scholar] [CrossRef]
- Mundschenk, N.A.; Miner, C.A.; Nastally, B.L. Effective Classroom Management. Interv. Sch. Clin. 2011, 47, 103–198. [Google Scholar] [CrossRef]
- Banire, B.; Al Thani, D.; Qaraqe, M.; Mansoor, B.; Makki, M. Impact of Mainstream Classroom Setting on Attention of Children with Autism Spectrum Disorder: An Eye-Tracking Study. Univ. Access Inf. Soc. 2021, 20, 785–795. [Google Scholar] [CrossRef]
- Hanley, M.; Khairat, M.; Taylor, K.; Wilson, R.; Cole-Fletcher, R.; Riby, D.M. Classroom Displays—Attraction or Distraction? Evidence of Impact on Attention and Learning from Children with and without Autism. Dev. Psychol. 2017, 53, 1265–1275. [Google Scholar] [CrossRef]
- Young, M.; Robinson, S.; Alberts, P. Students Pay Attention! Act. Learn. High. Educ. 2009, 10, 41–55. [Google Scholar] [CrossRef]
- May, K.E.; Elder, A.D. Efficient, Helpful, or Distracting? A Literature Review of Media Multitasking in Relation to Academic Performance. Int. J. Educ. Technol. High. Educ. 2018, 15, 13. [Google Scholar] [CrossRef]
- Fisher, A.; Godwin, K.E.; Seltman, H. Visual Environment, Attention Allocation, and Learning in Young Children. Psychol. Sci. 2014, 25, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Fredricks, J.A.; Blumenfeld, P.C.; Paris, A.H. School Engagement: Potential of the Concept, State of the Evidence. Rev. Educ. Res. 2004, 74, 59–109. [Google Scholar] [CrossRef]
- Rimm-Kaufman, S.E.; La Paro, K.M.; Downer, J.T.; Pianta, R.C. The Contribution of Classroom Setting and Quality of Instruction to Children’s Behavior in Kindergarten Classrooms. Elem. Sch. J. 2005, 105, 377–394. [Google Scholar] [CrossRef]
- Heinsen, R. Gaze Behavior Reveals Automaticity and Attention Allocation during Music Teaching vs. Observing. J. Eye Mov. Res. 2024, 17, 1–19. [Google Scholar] [CrossRef]
- Madsen, J.; Júlio, S.U.; Gucik, P.J.; Steinberg, R.; Parra, L.C. Synchronized Eye Movements Predict Test Scores in Online Video Education. Proc. Natl. Acad. Sci. USA 2021, 118, e2016980118. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Liu, X.; Gan, X.; Zeng, H. Using Synchronized Eye Movements to Predict Attention in Online Video Learning. Educ. Sci. 2024, 14, 548. [Google Scholar] [CrossRef]
- Chen, G.; Ji, J.; Wang, T. A Study of Classroom Learning Attention Discrimination Method Based on Head Posture Detection. In Proceedings of the 2023 8th International Conference on Intelligent Computing and Signal Processing (ICSP), Xi’an, China, 21–23 April 2023; pp. 2124–2128. [Google Scholar] [CrossRef]
- Sümer, Ö.; Goldberg, P.; D’Mello, S.; Gerjets, P.; Trautwein, U.; Kasneci, E. Multimodal Engagement Analysis From Facial Videos in the Classroom. IEEE Trans. Affect. Comput. 2023, 14, 1012–1027. [Google Scholar] [CrossRef]
- Thao, L.Q.; Kien, D.T.; Bach, N.C.; Thi Thanh Thuy, D.; Thi Minh Thuy, L.; Cuong, D.D.; Hieu, N.H.M.; Dang, N.H.T.; Bach, P.X.; Hieu, L.P.M. Monitoring and Improving Student Attention Using Deep Learning and Wireless Sensor Networks. Sens. Actuators A Phys. 2024, 367, 115055. [Google Scholar] [CrossRef]
- Trabelsi, Z.; Alnajjar, F.; Parambil, M.M.A.; Gochoo, M.; Ali, L. Real-Time Attention Monitoring System for Classroom: A Deep Learning Approach for Student’s Behavior Recognition. Big Data Cogn. Comput. 2023, 7, 48. [Google Scholar] [CrossRef]
- Zaletelj, J.; Košir, A. Predicting Students’ Attention in the Classroom from Kinect Facial and Body Features. EURASIP J. Image Video Process. 2017, 2017, 80. [Google Scholar] [CrossRef]
- Guedj, C.; Tyrand, R.; Badier, E.; Planchamp, L.; Stringer, M.; Zimmermann, M.O.; Férat, V.; Ha-Vinh Leuchter, R.; Grouiller, F. Self-Regulation of Attention in Children in a Virtual Classroom Environment: A Feasibility Study. Bioengineering 2023, 10, 1352. [Google Scholar] [CrossRef]
- Sulaiman, N.; Ismail, N.; Islam, M.N.; Rashid, M.; Jadin, M.S.; Mustafa, M.; Samsuri, F. Development of EEG-Based System to Identify Student Learning Attention Ability. In Proceedings of the 12th National Technical Seminar on Unmanned System Technology 2020, Online, 27–28 October 2020; Lecture Notes in Electrical Engineering. Springer: Singapore, 2022; Volume 770, pp. 627–639, ISBN 9789811624056. [Google Scholar]
- Verma, D.; Bhalla, S.; Sai Santosh, S.V.; Yadav, S.; Parnami, A.; Shukla, J. AttentioNet: Monitoring Student Attention Type in Learning with EEG-Based Measurement System. In Proceedings of the 2023 11th International Conference on Affective Computing and Intelligent Interaction (ACII), Cambridge, MA, USA, 10–13 September 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 1–8. [Google Scholar]
- Landau, A.N.; Fries, P. Attention Samples Stimuli Rhythmically. Curr. Biol. 2012, 22, 1000–1004. [Google Scholar] [CrossRef]
- Sali, A.W.; Courtney, S.M.; Yantis, S. Spontaneous Fluctuations in the Flexible Control of Covert Attention. J. Neurosci. 2016, 36, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Nastase, S.A.; Goldstein, A.; Hasson, U. Keep It Real: Rethinking the Primacy of Experimental Control in Cognitive Neuroscience. NeuroImage 2020, 222, 117254. [Google Scholar] [CrossRef]
- Bruer, J.T. Education and the Brain: A Bridge Too Far. Educ. Res. 1997, 26, 4–16. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G.; Mendelsohn, A. Real-Life Neuroscience: An Ecological Approach to Brain and Behavior Research. Perspect. Psychol. Sci. 2019, 14, 841–859. [Google Scholar] [CrossRef]
- Tommerdahl, J. A Model for Bridging the Gap between Neuroscience and Education. Oxf. Rev. Educ. 2010, 36, 109–197. [Google Scholar] [CrossRef]
- Dikker, S.; Wan, L.; Davidesco, I.; Kaggen, L.; Oostrik, M.; McClintock, J.; Rowland, J.; Michalareas, G.; Van Bavel, J.J.; Ding, M.; et al. Brain-to-Brain Synchrony Tracks Real-World Dynamic Group Interactions in the Classroom. Curr. Biol. 2017, 27, 1375–1380. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, B. Review on Portable EEG Technology in Educational Research. Comput. Hum. Behav. 2018, 81, 340–349. [Google Scholar] [CrossRef]
- Janssen, T.W.P.; Grammer, J.K.; Bleichner, M.G.; Bulgarelli, C.; Davidesco, I.; Dikker, S.; Jasińska, K.K.; Siugzdaite, R.; Vassena, E.; Vatakis, A.; et al. Opportunities and Limitations of Mobile Neuroimaging Technologies in Educational Neuroscience. Mind Brain Educ. 2021, 15, 354–370. [Google Scholar] [CrossRef]
- Tenório, K.; Pereira, E.; Remigio, S.; Costa, D.; Oliveira, W.; Dermeval, D.; da Silva, A.P.; Bittencourt, I.I.; Marques, L.B. Brain-Imaging Techniques in Educational Technologies: A Systematic Literature Review. Educ. Inf. Technol. 2022, 27, 1183–1212. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Ma, F.; Cui, H.; Cheng, X.; Pan, Y. Interpersonal Educational Neuroscience: A Scoping Review of the Literature. Educ. Res. Rev. 2024, 42, 100593. [Google Scholar] [CrossRef]
- Tan, S.H.J.; Wong, J.N.; Teo, W.-P. Is Neuroimaging Ready for the Classroom? A Systematic Review of Hyperscanning Studies in Learning. NeuroImage 2023, 281, 120367. [Google Scholar] [CrossRef]
- Nouri, A. A Scoping Review of Educational Neurotechnology: Methods, Applications, Opportunities, and Challenges. Rev. Educ. 2025, 13, e70070. [Google Scholar] [CrossRef]
- Bevilacqua, D.; Davidesco, I.; Wan, L.; Chaloner, K.; Rowland, J.; Ding, M.; Poeppel, D.; Dikker, S. Brain-to-Brain Synchrony and Learning Outcomes Vary by Student–Teacher Dynamics: Evidence from a Real-World Classroom Electroencephalography Study. J. Cogn. Neurosci. 2019, 31, 401–411. [Google Scholar] [CrossRef]
- Dikker, S.; Haegens, S.; Bevilacqua, D.; Davidesco, I.; Wan, L.; Kaggen, L.; McClintock, J.; Chaloner, K.; Ding, M.; West, T.; et al. Morning Brain: Real-World Neural Evidence That High School Class Times Matter. Soc. Cogn. Affect. Neurosci. 2020, 15, 1193–1202. [Google Scholar] [CrossRef]
- Grammer, J.K.; Xu, K.; Lenartowicz, A. Effects of Context on the Neural Correlates of Attention in a College Classroom. npj Sci. Learn. 2021, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Torgrimson, S.J.; Torres, R.; Lenartowicz, A.; Grammer, J.K. EEG Data Quality in Real-World Settings: Examining Neural Correlates of Attention in School-Aged Children. Mind Brain Educ. 2022, 16, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Stangl, M.; Maoz, S.L.; Suthana, N. Mobile Cognition: Imaging the Human Brain in the ‘Real World’. Nat. Rev. Neurosci. 2023, 24, 347–362. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for Conducting Systematic Scoping Reviews. JBI Evid. Implement. 2015, 13, 141. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Zeng, H. Neural Correlates of Growth Mindset: A Scoping Review of Brain-Based Evidence. Brain Sci. 2025, 15, 200. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Chen, C.-M. Improving Effectiveness of Learners’ Review of Video Lectures by Using an Attention-Based Video Lecture Review Mechanism Based on Brainwave Signals. Interact. Learn. Environ. 2019, 27, 86–102. [Google Scholar] [CrossRef]
- Poulsen, A.T.; Kamronn, S.; Dmochowski, J.; Parra, L.C.; Hansen, L.K. EEG in the Classroom: Synchronised Neural Recordings during Video Presentation. Sci. Rep. 2017, 7, 43916. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Lamba, M.; Verma, K.; Khuttan, S.; Gautam, H. A Preliminary Investigation for Assessing Attention Levels for Massive Online Open Courses Learning Environment Using EEG Signals: An Experimental Study. Hum. Behav. Emerg. Technol. 2021, 3, 933–941. [Google Scholar] [CrossRef]
- Chen, J.; Xu, B.; Zhang, D. Inter-Brain Coupling Analysis Reveals Learning-Related Attention of Primary School Students. Educ. Technol. Res. Dev. 2024, 72, 541–555. [Google Scholar] [CrossRef]
- Davidesco, I.; Laurent, E.; Valk, H.; West, T.; Milne, C.; Poeppel, D.; Dikker, S. The Temporal Dynamics of Brain-to-Brain Synchrony Between Students and Teachers Predict Learning Outcomes. Psychol. Sci. 2023, 34, 633–643. [Google Scholar] [CrossRef]
- Dhindsa, K.; Acai, A.; Wagner, N.; Bosynak, D.; Kelly, S.; Bhandari, M.; Petrisor, B.; Sonnadara, R.R. Individualized Pattern Recognition for Detecting Mind Wandering from EEG during Live Lectures. PLoS ONE 2019, 14, e0222276. [Google Scholar] [CrossRef]
- Horowitz-Kraus, T.; Heyd-Metzuyanim, E.; Zivan, M. Face-to-face Classroom Learning Produced Greater Brain Synchronisation in Children than a Zoom-based Online Session. Acta Paediatr. 2023, 112, 1266–1268. [Google Scholar] [CrossRef]
- Juárez-Varón, D.; Bellido-García, I.; Gupta, B.-B. Analysis of Stress, Attention, Interest, and Engagement in Onsite and Online Higher Education: A Neurotechnological Study. Comun. Rev. Científica de Comun. Y Educ. 2023, 31, 21–34. [Google Scholar] [CrossRef]
- Ko, L.-W.; Komarov, O.; Hairston, W.D.; Jung, T.-P.; Lin, C.-T. Sustained Attention in Real Classroom Settings: An EEG Study. Front. Hum. Neurosci. 2017, 11, 388. [Google Scholar] [CrossRef]
- Kosmyna, N.; Maes, P. AttentivU: An EEG-Based Closed-Loop Biofeedback System for Real-Time Monitoring and Improvement of Engagement for Personalized Learning. Sensors 2019, 19, 5200. [Google Scholar] [CrossRef]
- Sun, J.C.-Y. Influence of Polling Technologies on Student Engagement: An Analysis of Student Motivation, Academic Performance, and Brainwave Data. Comput. Educ. 2014, 72, 80–89. [Google Scholar] [CrossRef]
- Sun, J.C.-Y.; Chen, A.Y.-Z.; Yeh, K.P.-C.; Cheng, Y.-T.; Lin, Y.-Y. Is Group Polling Better? An Investigation of the Effect of Individual and Group Polling Strategies on Students’ Academic Performance, Anxiety, and Attention. Int. Forum Educ. Technol. Soc. 2018, 21, 12–24. [Google Scholar]
- Sun, J.C.-Y.; Hwang, G.-J.; Lin, Y.-Y.; Yu, S.-J.; Pan, L.-C.; Chen, A.Y.-Z. A Votable Concept Mapping Approach to Promoting Students’ Attentional Behavior. J. Educ. Technol. Soc. 2018, 21, 177–191. [Google Scholar]
- Janssen, T.W.P.; Van Atteveldt, N. Explore Your Brain: A Randomized Controlled Trial into the Effectiveness of a Growth Mindset Intervention with Psychosocial and Psychophysiological Components. Br. J. Edu. Psychol. 2025, 95, 280–302. [Google Scholar] [CrossRef] [PubMed]
- Egner, T.; Gruzelier, J.H. EEG Biofeedback of Low Beta Band Components: Frequency-Specific Effects on Variables of Attention and Event-Related Brain Potentials. Clin. Neurophysiol. 2004, 115, 131–139. [Google Scholar] [CrossRef]
- Kober, S.; Schweiger, D.; Reichert, J.; Neuper, C.; Wood, G. Upper Alpha Based Neurofeedback Training in Chronic Stroke: Brain Plasticity Processes and Cognitive Effects. Appl. Psychophysiol. Biofeedback 2017, 42, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Segalowitz, S.J.; Santesso, D.L.; Jetha, M.K. Electrophysiological Changes during Adolescence: A Review. Brain Cogn. 2010, 72, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Marcuse, L.V.; Schneider, M.; Mortati, K.A.; Donnelly, K.M.; Arnedo, V.; Grant, A.C. Quantitative Analysis of the EEG Posterior-Dominant Rhythm in Healthy Adolescents. Clin. Neurophysiol. 2008, 119, 1778–1781. [Google Scholar] [CrossRef]
- Schanzenbach, D.W. Limitations of Experiments in Education Research. Educ. Financ. Policy 2012, 7, 219–232. [Google Scholar] [CrossRef]
- Brockington, G.; Balardin, J.B.; Zimeo Morais, G.A.; Malheiros, A.; Lent, R.; Moura, L.M.; Sato, J.R. From the Laboratory to the Classroom: The Potential of Functional Near-Infrared Spectroscopy in Educational Neuroscience. Front. Psychol. 2018, 9, 1840. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, T.; Sakaki, K.; Ikeda, S.; Jeong, H.; Yamazaki, S.; Kawata, K.H.D.S.; Kawata, N.Y.D.S.; Sasaki, Y.; Kulason, K.; Hirano, K.; et al. Prior Physical Synchrony Enhances Rapport and Inter-Brain Synchronization during Subsequent Educational Communication. Sci. Rep. 2019, 9, 12747. [Google Scholar] [CrossRef] [PubMed]
- León, F.; Szanto, T.; Zahavi, D. Emotional Sharing and the Extended Mind. Synthese 2019, 196, 4847–4867. [Google Scholar] [CrossRef]
- Christou, A.I.; Fanti, K.; Mavrommatis, I.; Soursou, G. Parent–Child Eye Gaze Congruency to Emotional Expressions Mediated by Child Aesthetic Sensitivity. Children 2025, 12, 839. [Google Scholar] [CrossRef]
- Thompson, K.I.; Schneider, C.J.; Rocha-Hidalgo, J.; Jeyaram, S.; Mata-Centeno, B.; Furtado, E.; Vachhani, S.; Pérez-Edgar, K.; Perlman, S.B. Constructing the “Family Personality”: Can Family Functioning Be Linked to Parent–Child Interpersonal Neural Synchronization? J. Personal. 2025, 93, 755–766. [Google Scholar] [CrossRef] [PubMed]
| Criterion | Inclusion | Exclusion |
|---|---|---|
| Article type | Original research, including peer-reviewed journal articles, dissertations, and conference papers | Articles that were not original research (e.g., literature reviews, meta-analyses, editorials, book reviews, or reports) |
| Literature focus | Investigate the neural activity/neural mechanism/brain signal of attention/engagement in face-to-face classroom settings | Behavioral studies, pure online learning/e-learning studies, research topics unrelated to attention |
| Language | English | Non-English |
| Publication date | 2001–2024 | Studies outside this period |
| Age group | All age groups | - |
| Population type | Healthy participants | Participants with neural conditions (e.g., ADHD) |
| Education level | Kindergarten, K–12, higher education, and professional training | - |
| Disciplinary field | All fields | - |
| Characteristics | Information | Relevant RQ |
|---|---|---|
| Author | Name of the first author | Publication characteristics |
| Publication year | The time when the article was published | Publication characteristics |
| Region | The place where the research was conducted | Publication characteristics |
| Publication type | Peer-reviewed journal article, dissertation, or conference paper | Publication characteristics |
| Objectives | Purpose of the selected study | RQ1 |
| Outcomes | Outcomes of the selected study | RQ1 |
| Technique and device | Electroencephalogram (EEG), functional near-infrared spectroscopy (fNIRS), or other techniques and their devices | RQ2 |
| Neural measurement of attention | Neural indicator to measure attention level | RQ3 |
| Behavioral measurement of attention | Whether included behavioral measurement of attention. If yes, how? | RQ3 |
| Classroom activities | Lectures, videos, group discussion, polling, etc. Neural correlates of attention during classroom activities | RQ4 |
| Education level | Kindergarten, K–12, higher education, or professional training | RQ5 |
| Disciplinary field | Disciplinary fields | RQ5 |
| Sample size | Sample sizes of neural data collected | RQ5 |
| Duration | Number of sessions and duration of each session | RQ5 |
| No | Authors | Year | Publication Type | Technique | Device | Channel |
|---|---|---|---|---|---|---|
| 1 | Aggarwal et al. [47] | 2021 | Journal paper | EEG | Neurosky Mindwave | 1 |
| 2 | Bevilacqua et al. [36] | 2019 | Journal paper | EEG | Emotiv EPOC | 14 * |
| 3 | Chen et al. [48] | 2024 | Journal paper | EEG | NeuroSky | 1 |
| 4 | Davidesco et al. [49] | 2023 | Journal paper | EEG | Neuroelectrics | 32 |
| 5 | Dhindsa et al. [50] | 2019 | Journal paper | EEG | Not reported | 16 |
| 6 | Dikker et al. [29] | 2017 | Journal paper | EEG | Emotiv EPOC | 14 * |
| 7 | Dikker et al. [37] | 2020 | Journal paper | EEG | Emotiv EPOC | 14 |
| 8 | Grammer et al. [38] | 2021 | Journal paper | EEG | SMARTING mobile EEG | 24 |
| 9 | Horowitz-Kraus et al. [51] | 2023 | Journal paper | EEG | Not reported | Not reported |
| 10 | Juárez-Varón et al. [52] | 2023 | Journal paper | EEG | Emotiv EPOC | 14 |
| 11 | Ko et al. [53] | 2017 | Journal paper | EEG | NeuroScan | 32 |
| 12 | Kosmyna et al. [54] | 2019 | Journal paper | EEG | BrainCo Focus 1 | 1 |
| 13 | Sun [55] | 2014 | Journal paper | EEG | Not reported | Not reported |
| 14 | Sun, Chen et al. [56] | 2018 | Journal paper | EEG | NeuroSky | 1 |
| 15 | Sun, Hwang, et al. [57] | 2018 | Journal paper | EEG | NeuroSky | 1 |
| 16 | Xu et al. [39] | 2022 | Journal paper | EEG | Study 2: SMARTING mobile EEG | 24 |
| No. | Author | Classroom Activities | Neural Activities for Different Classroom Activities |
|---|---|---|---|
| 2 | Bevilacqua et al. [36] | Videos and lectures | Student-to-group synchrony, student–teacher synchrony, and engagement were all higher for videos as compared with lectures. |
| 6 | Dikker et al. (2017) [29] | Videos, lectures, and group discussions, and the teacher reading aloud | Student-to-group synchrony was higher for video and group discussions than lecture sessions. |
| 7 | Dikker et al. (2020) [37] | School 1: Videos, lectures, group discussions, and the teacher reading aloud; School 2: Videos and lectures | Lower alpha power during videos compared to lectures. Theta power did not vary by class activity. |
| 8 | Grammer et al. [38] | Teacher-led activities (videos and lectures) and student-led activities (group work and independent work) | Higher alpha power over the occipital cortex during video watching compared to lectures and student-led activities. Lower alpha, higher beta, and higher gamma power during student-led activities than teacher-led activities. |
| 13 | Sun [55] | Lectures and polling (clickers and mobile polling) | Students’ brainwave data related to attention increased during polling activities as opposed to the lecture in general. |
| 14 | Sun, Chen et al. [56] | Lectures, polling, concept mapping drawing, votable concept mapping | The attentional neural activities of the three students varied across different types of instructional methods and activities. |
| 15 | Sun, Hwang, et al. [57] | Lectures and polling (clickers, group polling, group polling with competition) | The attentional neural activities of the three students varied across different types of instructional methods and activities. |
| 16 | Xu et al. [39] | Mindfulness, teacher-led activities (lecture), and student-led activities (card games played in pairs and crafting activities done individually) | Alpha power was highest during mindfulness. No significant difference between teacher-led and student-led activities. |
| No. | Author | Neural Measurement | Behavioral Measurement |
|---|---|---|---|
| 1 | Aggarwal et al. [47] | Self-trained classification model based on eight frequency bands (high α, mid α, low α, high β, low β, γ, θ, and δ) of the EEG signal and R1: (low α/low β), R2: (high α/ high β) | Students’ self-feedback about their state of mind as seen in the video and other distractions like losing eye contact with the teacher or teaching board were also considered |
| 2 | Bevilacqua et al. [36] | Brain-to-brain synchrony (student-to-group synchrony, student–teacher synchrony) at F3, F4, P7, P8, O1, and O2 sites | 1–7 Likert engagement rating by students |
| 3 | Chen et al. [48] | Brain-to-brain synchrony (student-to-group synchrony, based on attention level provided by NeuroSky) | - |
| 4 | Davidesco et al. [49] | Brain-to-brain synchrony (based on phase) at center, frontal, and posterior electrodes | - |
| 5 | Dhindsa et al. [50] | Self-trained classification model based on four frequency (θ, α, β1, βa2) bands of the EEG signal | Thought probes |
| 6 | Dikker et al. (2017) [29] | Brain-to-brain synchrony (student-to-group synchrony) at F3, F4, P7, P8, O1, and O2 sites | 1–7 Likert engagement rating by students |
| 7 | Dikker et al. (2020) [37] | α power (highest local maximum) at all occipital electrodes | Self-reported focus scores |
| 8 | Grammer et al. [38] | α power on Pz, POz, O1, and O2 electrodes (β, θ, δ power reported in the supplementary information) | Observer ratings based on behavioral cues, including body positioning, eye gaze, and activity engagement in 1 min intervals |
| 9 | Horowitz-Kraus et al. [51] | Brain-to-brain synchrony (teacher-students synchrony) at α and β frequency at frontal electrodes (AF3, AF4, F4, F3, F8, and F7) | - |
| 10 | Juárez-Varón et al. [52] | Not reported | - |
| 11 | Ko et al. [53] | α, β, θ, δ power | Sustained attention task |
| 12 | Kosmyna et al. [54] | Index E = β/(α + θ) | Self-report engagement score |
| 13 | Sun [55] | Not reported | - |
| 14 | Sun, Chen et al. [56] | Attention level provided by NeuroSky | Three observers coded the attentional behavior of the participants |
| 15 | Sun, Hwang, et al. [57] | Attention level provided by NeuroSky | - |
| 16 | Xu et al. [39] | α power on Pz, POz, O1, and O2 electrodes (β, θ, δ power reported in the supplementary information) | - |
| No. | Authors | Education Level | Disciplinary Domain | Duration | Sample Size |
|---|---|---|---|---|---|
| 1 | Aggarwal et al. [47] | Higher education | Machine learning | 1 session of 15–20 min | 12 |
| 2 | Bevilacqua et al. [36] | High school | Biology | 6 sessions of 80 min | 12 |
| 3 | Chen et al. [48] | Primary school | Brain sciences | 5 sessions of 20 min | 24 |
| 4 | Davidesco et al. [49] | High school and higher education | Biology and Chemistry | 4 sessions of 7-min | 31 |
| 5 | Dhindsa et al. [50] | Higher education | Medicine | 2 sessions of 30 min | 15 |
| 6 | Dikker et al. [29] | High school | Biology | 11 sessions of 50 min | 12 |
| 7 | Dikker et al. [37] | High school | Biology | School 1: same as Study 5; School 2: same as Study 2 | 22 |
| 8 | Grammer et al. [38] | Higher education | Educational neuroscience | 1 session of 40–60 min | 23 |
| 9 | Horowitz-Kraus et al. [51] | Primary school | Science | 2 sessions of 15 min | 6 |
| 10 | Juárez-Varón et al. [52] | Higher education | Consumer behavior | 5 sessions of 45 min | 20 |
| 11 | Ko et al. [53] | Higher education | Not reported | 2–8 sessions of 50 min (mean 5.4 sessions) | 18 |
| 12 | Kosmyna et al. [54] | Higher education | VR | 3 sessions of 40–50 min | 12 |
| 13 | Sun [55] | Higher education | Educational research methods and sociology of education | 16 sessions | 32 |
| 14 | Sun, Chen et al. [56] | Higher education | Educational research methodology | 3 sessions of 100 min | 3 |
| 15 | Sun, Hwang, et al. [57] | Higher education | Not reported | 6 sessions of 100 min | 3 |
| 16 | Xu et al. [39] | kindergarten to 4th-grade students | Neuroscience | 3 sessions of 24 min | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; Huang, X.; Liu, Y.; Gu, X. Exploring Neural Evidence of Attention in Classroom Environments: A Scoping Review. Brain Sci. 2025, 15, 860. https://doi.org/10.3390/brainsci15080860
Zeng H, Huang X, Liu Y, Gu X. Exploring Neural Evidence of Attention in Classroom Environments: A Scoping Review. Brain Sciences. 2025; 15(8):860. https://doi.org/10.3390/brainsci15080860
Chicago/Turabian StyleZeng, Hang, Xinmei Huang, Yelin Liu, and Xiaojing Gu. 2025. "Exploring Neural Evidence of Attention in Classroom Environments: A Scoping Review" Brain Sciences 15, no. 8: 860. https://doi.org/10.3390/brainsci15080860
APA StyleZeng, H., Huang, X., Liu, Y., & Gu, X. (2025). Exploring Neural Evidence of Attention in Classroom Environments: A Scoping Review. Brain Sciences, 15(8), 860. https://doi.org/10.3390/brainsci15080860






