Advances in Research on Brain Structure and Activation Characteristics in Patients with Anterior Cruciate Ligament Reconstruction: A Systematic Review
Abstract
1. Introduction
- (1)
- Typical patterns of structural brain remodeling and alterations in functional brain activation following ACLR;
- (2)
- The relationship between neuroadaptive changes and subsequent clinical functional outcomes;
- (3)
- Methodological limitations within the field of neurorehabilitation and their implications for translational applications.
2. Methods
2.1. Study Design
2.2. Search Methods
2.3. Eligibility Criteria
2.4. Selection Process
2.5. Extraction of Data
2.6. Risk of Bias Assessment
2.7. Statistical Analysis
3. Results
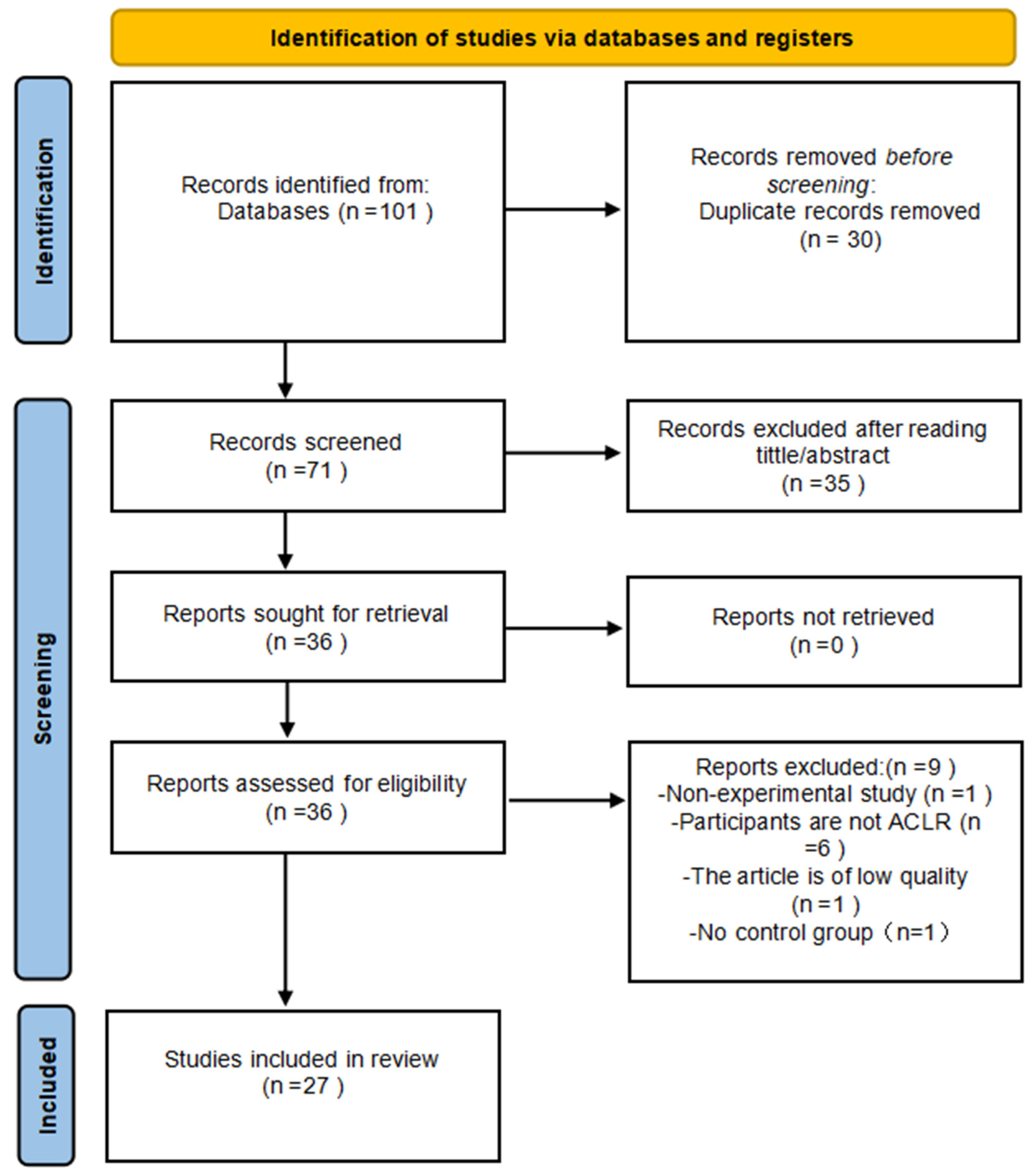
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Synthesis of Findings
- (1)
- Methodological diversity: Varied neuroimaging techniques (e.g., EEG, TMS, fMRI, fNIRS) measured fundamentally distinct neural phenomena;
- (2)
- Outcome metric inconsistency: Studies reported non-comparable units (e.g., % signal change vs. threshold values);
- (3)
- Clinical heterogeneity: Substantial variations in patient characteristics (38% unreported graft types; postoperative duration range: 1.5 months to 5.5 years) and control group designs (healthy controls vs. within-subject uninjured limbs).
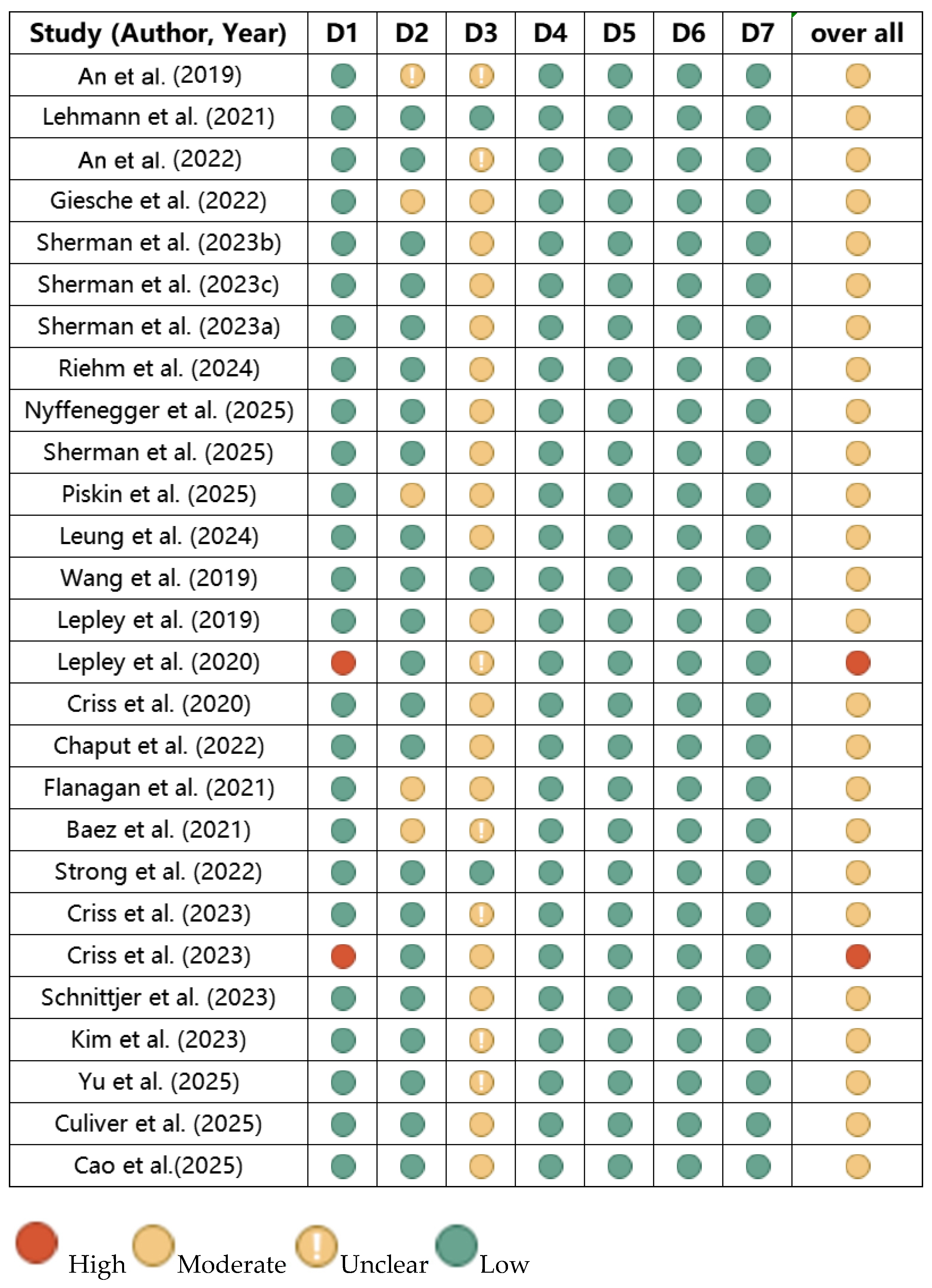
3.4. Risk of Bias Assessment of the Included Literature
4. Discussion
4.1. Characteristics of Cortical Activity Changes in ACLR
4.1.1. Altered Somatosensory Motor Cortex Activity
4.1.2. Increased Visual Cortex Activation
4.1.3. Diminished Activation Efficiency in the Motor Cortex
4.2. Cortical Activation Under Different States
4.2.1. Characteristics of Cortical Activation During Motor Actions
4.2.2. Characteristics of Cortical Activation During Resting Condition
4.2.3. Cortical Activation Related to Emotion
4.3. Brain Structural Changes
4.4. Changes in Neural Activity Patterns After ACLR and Their Implications
4.4.1. The Impact of Postoperative Time and Graft Type on Neural Activation Patterns
4.4.2. The Association Between Brain Activity Alterations and Clinical Functional Outcomes
4.5. Limitations and Future Directions
4.6. Recommendations in Rehabilitation Therapy
5. Conclusions
- (1)
- Alterations in sensory cortex activation, augmented visual cortex activation, and diminished efficiency of motor cortex activation: Patients exhibit an increased dependence on visual input for motor control during task execution.
- (2)
- Modifications in neural network connectivity: Augmented connectivity among the prefrontal, parietal, and occipital lobes, yet diminished cognitive–motor efficiency. Attenuated inhibitory function of the emotion-related default network, coupled with heightened activation in limbic system regions.
- (3)
- Diminished volume of the corticospinal tract corresponding to the injured limb and reduced thickness of the sensorimotor cortex: These structural alterations further compromise the patient’s motor function.
- (4)
- Augmented activation in sensory integration areas and visual cognition-related regions among ACLR patients, correlated with enhanced lower limb function: This suggests that compensatory alterations in brain activation post-ACLR partially facilitate functional recovery.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mall, N.A.; Chalmers, P.N.; Moric, M.; Tanaka, M.J.; Cole, B.J.; Bach, B.R.; Paletta, G.A. Incidence and Trends of Anterior Cruciate Ligament Reconstruction in the United States. Am. J. Sports Med. 2014, 42, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Banios, K.; Raoulis, V.; Fyllos, A.; Chytas, D.; Mitrousias, V.; Zibis, A. Anterior and Posterior Cruciate Ligaments Mechanoreceptors: A Review of Basic Science. Diagnostics 2022, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, M.S.; Bali, K.; Prabhakar, S. Proprioception in Anterior Cruciate Ligament Deficient Knees and Its Relevance in Anterior Cruciate Ligament Reconstruction. Indian J. Orthop. 2011, 45, 294–300. [Google Scholar] [CrossRef]
- Hewett, T.E.; Di Stasi, S.L.; Myer, G.D. Current Concepts for Injury Prevention in Athletes after Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2013, 41, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.; Pereira, R.; van Cingel, R.; Staal, J.B.; Espregueira-Mendes, J. How Should Clinicians Rehabilitate Patients after ACL Reconstruction? A Systematic Review of Clinical Practice Guidelines (CPGs) with a Focus on Quality Appraisal (AGREE II). Br. J. Sports Med. 2020, 54, 512–519. [Google Scholar] [CrossRef]
- Frobell, R.B.; Roos, H.P.; Roos, E.M.; Roemer, F.W.; Ranstam, J.; Lohmander, L.S. Treatment for Acute Anterior Cruciate Ligament Tear: Five Year Outcome of Randomised Trial. Br. J. Sports Med. 2015, 49, 700. [Google Scholar] [CrossRef]
- Paterno, M.V.; Rauh, M.J.; Schmitt, L.C.; Ford, K.R.; Hewett, T.E. Incidence of Contralateral and Ipsilateral Anterior Cruciate Ligament (ACL) Injury After Primary ACL Reconstruction and Return to Sport. Clin. J. Sport Med. 2012, 22, 116–121. [Google Scholar] [CrossRef]
- Ifran, N.N.; Mok, Y.R.; Krishna, L. Tear Rates of the Ipsilateral ACL Graft and the Contralateral Native ACL Are Similar Following ACL Reconstruction. J. Knee Surg. 2022, 35, 308–311. [Google Scholar] [CrossRef]
- Culvenor, A.G.; Eckstein, F.; Wirth, W.; Lohmander, L.S.; Frobell, R. Loss of Patellofemoral Cartilage Thickness over 5 Years Following ACL Injury Depends on the Initial Treatment Strategy: Results from the KANON Trial. Br. J. Sports Med. 2019, 53, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- van Meer, B.L.; Meuffels, D.E.; van Eijsden, W.A.; Verhaar, J.A.N.; Bierma-Zeinstra, S.M.A.; Reijman, M. Which Determinants Predict Tibiofemoral and Patellofemoral Osteoarthritis after Anterior Cruciate Ligament Injury? A Systematic Review. Br. J. Sports Med. 2015, 49, 975–983. [Google Scholar] [CrossRef]
- Lepley, A.S.; Kuenze, C.M. Hip and Knee Kinematics and Kinetics During Landing Tasks After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. J. Athl. Train. 2018, 53, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.; Rine, R.M.; Kroll, P. Central Somatosensory Changes and Altered Muscle Synergies in Subjects with Anterior Cruciate Ligament Deficiency. Gait Posture 2005, 22, 69–74. [Google Scholar] [CrossRef]
- Slater, L.V.; Hart, J.M.; Kelly, A.R.; Kuenze, C.M. Progressive Changes in Walking Kinematics and Kinetics After Anterior Cruciate Ligament Injury and Reconstruction: A Review and Meta-Analysis. J. Athl. Train. 2017, 52, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.L.; Felix, E.C.R.; Bessa, F.; Luna, N.M.; Sugimoto, D.; Greve, J.M.D.; Hernandez, A.J. Evaluation of Static and Dynamic Balance in Athletes with Anterior Cruciate Ligament Injury—A Controlled Study. Clinics 2016, 71, 425–429. [Google Scholar] [CrossRef]
- Butler, R.J.; Minick, K.I.; Ferber, R.; Underwood, F. Gait Mechanics after ACL Reconstruction: Implications for the Early Onset of Knee Osteoarthritis. Br. J. Sports Med. 2009, 43, 366–370. [Google Scholar] [CrossRef]
- Palmieri-Smith, R.M.; Thomas, A.C. A Neuromuscular Mechanism of Posttraumatic Osteoarthritis Associated with ACL Injury. Exerc. Sport Sci. Rev. 2009, 37, 147–153. [Google Scholar] [CrossRef]
- Chaudhari, A.M.W.; Briant, P.L.; Bevill, S.L.; Koo, S.; Andriacchi, T.P. Knee Kinematics, Cartilage Morphology, and Osteoarthritis after ACL Injury. Med. Sci. Sports Exerc. 2008, 40, 215–222. [Google Scholar] [CrossRef]
- Swanik, C.B.; Lephart, S.M.; Giannantonio, F.P.; Fu, F.H. Reestablishing Proprioception and Neuromuscular Control in the ACL-Injured Athlete. J. Sport Rehabil. 1997, 6, 182–206. [Google Scholar] [CrossRef]
- Grooms, D.; Appelbaum, G.; Onate, J. Neuroplasticity Following Anterior Cruciate Ligament Injury: A Framework for Visual-Motor Training Approaches in Rehabilitation. J. Orthop. Sports Phys. Ther. 2015, 45, 381–393. [Google Scholar] [CrossRef]
- Nashner, L.; Berthoz, A. Visual Contribution to Rapid Motor Responses during Postural Control. Brain Res. 1978, 150, 403–407. [Google Scholar] [CrossRef]
- Georgiev, G.P.; Yordanov, Y.; Gaydarski, L.; Tubbs, R.S.; Olewnik, Ł.; Zielinska, N.; Piagkou, M.; Ananiev, J.; Dimitrova, I.N.; Slavchev, S.A.; et al. Are There Any Differences in the Healing Capacity between the Medial Collateral Ligament’s (MCL) Proximal and Distal Parts in the Human Knee? Quantitative and Immunohistochemical Analysis of CD34, α-Smooth Muscle Actin (α-SMA), and Vascular Endothelial Growth Factor (VEGF) Expression Regarding the Epiligament (EL) Theory. Biomedicines 2024, 12, 659. [Google Scholar] [CrossRef]
- Georgiev, G.P.; Yordanov, Y.; Olewnik, Ł.; Tubbs, R.S.; LaPrade, R.F.; Ananiev, J.; Slavchev, S.A.; Dimitrova, I.N.; Gaydarski, L.; Landzhov, B. Do the Differences in the Epiligament of the Proximal and Distal Parts of the Anterior Cruciate Ligament Explain Their Different Healing Capacities? Quantitative and Immunohistochemical Analysis of CD34 and α-SMA Expression in Relation to the Epiligament Theory. Biomedicines 2024, 12, 156. [Google Scholar] [CrossRef]
- Georgiev, G.P.; Gaydarski, L.; Landzhov, B. Should We Accept the Epiligament Theory About the Differences in the Healing Potential of the Medial Collateral and the Anterior Cruciate Ligament? Biomedicines 2025, 13, 522. [Google Scholar] [CrossRef] [PubMed]
- Pietrosimone, B.G.; Lepley, A.S.; Ericksen, H.M.; Clements, A.; Sohn, D.H.; Gribble, P.A. Neural Excitability Alterations After Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2015, 50, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Grooms, D.R.; Diekfuss, J.A.; Slutsky-Ganesh, A.B.; DiCesare, C.A.; Bonnette, S.; Riley, M.A.; Kiefer, A.W.; Wohl, T.R.; Criss, C.R.; Lamplot, J.; et al. Preliminary Report on the Train the Brain Project, Part II: Neuroplasticity of Augmented Neuromuscular Training and Improved Injury-Risk Biomechanics. J. Athl. Train. 2022, 57, 911–920. [Google Scholar] [CrossRef] [PubMed]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat.-Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. Br. Med. J. 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without Meta-Analysis (SWiM) in Systematic Reviews: Reporting Guideline. Br. Med. J. 2020, 368, l6890. [Google Scholar] [CrossRef]
- An, Y.W.; Lobacz, A.D.; Lehmann, T.; Baumeister, J.; Rose, W.C.; Higginson, J.S.; Rosen, J.; Swanik, C.B. Neuroplastic Changes in Anterior Cruciate Ligament Reconstruction Patients from Neuromechanical Decoupling. Scand. J. Med. Sci. Sports 2019, 29, 251–258. [Google Scholar] [CrossRef]
- Lehmann, T.; Büchel, D.; Mouton, C.; Gokeler, A.; Seil, R.; Baumeister, J. Functional Cortical Connectivity Related to Postural Control in Patients Six Weeks After Anterior Cruciate Ligament Reconstruction. Front. Hum. Neurosci. 2021, 15, 655116. [Google Scholar] [CrossRef]
- An, Y.W.; Kang, Y.; Jun, H.-P.; Chang, E. Anterior Cruciate Ligament Reconstructed Patients Who Recovered Normal Postural Control Have Dissimilar Brain Activation Patterns Compared to Healthy Controls. Biology 2022, 11, 119. [Google Scholar] [CrossRef] [PubMed]
- Giesche, F.; Vieluf, S.; Wilke, J.; Engeroff, T.; Niederer, D.; Banzer, W. Cortical Motor Planning and Biomechanical Stability During Unplanned Jump Landings in Men With Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2022, 57, 547–556. [Google Scholar] [CrossRef]
- Sherman, D.A.; Baumeister, J.; Stock, M.S.; Murray, A.M.; Bazett-Jones, D.M.; Norte, G.E. Inhibition of Motor Planning and Response Selection after Anterior Cruciate Ligament Reconstruction. Med. Sci. Sports Exerc. 2023, 55, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.A.; Baumeister, J.; Stock, M.S.; Murray, A.M.; Bazett-Jones, D.M.; Norte, G.E. Weaker Quadriceps Corticomuscular Coherence in Individuals after ACL Reconstruction during Force Tracing. Med. Sci. Sports Exerc. 2023, 55, 625–632. [Google Scholar] [CrossRef]
- Sherman, D.A.; Baumeister, J.; Stock, M.S.; Murray, A.M.; Bazett-Jones, D.M.; Norte, G.E. Brain Activation and Single-Limb Balance Following Anterior Cruciate Ligament Reconstruction. Neurophysiol. Clin. 2023, 149, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Riehm, C.D.; Bonnette, S.; Rush, J.L.; Diekfuss, J.A.; Koohestani, M.; Myer, G.D.; Norte, G.E.; Sherman, D.A. Corticomuscular Cross-Recurrence Analysis Reveals between-Limb Differences in Motor Control among Individuals with ACL Reconstruction. Exp. Brain Res. 2024, 242, 355–365. [Google Scholar] [CrossRef]
- Nyffenegger, D.; Baur, H.; Henle, P.; Busch, A. Cortical Activity during the First 4 Months after Anterior Cruciate Ligament Reconstruction While Performing an Active Knee Joint Position Sense Test: A Pilot Study. Knee 2025, 55, 168–178. [Google Scholar] [CrossRef]
- Sherman, D.A.; Beausejour, J.P.; Koohestani, M.; Stock, M.S.; Norte, G.E. Quadriceps Motor Unit Properties Following ACL Reconstruction Are Associated with Corticospinal Excitability and Motor Cortex Activations. J. Appl. Physiol. 2025, 138, 1011–1023. [Google Scholar] [CrossRef]
- Piskin, D.; Cobani, G.; Lehmann, T.; Büchel, D.; Baumeister, J. Cortical Changes Associated with an Anterior Cruciate Ligament Injury May Retrograde Skilled Kicking in Football: Preliminary EEG Findings. Sci. Rep. 2025, 15, 2208. [Google Scholar] [CrossRef]
- Leung, A.; Kantak, S.; Hammoud, S.; Abraham, R.; Zarzycki, R. Sex Differences in Corticospinal Excitability and Quadriceps Performance after ACL Reconstruction. J. Orthop. Res. 2024, 42, 769–776. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, H.; Wang, X.B.; Zhang, Y.Q.; Wang, S.Q.; Shi, Y.Q.; Zhang, M.; Zhao, X.H. A preliminary study on resting-state functional magnetic resonance imaging of brain after anterior cruciate ligament preservation reconstruction with autologous tendon. Chin. Med. J. 2019, 99, 1479–1483. [Google Scholar] [CrossRef]
- Lepley, A.S.; Grooms, D.R.; Burland, J.P.; Davi, S.M.; Kinsella-Shaw, J.M.; Lepley, L.K. Quadriceps Muscle Function Following Anterior Cruciate Ligament Reconstruction: Systemic Differences in Neural and Morphological Characteristics. Exp. Brain Res. 2019, 237, 1267–1278. [Google Scholar] [CrossRef]
- Lepley, A.S.; Ly, M.T.; Grooms, D.R.; Kinsella-Shaw, J.M.; Lepley, L.K. Corticospinal Tract Structure and Excitability in Patients with Anterior Cruciate Ligament Reconstruction: A DTI and TMS Study. NeuroImage Clin. 2020, 25, 102157. [Google Scholar] [CrossRef] [PubMed]
- Criss, C.R.; Onate, J.A.; Grooms, D.R. Neural Activity for Hip-Knee Control in Those with Anterior Cruciate Ligament Reconstruction: A Task-Based Functional Connectivity Analysis. Neurosci. Lett. 2020, 730, 134985. [Google Scholar] [CrossRef]
- Chaput, M.; Onate, J.A.; Simon, J.E.; Criss, C.R.; Jamison, S.; McNally, M.; Grooms, D.R. Visual Cognition Associated with Knee Proprioception, Time to Stability, and Sensory Integration Neural Activity after ACL Reconstruction. J. Orthop. Res. 2022, 40, 95–104. [Google Scholar] [CrossRef]
- Flanagan, S.D.; Proessl, F.; Dunn-Lewis, C.; Sterczala, A.J.; Connaboy, C.; Canino, M.C.; Beethe, A.Z.; Eagle, S.R.; Szivak, T.K.; Onate, J.A.; et al. Differences in Brain Structure and Theta Burst Stimulation-Induced Plasticity Implicate the Corticomotor System in Loss of Function after Musculoskeletal Injury. J. Neurophysiol. 2021, 125, 1006–1021. [Google Scholar] [CrossRef]
- Baez, S.; Andersen, A.; Andreatta, R.; Cormier, M.; Gribble, P.A.; Hoch, J.M. Neuroplasticity in Corticolimbic Brain Regions in Patients After Anterior Cruciate Ligament Reconstruction. J. Athl. Train. 2021, 56, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.; Grip, H.; Boraxbekk, C.-J.; Selling, J.; Häger, C.K. Brain Response to a Knee Proprioception Task Among Persons with Anterior Cruciate Ligament Reconstruction and Controls. Front. Hum. Neurosci. 2022, 16, 841874. [Google Scholar] [CrossRef] [PubMed]
- Criss, C.R.; Lepley, A.S.; Onate, J.A.; Clark, B.C.; Simon, J.E.; France, C.R.; Grooms, D.R. Brain Activity Associated with Quadriceps Strength Deficits after Anterior Cruciate Ligament Reconstruction. Sci. Rep. 2023, 13, 8043. [Google Scholar] [CrossRef]
- Criss, C.R.; Lepley, A.S.; Onate, J.A.; Simon, J.E.; France, C.R.; Clark, B.C.; Grooms, D.R. Neural Correlates of Self-Reported Knee Function in Individuals After Anterior Cruciate Ligament Reconstruction. Sports Health 2023, 15, 52–60. [Google Scholar] [CrossRef]
- Schnittjer, A.J.; Kim, H.; Lepley, A.S.; Onate, J.A.; Criss, C.R.; Simon, J.E.; Grooms, D.R. Organization of Sensorimotor Activity in Anterior Cruciate Ligament Reconstructed Individuals: An fMRI Conjunction Analysis. Front. Hum. Neurosci. 2023, 17, 1263292. [Google Scholar] [CrossRef]
- Kim, H.; Onate, J.A.; Criss, C.R.; Simon, J.E.; Mischkowski, D.; Grooms, D.R. The Relationship between Drop Vertical Jump Action-Observation Brain Activity and Kinesiophobia after Anterior Cruciate Ligament Reconstruction: A Cross-Sectional fMRI Study. Brain Behav. 2023, 13, e2879. [Google Scholar] [CrossRef]
- Yu, L.; Jin, Z.; Xue, X.; Tao, W.; Xu, X.; Xia, T.; Zhang, Y.; Yu, W.; Wang, R.; Wang, H.; et al. Clinical Features Post-Anterior Cruciate Ligament Reconstruction Associated with Structural Alterations in the Corticospinal Tract. J. Athl. Train. 2025, 60, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Culiver, A.M.; Grooms, D.R.; Caccese, J.B.; Hayes, S.M.; Schmitt, L.C.; Oñate, J.A. fMRI Activation in Sensorimotor Regions at 6 Weeks After Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2025, 53, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, H.; Wu, X.; Zhang, Y.; Yu, J.; Li, W. Brain Near-Infrared Study of Upstairs Movement after Anterior Cruciate Ligament Reconstruction. Front. Neurol. 2025, 15, 1500579. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xue, X.; Zheng, S.; Tao, W.; Li, Q.; Wang, Y.; Gu, X.; Sun, Y.; Wang, R.; Hua, Y. Failed Single-Leg Assessment of Postural Stability after Anterior Cruciate Ligament Injuries and Reconstruction: An Updated Systematic Review and Meta-Analysis. Sports Med. Health Sci. 2025, 7, 8–15. [Google Scholar] [CrossRef]
- Wu, M.-T.; Sheen, J.-M.; Chuang, K.-H.; Yang, P.; Chin, S.-L.; Tsai, C.-Y.; Chen, C.-J.; Liao, J.-R.; Lai, P.-H.; Chu, K.-A.; et al. Neuronal Specificity of Acupuncture Response: A fMRI Study with Electroacupuncture. Neuroimage 2002, 16, 1028–1037. [Google Scholar] [CrossRef]
- Lavender, A.; Laurence, A.S.; Bangash, I.H.; Smith, R.B. Cortical Evoked Potentials in the Ruptured Anterior Cruciate Ligament. Knee Surg. Sports Traumatol. Arthrosc. 1999, 7, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Valeriani, M.; Restuccia, D.; Di Lazzaro, V.; Franceschi, F.; Fabbriciani, C.; Tonali, P. Central Nervous System Modifications in Patients with Lesion of the Anterior Cruciate Ligament of the Knee. Brain 1996, 119, 1751–1762. [Google Scholar] [CrossRef]
- Grooms, D.R.; Page, S.J.; Nichols-Larsen, D.S.; Chaudhari, A.M.W.; White, S.E.; Onate, J.A. Neuroplasticity Associated with Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2017, 47, 180–189. [Google Scholar] [CrossRef]
- Kapreli, E.; Athanasopoulos, S.; Gliatis, J.; Papathanasiou, M.; Peeters, R.; Strimpakos, N.; Van Hecke, P.; Gouliamos, A.; Sunaert, S. Anterior Cruciate Ligament Deficiency Causes Brain Plasticity: A Functional MRI Study. Am. J. Sports Med. 2009, 37, 2419–2426. [Google Scholar] [CrossRef]
- Riemann, B.L.; Lephart, S.M. The Sensorimotor System, Part I: The Physiologic Basis of Functional Joint Stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar] [PubMed]
- Toth, A.J.; Harris, L.R.; Zettel, J.; Bent, L.R. Vision Can Recalibrate the Vestibular Reafference Signal Used to Re-Establish Postural Equilibrium Following a Platform Perturbation. Exp. Brain Res. 2017, 235, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Dauty, M.; Collon, S.; Dubois, C. Change in Posture Control after Recent Knee Anterior Cruciate Ligament Reconstruction? Clin. Physiol. Funct. Imaging 2010, 30, 187–191. [Google Scholar] [CrossRef]
- Dingenen, B.; Janssens, L.; Claes, S.; Bellemans, J.; Staes, F.F. Postural Stability Deficits during the Transition from Double-Leg Stance to Single-Leg Stance in Anterior Cruciate Ligament Reconstructed Subjects. Hum. Mov. Sci. 2015, 41, 46–58. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Song, K.; Pietrosimone, B.G.; Blackburn, J.T.; Padua, D.A. Visual Utilization During Postural Control in Anterior Cruciate Ligament- Deficient and -Reconstructed Patients: Systematic Reviews and Meta-Analyses. Arch. Phys. Med. Rehabil. 2017, 98, 2052–2065. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.W.; Dukas, R. The Behavioral Ecology of a Cognitive Constraint: Limited Attention. Behav. Ecol. 2003, 14, 151–156. [Google Scholar] [CrossRef]
- Ahmadi, P.; Salehi, R.; Mehravar, M.; Goharpey, S.; Negahban, H. Comparing the Effects of External Focus of Attention and Continuous Cognitive Task on Postural Control in Anterior Cruciate Ligament Reconstructed Athletes. Neurosci. Lett. 2020, 715, 134666. [Google Scholar] [CrossRef]
- Mohammadi-Rad, S.; Salavati, M.; Ebrahimi-Takamjani, I.; Akhbari, B.; Sherafat, S.; Negahban, H.; Lali, P.; Mazaheri, M. Dual-Tasking Effects on Dynamic Postural Stability in Athletes with and Without Anterior Cruciate Ligament Reconstruction. J. Sport Rehabil. 2016, 25, 324–329. [Google Scholar] [CrossRef]
- Negahban, H.; Ahmadi, P.; Salehi, R.; Mehravar, M.; Goharpey, S. Attentional Demands of Postural Control during Single Leg Stance in Patients with Anterior Cruciate Ligament Reconstruction. Neurosci. Lett. 2013, 556, 118–123. [Google Scholar] [CrossRef]
- Welling, W.; Benjaminse, A.; Seil, R.; Lemmink, K.; Gokeler, A. Altered Movement during Single Leg Hop Test after ACL Reconstruction: Implications to Incorporate 2-D Video Movement Analysis for Hop Tests. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3012–3019. [Google Scholar] [CrossRef]
- Bin Park, H.; Koh, M.; Cho, S.H.; Hutchinson, B.; Lee, B. Mapping the Rat Somatosensory Pathway from the Anterior Cruciate Ligament Nerve Endings to the Cerebrum. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2005, 23, 1419–1424. [Google Scholar] [CrossRef]
- Terada, M.; Johnson, N.; Kosik, K.; Gribble, P. Quantifying Brain White Matter Microstructure of People with Lateral Ankle Sprain. Med. Sci. Sports Exerc. 2019, 51, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Diekfuss, J.A.; Rhea, C.K.; Schmitz, R.J.; Grooms, D.R.; Wilkins, R.W.; Slutsky, A.B.; Raisbeck, L.D. The Influence of Attentional Focus on Balance Control over Seven Days of Training. J. Mot. Behav. 2019, 51, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Marmura, H.; Bryant, D.M.; Getgood, A.M. Infographic. Sex Differences and ACL Injuries. Br. J. Sports Med. 2021, 55, 1313–1314. [Google Scholar] [CrossRef]
- Jenz, S.T.; Pearcey, G.E.P. Sex Matters in Neuromuscular Control. Acta Physiol. 2022, 235, e13823. [Google Scholar] [CrossRef]
- Lee, H.; Han, S.; Hopkins, J.T. Ty Hopkins Balance Training with Stroboscopic Glasses Alters Neuromechanics in Chronic Ankle Instability Patients during Single Leg Drop. J. Athl. Train. 2023, 59, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Harkey, M.S.; Gribble, P.A.; Pietrosimone, B.G. Disinhibitory Interventions and Voluntary Quadriceps Activation: A Systematic Review. J. Athl. Train. 2014, 49, 411–421. [Google Scholar] [CrossRef]
- Collins, A.T.; Blackburn, J.T.; Olcott, C.W.; Miles, J.; Jordan, J.; Dirschl, D.R.; Weinhold, P.S. Stochastic Resonance Electrical Stimulation to Improve Proprioception in Knee Osteoarthritis. Knee 2011, 18, 317–322. [Google Scholar] [CrossRef]
- Sanders, T.L.; Maradit Kremers, H.; Bryan, A.J.; Larson, D.R.; Dahm, D.L.; Levy, B.A.; Stuart, M.J.; Krych, A.J. Incidence of Anterior Cruciate Ligament Tears and Reconstruction: A 21-Year Population-Based Study. Am. J. Sports Med. 2016, 44, 1502–1507. [Google Scholar] [CrossRef]
- Yin, Z.; Shen, Y.; Reinhardt, J.; Chen, C.-F.; Jiang, X.; Dai, W.; Zhang, W.; Machado, S.; Arias-Carrion, O.; Yuan, T.-F.; et al. 5 Hz Repetitive Transcranial Magnetic Stimulation with Maximum Voluntary Muscle Contraction Facilitates Cerebral Cortex Excitability of Normal Subjects. CNS Neurol. Disord. Drug Targets 2015, 14, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Han, S.; Page, G.; Bruening, D.A.; Seeley, M.K.; Hopkins, J.T. Effects of Balance Training with Stroboscopic Glasses on Postural Control in Chronic Ankle Instability Patients. Scand. J. Med. Sci. Sports 2022, 32, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Marshall, P.J.; Wright, W.G. The Impact of External and Internal Focus of Attention on Visual Dependence and EEG Alpha Oscillations during Postural Control. J. Neuroeng. Rehabil. 2022, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Research | Participant Information | Technical Specifications | Test Task | Results | |||
|---|---|---|---|---|---|---|---|
| Group/n (Male/Female) | Age (Years) | Injury/Postoperative Duration | Injury/Surgery Type | ||||
| Test Technique: EEG (n = 11) | |||||||
| An et al. (2019) [29] | ACLR group: 17 participants (No information); healthy controls group: 17 participants | 22.29 ± 3.77 26.94 ± 5.56 | 3.48 ± 2.06 years postoperative | No information | EEG spectral characteristics | Degree of relaxation in the three-dimensional spatial vertical translation of the knee | Compared with the healthy limb and matched limb of the control group, ACLR participants showed increased cortical activity (higher alpha-2 ERD) in the reconstructed limb during early weight-bearing (ERD1) phases, although there were no significant group differences in joint relaxation. During late joint load phases, ACLR participants demonstrated greater somatosensory cortical activity (ERD3) relative to the control group, without differences in relaxation. |
| Lehmann et al. (2021) [30] | ACLR group: 12 participants (7/5); healthy controls group: 12 participants (7/5) | 25.1 ± 3.2 25.5 ± 3.8 | 44.4 ± 4.5 days postoperative | 5 right knees 7 left knees HT grafts (4 cases with meniscal repair, 6 cases with secondary injury) | EEG spectral characteristics | Ten 30 s trials of single-leg standing | In the ACLR group, functional connectivity in the alpha-2 frequency band significantly increased while standing on the injured leg, involving frontal–parietal, frontal–occipital, occipital–motor, occipital–parietal, and parietal–occipital connections in the contralateral hemisphere; occipital–motor connections in the ipsilateral hemisphere; and interhemispheric frontal–frontal, occipital–motor, and occipital–parietal connections. The control group showed a similar trend in both legs. |
| An et al. (2022) [31] | ACLR group:15 participants (10/5). healthy control group: 17 participants (10/5) | 23.13 ± 3.20 23.07 ± 3.45 | 2.97 ± 2.28 years postoperative | No information | EEG spectral characteristics | During single-leg stance tasks on stable and unstable platforms (moving in all directions by 15°), the supporting knee was flexed at 5° and the non-supporting knee at 45° (only for the reconstructed or matched limb, not the healthy limb), with visual feedback on the center of pressure. | During single-leg stance, ACLR participants displayed higher theta power in the contralateral primary motor cortex, higher alpha-2 power in the ipsilateral prefrontal cortex, lower alpha-2 power in the central somatosensory cortex, and increased alpha-2 power in the primary visual cortex. |
| Giesche et al. (2022) [32] | ACLR group: 10 men; healthy control group: 17 men. | 28 ± 4 28 ± 4 | Postoperative period: 63 ± 35 months. | No information Any type of graft, including those with meniscal damage | EEG spectral characteristics | 43 ± 4 planned (landing leg displayed before takeoff) and 51 ± 5 unplanned (visual cue during flight) reactive jumps with single-leg landings. | ACLR participants showed slightly higher MRCP values in unplanned tasks, with moderate effect size. |
| Sherman et al. (2023b) [33] | ACLR group: 20 participants (8/12); healthy control group: 20 participants (8/12). | 21.2 ± 2.2 21.5 ± 2.2 | Postoperative period: 15.1 ± 9.6 months. | No information 10 BTB, 5 HT, 4 QT, 1 Allo | LRP/AMT | Lateralized choice reaction time task in a seated position. Based on the position of the ball and the color of the jersey on the screen, participants quickly kicked the ball with their left or right foot. EEG was used to record brain activity. | In the ACLR group, the stimulus-locked LRP area was smaller than that of the control group under both Go and NoGo conditions, indicating lower EEG activity amplitude in the response selection phase after stimulus onset. The ACLR group also had significantly higher AMT than the control group. |
| Sherman et al. (2023c) [34] | ACLR group: 20 participants (8/12); healthy control group: 20 participants (8/12). | 21.2 ± 2.2 21.5 ± 2.2 | Postoperative period: 15.1 ± 9.6 months. | No information 10 BTB, 5 HT, 4 QT, 1 Allo | Contralateral motor cortex and ipsilateral quadriceps CMC. | Knee extension torque control task at 50% MVC with 8 trapezoidal waveform traces. | The γ-band CMC was significantly lower in the ACLR group compared to the control group (vastus medialis: d = 0.8; vastus lateralis: d = 0.7), and the active motor threshold was higher (d = 1.0), indicating reduced cortical drive. |
| Sherman et al. (2023a) [35] | ACLR group: 20 participants (8/12); healthy control group: 20 participants (8/12). | 21.2 ± 2.2 21.5 ± 2.2 | Postoperative period: 15.1 ± 9.6 months. | No information 10 BTB, 5 HT, 4 QT, 1 Allo | EEG spectral characteristics. | Single-limb balance tasks were performed under four conditions: internal focus (IF), object-based external focus (EF), goal-based external focus (EF), and transcutaneous electrical nerve stimulation (TENS). | Under all conditions, parietal α-2 frequency power was higher, as was bilateral motor cortex α-2 frequency power, along with θ frequency power. |
| Riehm et al. (2024) [36] | ACLR group: 20 participants (8/12); healthy control group: 20 participants (8/12). | 21.2 ± 2.2 21.5 ± 2.2 | Postoperative period: 15.1 ± 9.6 months. | No information 10 BTB, 5 HT, 4 QT, 1 Allo | CM-cRQA | Knee extension torque control task at 50% maximal voluntary contraction (MVC) with 8 trapezoidal waveform traces. | Significant asymmetry in cortico-muscular dynamics was observed between the affected and unaffected legs in the ACLR group, whereas the control group showed no such asymmetry in cortico-muscular dynamics. |
| Nyffenegger et al. (2025) [37] | ACLR group: 12 participants (9/3); healthy control group: 12 participants (9/3). | 25.3 ± 6.4 28.8 ± 9.7 | First test (M1): 5–8 weeks after surgery Second test (M2): 12–16 weeks after surgery | 4 participants injured dominant leg 1HT,11QT | EEG spectral characteristics. | Active knee Joint Position Sense (JPS) tests were performed in the open kinetic chain, starting with a knee flexion angle of 90° and targeting an angle of 50° | Participants with ACLR exhibited significantly higher central θ power during JPS testing with their uninvolved leg at M1 compared with M2. |
| Sherman et al. (2025) [38] | ACLR group: 20 participants (8 males/12 females); healthy control group: 20 participants (8 males/12 females). | 21.2 ± 2.2 21.5 ± 2.2 | Postoperative period: 15.1 ± 9.6 months. | No information 10 BTB, 5 HT, 4 QT, 1 Allo | CM-cRQA | Knee extension torque control task at 50% maximal voluntary contraction (MVC) with 8 trapezoidal waveform traces. | Participants with ACLR had lower corticospinal excitability and lower contralateral hemisphere motor cortex activations during quadriceps contractions. Lower corticospinal excitability and lower activations in the sensory and motor cortices were weakly associated with smaller MU action potential amplitudes, whereas group was not. |
| Piskin et al. (2025) [39] | ACLR group: 10 players (6 males/4 females); healthy control group: 15 players (9 males/6 females). | 25.5 ± 3.0 20.5 ± 4.5 | Postoperative period:5.05 ± 2.6 months. | No information 3 BTB, 6 HT, 1 QT | EEG (ERSP) | short-distance passing motion | The ACLR group exhibited significantly higher entropy values in foot external rotation. Source-derived event-related spectral perturbations indicated significant differences in posterior α (healthy players showed stronger α desynchronization, with significant differences observed between 1750 and 2250 ms) and frontal θ oscillations (ACLR players had more pronounced θ synchronization, with significant differences observed between 750 and 1000 ms) between the two groups. |
| Testing technique: TMS. (n = 1) | |||||||
| Leung et al. (2024) [40] | ACL injury group: 20 participants (9 males/11 females)h healthy control group: 18 participants (9 males/11 females). | 23.9 ± 6.3 23.7 ± 5.9 | Postoperative period: 5.4 ± 1.1 months. | No information 14 BTB, 2 HT, 1 QT, 3 Allo | AMT assessment of the CSE | Participants were seated with the hip to 90° and the knee to 60° to perform 3 MVIC, each lasting 5 s. | No significant between group differences in AMT on either side (p ≥ 0.395). There were significant between-group differences in the slope of the stimulus-response curve of the surgical side (p = 0.007), |
| Testing technique: MRI (n = 14) | |||||||
| Wang et al. (2019) [41] | ACLR group: 18 participants (10 males/8 females); healthy control group: 17 participants (10 males/7 females). | 36 ± 10 35 ± 11 | Postoperative period: 2–12 weeks. | Left knee ACL reconstruction with autograft preservation. | rs-fMRI | Eyes closed, staying alert, avoiding active mental activity. | The ACLR group showed higher ALFF in bilateral middle cingulate gyri, involving the supplementary motor area. fALFF activation cluster 1 was higher in the right postcentral gyrus, involving the right inferior parietal lobule and right supramarginal gyrus; activation cluster 2 was higher in the right middle cingulate gyrus, involving the right supplementary motor area. |
| Lepley et al. (2019) [42] | ACLR group: 11 participants (5 males/6 females); healthy control group: 11 participants (5 males/6 females). | 22.6 ± 1.8 23.2 ± 1.6 | Postoperative period: 69.4 ± 22.4 months. | No information 9 BTB grafts 2 HT grafts. | fMRI/AMT | Independent cyclic flexion-extension movement of the right knee from 0° to 45°; MEP amplitude induced at 120% AMT. | The ACLR group showed higher activation in the gyrus rectus, inferior frontal pole, paracingulate gyrus, and anterior cingulate gyrus. The AMT was higher bilaterally in the ACLR group, and MEP was smaller in the injured limb. |
| Lepley et al. (2020) [43] | ACLR group: 10 participants (4 males/6 females), comparing injured and uninjured sides. | 22.6 ± 1.9 | Postoperative period: 70.0 ± 23.6 months (66.6–96.5 months). | No information. | DTI/MEP | DTI was used to scan the structural characteristics of the corticospinal tract. At 120% AMT intensity, 5 MEPs were induced, and the peak-to-peak MEP amplitude average was calculated. | Compared to the uninjured limb, the hemisphere of the ACLR-injured limb showed smaller corticospinal tract volume, lower FA values, and higher MD values, indicating white matter damage and reduced corticospinal tract excitability. Corticospinal tract volume was significantly positively correlated with excitability. |
| Criss et al. (2020) [44] | ACLR group: 15 participants (7 males/8 females); healthy control group: 15 participants (7 males/8 females). | 20.94 ± 2.68 22.53 ± 2.47 | Postoperative period: 43.33 ± 33.14 months. | Left knees 14 HT grafts, 1 BTB grafts. | fMRI | Unilateral left knee (ACLR knee or matched healthy control knee) cyclic knee and hip extension/flexion (similar to heel slides). | The ACL group showed increased BOLD signal in the superior parietal lobe and left fusiform gyrus in the occipital lobe and visual pathway, as well as in the left lateral occipital cortex, angular gyrus, and parietal lobe. |
| Chaput et al. (2022) [45] | ACLR group: 16 participants (6 males/10 females); healthy control group: 15 participants (6 males/9 females). | 21.5 ± 2.6 2.9 ± 3.03 | Postoperative period: 41.4 ± 33.0 months. | Left knees 13 HT grafts, 3 BTB grafts. | fMRI | Independent cyclic flexion-extension movement of the right knee from 0° to 45°. | Visual cognition was associated with increased neural activity in the precuneus and posterior cingulate cortex in the ACLR group, but not in the control group. Visual memory and visuomotor abilities were negatively correlated with proprioceptive error and landing stability time. |
| Flanagan et al. (2021) [46] | ACLR group: 9 female participants; healthy control group: 11 female participants. | 20.6 ± 1.4 20.6 ± 2.3 | 3.2 ± 1.1 years. | 5 left knees and 6 right knees; 5 HT grafts and 4 BTB grafts. | MRI (DWI) | Resting-state scanning. | Cortical thickness was reduced in the somatosensory cortex, particularly in the paracentral lobule (PCL), in the ACLR group. The topological structure of the cortical motor network changed in the ACLR group, with increased importance in non-limb motor areas and decreased effectiveness and eccentricity in primary somatosensory regions. |
| Baez et al. (2021) [47] | ACLR group: 12 female participants; healthy control group: 12 female participants. | 21.5 ± 6.8 23.0 ± 1.8 | Duration: 5.5 ± 4.2 years. | Left knee No information. | fMRI | Motor imagery and daily life activity imagery tasks were conducted. | In the ACLR group, the following changes were observed in the cortical-limbic system regions during imagery tasks: Increased activity in the inferior parietal lobule and dorsomedial thalamus. Decreased inhibition of the default mode network, with an inability to suppress the default mode network, including the posterior cingulate cortex, precuneus, and ventromedial prefrontal cortex. Task-specific changes: Increased activity in the cerebellum and inferior occipital cortex when imagining motor-related images. |
| Strong et al. (2022) [48] | ACLR group: 21 participants (8 males/13 females); healthy control group: 19 participants(7 males/12 females). | Right knee injury (24.8 ± 4.2); left knee (28.2 ± 4.7). Healthy participants 27.1 ± 4.6 | Average postoperative period: 23 months. | 10 right knee (4 males/6 females, 20.0 ± 9.7 months); left knee, 11 cases (4 males/7 females, 28.5 ± 18.6 months); HT grafts. | fMRI | Knee joint position sense (JPS) testing was performed during functional magnetic resonance imaging (fMRI). | Both groups activated proprioception-related brain regions, such as the somatosensory cortex, prefrontal cortex, and insula, during the JPS test. However, no significant differences were found in brain responses between the groups. JPS error was positively correlated with activation in the contralateral insula, anterior cingulate cortex, and supramarginal gyrus. |
| Criss et al. (2023) [49] | ACLR group: 22 participants (8 males/14 females); healthy control group: 22 participants (8 males/14 females). | 22.1 ± 2.6 22.9 ± 2.7 | Postoperative period: 4.6 ± 2.6 years. | 7 right knees and 15 left knees. No information. | fMRI | Independent cyclic flexion-extension movement of the right knee from 0° to 45°. | In the ACLR group, increased activation was observed in the lingual gyrus, contralateral premotor cortex, and contralateral parietal lobe. |
| Criss et al. (2023) [50] | ACLR group: 25 participants (10 males/15 females), comparing injured and uninjured sides. | 21.8 ± 2.6 | Postoperative period: 51.6 ± 32.9 months. | 7 right knees and 18 left knees; 15 HT grafts 10 BTB grafts. | fMRI | Independent cyclic flexion-extension movement of the right knee from 0° to 45°. | Compared to the injured side, the uninjured side showed higher activation in the primary motor, primary somatosensory, premotor, secondary somatosensory cortices, supplementary motor area (SMA), and cerebellum. |
| Schnittjer et al. (2023) [51] | ACLR group: 18 participants (8 males/10 females); healthy control group: 18 participants (7 males/11 females). | 21.00 ± 5.25 22.00 ± 4.00 | Postoperative period: 45.94 ± 7.55 months. | Left knee (No information). | fMRI | Independent cyclic flexion-extension movement of the right knee from 0° to 45°. | During movement of the affected limb in the ACLR group, motor cortex activity increased, activating unique parietal regions, including the inferior parietal lobule and lateral occipital cortex (upper region). The peak voxel position in the frontal and parietal cortices was more medial and superior, while the parietal peak voxel position was more posterior and superior. Cerebellar activity was more focused. |
| Kim et al. (2023) [52] | ACLR group: 13 participants (5 males/8 females); healthy control group: 13 participants (5 males/8 females). | 20.62 ± 1.93 22.92 ± 3.17 | Postoperative period: 1–10 years. | No information. | fMRI | Action observation drop vertical jump (AO-DVJ) paradigm; participants observed and imagined performing a drop vertical jump. | In the ACLR group, right ventrolateral prefrontal cortex activity was lower. Brain activity in certain cerebellar regions, amygdala, middle temporal gyrus, and temporal pole positively correlated with TSK-11 scores. No correlation between brain activity and TSK-11 scores was found in the control group. |
| Yu et al. (2025) [53] | ACLR group: 26 participants (23 males/3 females); healthy control group: 26 participants (22 males/4 females). | 36.35 ± 6.39 32.85 ± 9.20 | Postoperative period:15.0 months (interquartile range = 12.0–19.5 months) | 14 right knees and 12 left knees No information. | DTI assessment of the CST structure. | Resting-state scanning. | ACLR had moderately lower fractional anisotropy (Cohen d = −0.666; 95% CI = −1.221, −0.104; p = 0.01), lower axial diffusivity (Cohen d = −0.526; 95% CI = −1.077, 0.030; p = 0.03), higher radial diffusivity (RD; Cohen d = 0.514; 95% CI = −0.042, 1.064; p = 0.04), and smaller Y-Balance Test anterior-reach distance (Cohen d = −0.743; 95% CI = −1.302, −0.177; p = 0.005) compared with healthy controls. The RD values were correlated with the postoperative duration (r = 0.623, p < 0.001) after controlling for age, sex, and body mass index in patients with ACLR. |
| Culiver et al. (2025) [54] | ACLR group: 15 participants (7 males/8 females); healthy control group: 15 participants (7 males/8 females) | 21.9 ± 4.3 23.1 ± 3.1 | Postoperative period:6.8 ± 1.2 weeks | 4 Right knees 11 left knees 10 BTB grafts 5 HT grafts. | fMRI | Independent cyclic flexion-extension movement of the right knee from 0° to 45°. | Compared with the control group, during movement of the ACLR-involved and uninvolved knees, there was a significant reduction in the following regions: ipsilateral M1, ipsilateral/contra-lateral S1, SMA, and precuneus (p < 0.05, effect size 0.5–1.4). |
| Testing technique: fnirs(n = 1) | |||||||
| Cao et al. (2025) [55] | ACLR group: 27 participants (23 males/4 females); healthy control group: 15 participants (23 males/4 females) | 25.6 ± 2.3 25.8 ± 2.8 | Postoperative period:4.6 ± 1.4 months | Right knees No information. | fNIRS | Repeat the upstairs task. Go up the stairs for 15 s. Then, after a 20 s rest, repeat the upstairs task for 15 s; a total of 4 sets were completed. | The ACLR group showed a significant decrease in channel 25β values (corresponding to the premotor and supplementary motor cortices), while the healthy control group had significantly higher β values in channel 33 (primary somatosensory cortex) |
| Neural Alteration | Number of Studies | Primary Techniques | Key Clinical Correlations |
|---|---|---|---|
| Sensory Cortex Changes | 4 | EEG (2), fMRI (2) | • Enhanced proprioceptive awareness during weight-bearing [29] • Reduced activation during knee flexion [54] |
| Increased visual cortex activation | 5 | EEG (1), fMRI (4) | • Greater dependence on visual feedback for balance [31] • Correlated with reduced postural stability in visually deprived conditions [44] |
| Reduced Motor Cortex Efficiency | 8 | TMS (3), EEG (3), fMRI (2) | • Elevated AMT linked to quadriceps weakness [42] • Lower γ-band CMC associated with reduced cortical drive [34] |
| Fronto-Parietal-Occipital Connectivity | 1 | EEG (1) | • Compensatory mechanism for postural control [30] |
| Default Mode Network Inhibition | 1 | fMRI (1) | • Inability to suppress DMN during motor imagery [47] |
| Limbic System Activation | 2 | fMRI (2) | • Positive correlation with TSK-11 scores (fear of movement) [52] |
| Corticospinal Tract Atrophy | 2 | DTI (2) | • Reduced volume correlated with lower excitability [43] • RD values increased with postoperative duration [53] |
| Sensorimotor Cortex Thinning | 1 | MRI-DWI (1) | • Altered motor network topology [46] |
| Sensory/Visual Integration and Function | 2 | fMRI (2) | • Positive correlation with IKDC scores [50] • Negative correlation with proprioceptive error [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jia, Y.; Li, Q.; Li, L.; Dong, Q.; Fu, Q. Advances in Research on Brain Structure and Activation Characteristics in Patients with Anterior Cruciate Ligament Reconstruction: A Systematic Review. Brain Sci. 2025, 15, 831. https://doi.org/10.3390/brainsci15080831
Wang J, Jia Y, Li Q, Li L, Dong Q, Fu Q. Advances in Research on Brain Structure and Activation Characteristics in Patients with Anterior Cruciate Ligament Reconstruction: A Systematic Review. Brain Sciences. 2025; 15(8):831. https://doi.org/10.3390/brainsci15080831
Chicago/Turabian StyleWang, Jingyi, Yaxiang Jia, Qiner Li, Longhui Li, Qiuyu Dong, and Quan Fu. 2025. "Advances in Research on Brain Structure and Activation Characteristics in Patients with Anterior Cruciate Ligament Reconstruction: A Systematic Review" Brain Sciences 15, no. 8: 831. https://doi.org/10.3390/brainsci15080831
APA StyleWang, J., Jia, Y., Li, Q., Li, L., Dong, Q., & Fu, Q. (2025). Advances in Research on Brain Structure and Activation Characteristics in Patients with Anterior Cruciate Ligament Reconstruction: A Systematic Review. Brain Sciences, 15(8), 831. https://doi.org/10.3390/brainsci15080831






