Gender Differences in the Effects of Exercise Interventions on Alzheimer’s Disease
Abstract
1. Introduction
2. Gender Differences in AD Formation
2.1. Epidemiological Differences
2.2. Phenotypic Differences
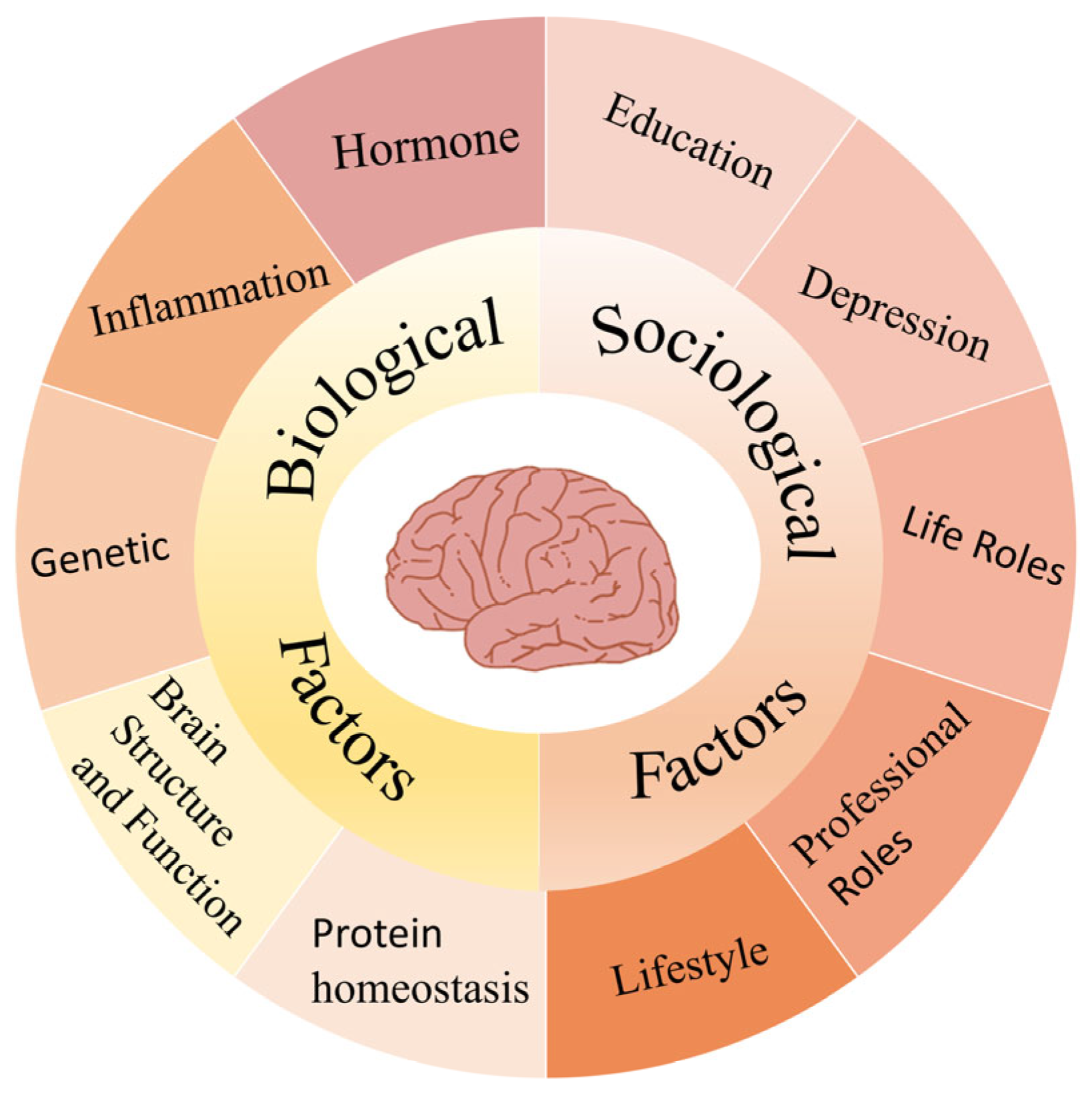
2.3. Contributing Factors to Gender Differences in AD Formation
2.3.1. Biological Factors
| Category | Female | Male | Reference |
|---|---|---|---|
| Age of Onset and Disease Course | 1. Higher AD prevalence with increasing age. 2. Greater disease burden among older death age groups. | 1. Earlier cognitive symptom onset. 2. Shorter disease duration. 3. More atypical (non-amnestic) presentations. | [30] |
| Pathological Accumulation | 1. Greater brain region involvement from late middle age. 2. Extensive accumulation of SP was detected at NFT stages I, II, and III. (Specifically observed in females carrying the APOE4 allele). | 1. Extensive accumulation of SP was detected at NFTs stages IV, V, and VI. | [31] |
| Tau accumulation in high Aβ or APOEε4 | 1. Faster tau accumulation in inferior temporal, temporal fusiform, and lateral occipital regions. 2. In APOEε4 carriers (inferior temporal). | 1. Slower tau accumulation in these regions. | [32] |
| Neurodegeneration Biomarkers | 1. Higher brain glucose metabolism. 2. Greater cortical thickness. 3. Elevated CSF T-tau levels. | 1. Higher lifelong levels of neurofilament light chain (NfL). | [33] |
| Aβ burden (Animal Model) | 1. Female mice exhibit significantly greater senile plaque burden. 2. Higher brain levels of Aβ40 and Aβ42 compared to male mice. | N/A | [34] |
| Cognitive Decline Progression | 1. Greater decline in ADAS-Cog11 scores in both APOE ε4 carriers and non-carriers. 2. Faster cognitive decline with lower CSF Aβ42 levels. 3. More pronounced cognitive deterioration during the MCI stage; smaller baseline hippocampal volume but larger after normalization. 4. Greater cognitive decline in APOEε4 positive MCI subjects. | 1. Smaller decline in ADAS-Cog11 scores in both APOEε4 carriers and non-carriers compared to females. 2. Less cognitive deterioration during MCI stage; larger baseline hippocampal volume but smaller after intracranial volume normalization. 3. Slower cognitive decline in APOEε4 positive MCI subjects. | [36] |
| Clinical Cognitive Performance | 1. Worse performance in visuospatial, language, and semantic memory tasks. | 1. Advantages in visuospatial, linguistic, and semantic memory tasks. | [37] |
| Hippocampal Asymmetry | 1. Lower hippocampal volumetric asymmetry. | 1. Higher hippocampal volumetric asymmetry. | [38] |
| Hippocampal Atrophy Based on Biomarker Status | 1. More pronounced left hippocampal atrophy with a decrease in Aβ42 in CSF 2. Memory and executive function decline faster. 3. Tau is elevated in CSF, and left hippocampus is atrophied. | N/A | [39] |
| Temporal Lobe Glucose Metabolic Rate and Verbal Memory | 1. Higher Temporal Lobe Glucose Metabolic Rate (TLGluMR) is associated with better verbal memory performance. 2. The female advantage in verbal memory is most pronounced at moderate to high TLGluMR levels. | N/A | [40] |
| Subjective Cognitive Decline/ Self-Memory Complaint (SCD/SMC) | 1. SMC is significantly associated with increased dementia risk across all risk periods, including long-term follow-up. 2. After adjustment for education, marital status, depressive symptoms, and global cognition, SMC independently predicts dementia risk, whereas IADL limitations are not associated. | 1. IADL limitations are associated with an increased risk of dementia, limited to the first 5 years. 2. After adjustment for education, marital status, depressive symptoms, and global cognition, only IADL limitations remain significantly associated with dementia risk, while SMC is not predictive. | [41] |
| Neuropsychiatric Symptoms (NPS) | 1. Greater NPS burden. 2. More frequent depression, anxiety, and delusions. | 1. It is often associated with indifference. | [42] |
| Clinicopathological Correlation | 1. Stronger correlation between AD pathology and clinical symptoms. | 1. Weaker correlation between AD pathology and clinical symptoms. | [43] |
| Indicator | Female | Male | Reference |
|---|---|---|---|
| Age distribution curve of AD | U-shape (intersecting at age 70) | Inverted U-shape | [30] |
| AD subtype | Limbic predominant subtype | Hippocampus-sparing subtype | [30] |
| Regional distribution of hippocampal NFT counts | Gradual increase | Gradual decrease | [30] |
| Regional distribution of neocortical NFT counts | Gradual decrease | Gradual decrease | [30] |
| Braak NFTs stage | Higher | N/A | [30] |
| Amyloid plaque burden | Slightly higher | N/A | [30] |
| Hippocampal asymmetry value | 3.46% | 5.5% | [38] |
| Number of individuals meeting probable AD clinical criteria | 34 individuals | 23 individuals | [43] |
| Association between pathology and clinical diagnosis | Each unit increase in AD pathology increases the likelihood of clinical AD diagnosis by nearly 20 times | Each unit increase in AD pathology triples the likelihood of clinical AD diagnosis | [43] |
2.3.2. Sociological Factors
3. Gender-Specific Effects of Exercise Interventions on AD Risk
3.1. Baseline Gender Differences in Exercise Interventions
3.2. Gender-Specific Manifestations of Exercise Intervention in AD Pathological Scenarios
3.3. Mechanisms Underlying Gender Differences in Exercise Effects on AD: Evidence from Human and Animal Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | amyloid-beta |
| NFT | neurofibrillary tangles |
| MCI | mild cognitive impairment |
| NPS | neuropsychiatric symptoms |
| APOE4 | Apolipoprotein E4 |
| T-tau | total tau |
| P-tau | phosphorylated tau |
| SCD | subjective cognitive decline |
| SMC | subjective memory complaints |
| BDNF | brain-derived neurotrophic factor |
| HRT | hormone replacement therapy |
| 1L-1β | Interleukin-1β |
| 1L-6 | Interleukin-6 |
| BMI | body mass index |
| CK | creatine kinase |
| LDL | low-Denisty Lipoprotein |
| HIIT | high-intensity interval training |
| TMT | Trail Making Test |
| IGF-1 | insulin-like growth factor-1 |
| TNF-α | tumor necrosis factor-alpha |
| CREB | cyclic AMP response element-binding |
References
- Stefaniak, O.; Dobrzyńska, M.; Drzymała-Czyż, S.; Przysławski, J. Diet in the Prevention of Alzheimer’s Disease: Current Knowledge and Future Research Requirements. Nutrients 2022, 14, 4564. [Google Scholar] [CrossRef]
- Podcasy, J.L.; Epperson, C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016, 18, 437–446. [Google Scholar] [CrossRef]
- Kheloui, S.; Jacmin-Park, S.; Larocque, O.; Kerr, P.; Rossi, M.; Cartier, L.; Juster, R.P. Sex/gender differences in cognitive abilities. Neurosci. Biobehav. Rev. 2023, 152, 105333. [Google Scholar] [CrossRef] [PubMed]
- Rosende-Roca, M.; García-Gutiérrez, F.; Cantero-Fortiz, Y.; Alegret, M.; Pytel, V.; Cañabate, P.; González-Pérez, A.; de Rojas, I.; Vargas, L.; Tartari, J.P.; et al. Exploring sex differences in Alzheimer’s disease: A comprehensive analysis of a large patient cohort from a memory unit. Alzheimers Res. Ther. 2025, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Davies, D.A.; Albensi, B.C. The Interaction Between NF-κB and Estrogen in Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Levey, A. Gender Differences: A Lifetime Analysis of the Economic Burden of Alzheimer’s Disease. Womens Health Issues 2015, 25, 436–440. [Google Scholar] [CrossRef]
- 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021, 17, 327–406. [CrossRef]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 6, Cd001190. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Dou, K.X.; Tan, M.S.; Tan, C.C.; Cao, X.P.; Hou, X.H.; Guo, Q.H.; Tan, L.; Mok, V.; Yu, J.T. Comparative safety and effectiveness of cholinesterase inhibitors and memantine for Alzheimer’s disease: A network meta-analysis of 41 randomized controlled trials. Alzheimers Res. Ther. 2018, 10, 126. [Google Scholar] [CrossRef]
- Whittington, M.D.; Campbell, J.D.; Rind, D.; Fluetsch, N.; Lin, G.A.; Pearson, S.D. Cost-Effectiveness and Value-Based Pricing of Aducanumab for Patients With Early Alzheimer Disease. Neurology 2022, 98, e968–e977. [Google Scholar] [CrossRef] [PubMed]
- López-Ortiz, S.; Lista, S.; Valenzuela, P.L.; Pinto-Fraga, J.; Carmona, R.; Caraci, F.; Caruso, G.; Toschi, N.; Emanuele, E.; Gabelle, A.; et al. Effects of physical activity and exercise interventions on Alzheimer’s disease: An umbrella review of existing meta-analyses. J. Neurol. 2023, 270, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; de la Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Calmaestra, R.; Martínez-Amat, A.; Aibar-Almazán, A.; Hita-Contreras, F.; de Miguel Hernando, N.; Achalandabaso-Ochoa, A. Effectiveness of Physical Exercise on Alzheimer’s disease. A Systematic Review. J. Prev. Alzheimers Dis. 2022, 9, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Lara, A.; Sepúlveda, P.; Marzuca-Nassr, G.N. Resistance Exercise Training as a New Trend in Alzheimer’s Disease Research: From Molecular Mechanisms to Prevention. Int. J. Mol. Sci. 2024, 25, 7084. [Google Scholar] [CrossRef]
- Coutinho, L.A.; Leão, L.L.; Cassilhas, R.C.; de Paula, A.M.B.; Deslandes, A.C.; Monteiro-Junior, R.S. Alzheimer’s disease genes and proteins associated with resistance and aerobic training: An in silico analysis. Exp. Gerontol. 2022, 168, 111948. [Google Scholar] [CrossRef]
- Tsai, C.L.; Pai, M.C.; Ukropec, J.; Ukropcová, B. Distinctive Effects of Aerobic and Resistance Exercise Modes on Neurocognitive and Biochemical Changes in Individuals with Mild Cognitive Impairment. Curr. Alzheimer Res. 2019, 16, 316–332. [Google Scholar] [CrossRef]
- Stefanacci, R.G. The costs of Alzheimer’s disease and the value of effective therapies. Am. J. Manag. Care 2011, 17 (Suppl. S13), S356–S362. [Google Scholar]
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024, 20, 3708–3821. [CrossRef]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Ji, Q.; Chen, J.; Li, Y.; Tao, E.; Zhan, Y. Incidence and prevalence of Alzheimer’s disease in China: A systematic review and meta-analysis. Eur. J. Epidemiol. 2024, 39, 701–714. [Google Scholar] [CrossRef]
- Seshadri, S.; Wolf, P.A.; Beiser, A.; Au, R.; McNulty, K.; White, R.; D’Agostino, R.B. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology 1997, 49, 1498–1504. [Google Scholar] [CrossRef]
- Hebert, L.E.; Scherr, P.A.; McCann, J.J.; Beckett, L.A.; Evans, D.A. Is the risk of developing Alzheimer’s disease greater for women than for men? Am. J. Epidemiol. 2001, 153, 132–136. [Google Scholar] [CrossRef]
- Wang, X.; Feng, S.; Deng, Q.; Wu, C.; Duan, R.; Yang, L. The role of estrogen in Alzheimer’s disease pathogenesis and therapeutic potential in women. Mol. Cell Biochem. 2025, 480, 1983–1998. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hong, F.; Yang, S. Amyloidosis in Alzheimer’s Disease: Pathogeny, Etiology, and Related Therapeutic Directions. Molecules 2022, 27, 1210. [Google Scholar] [CrossRef]
- Arenaza-Urquijo, E.M.; Boyle, R.; Casaletto, K.; Anstey, K.J.; Vila-Castelar, C.; Colverson, A.; Palpatzis, E.; Eissman, J.M.; Kheng Siang Ng, T.; Raghavan, S.; et al. Sex and gender differences in cognitive resilience to aging and Alzheimer’s disease. Alzheimers Dement. 2024, 20, 5695–5719. [Google Scholar] [CrossRef]
- Nebel, R.A.; Aggarwal, N.T.; Barnes, L.L.; Gallagher, A.; Goldstein, J.M.; Kantarci, K.; Mallampalli, M.P.; Mormino, E.C.; Scott, L.; Yu, W.H.; et al. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. 2018, 14, 1171–1183. [Google Scholar] [CrossRef]
- Liesinger, A.M.; Graff-Radford, N.R.; Duara, R.; Carter, R.E.; Hanna Al-Shaikh, F.S.; Koga, S.; Hinkle, K.M.; DiLello, S.K.; Johnson, M.F.; Aziz, A.; et al. Sex and age interact to determine clinicopathologic differences in Alzheimer’s disease. Acta Neuropathol. 2018, 136, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Corder, E.H.; Ghebremedhin, E.; Taylor, M.G.; Thal, D.R.; Ohm, T.G.; Braak, H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: Modification by age, sex, and APOE polymorphism. Ann. N. Y. Acad. Sci. 2004, 1019, 24–28. [Google Scholar] [CrossRef]
- Coughlan, G.T.; Klinger, H.M.; Boyle, R.; Betthauser, T.J.; Binette, A.P.; Christenson, L.; Chadwick, T.; Hansson, O.; Harrison, T.M.; Healy, B.; et al. Sex Differences in Longitudinal Tau-PET in Preclinical Alzheimer Disease: A Meta-Analysis. JAMA Neurol. 2025, 82, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Aggarwal, N.T.; Vila-Castelar, C.; Agarwal, P.; Arenaza-Urquijo, E.M.; Brett, B.; Brugulat-Serrat, A.; DuBose, L.E.; Eikelboom, W.S.; Flatt, J.; et al. Consideration of sex and gender in Alzheimer’s disease and related disorders from a global perspective. Alzheimers Dement. 2022, 18, 2707–2724. [Google Scholar] [CrossRef]
- Callahan, M.J.; Lipinski, W.J.; Bian, F.; Durham, R.A.; Pack, A.; Walker, L.C. Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am. J. Pathol. 2001, 158, 1173–1177. [Google Scholar] [CrossRef]
- Emrani, S.; Sundermann, E.E. Sex/gender differences in the clinical trajectory of Alzheimer’s disease: Insights into diagnosis and cognitive reserve. Front. Neuroendocrinol. 2025, 77, 101184. [Google Scholar] [CrossRef]
- Sohn, D.; Shpanskaya, K.; Lucas, J.E.; Petrella, J.R.; Saykin, A.J.; Tanzi, R.E.; Samatova, N.F.; Doraiswamy, P.M. Sex Differences in Cognitive Decline in Subjects with High Likelihood of Mild Cognitive Impairment due to Alzheimer’s disease. Sci. Rep. 2018, 8, 7490. [Google Scholar] [CrossRef]
- Irvine, K.; Laws, K.R.; Gale, T.M.; Kondel, T.K. Greater cognitive deterioration in women than men with Alzheimer’s disease: A meta analysis. J. Clin. Exp. Neuropsychol. 2012, 34, 989–998. [Google Scholar] [CrossRef]
- Ardekani, B.A.; Hadid, S.A.; Blessing, E.; Bachman, A.H. Sexual Dimorphism and Hemispheric Asymmetry of Hippocampal Volumetric Integrity in Normal Aging and Alzheimer Disease. AJNR Am. J. Neuroradiol. 2019, 40, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Koran, M.E.I.; Wagener, M.; Hohman, T.J. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2017, 11, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, E.E.; Maki, P.M.; Rubin, L.H.; Lipton, R.B.; Landau, S.; Biegon, A. Female advantage in verbal memory: Evidence of sex-specific cognitive reserve. Neurology 2016, 87, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Pérès, K.; Helmer, C.; Amieva, H.; Matharan, F.; Carcaillon, L.; Jacqmin-Gadda, H.; Auriacombe, S.; Orgogozo, J.M.; Barberger-Gateau, P.; Dartigues, J.F. Gender differences in the prodromal signs of dementia: Memory complaint and IADL-restriction. a prospective population-based cohort. J. Alzheimers Dis. 2011, 27, 39–47. [Google Scholar] [CrossRef]
- Eikelboom, W.S.; Pan, M.; Ossenkoppele, R.; Coesmans, M.; Gatchel, J.R.; Ismail, Z.; Lanctôt, K.L.; Fischer, C.E.; Mortby, M.E.; van den Berg, E.; et al. Sex differences in neuropsychiatric symptoms in Alzheimer’s disease dementia: A meta-analysis. Alzheimers Res. Ther. 2022, 14, 48. [Google Scholar] [CrossRef]
- Barnes, L.L.; Wilson, R.S.; Bienias, J.L.; Schneider, J.A.; Evans, D.A.; Bennett, D.A. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psychiatry 2005, 62, 685–691. [Google Scholar] [CrossRef]
- Wang, X. Analysis on Risk Factors of Women in Alzheimer’s Disease. In Proceedings of the 2020 International Conference on Public Health and Data Science (ICPHDS), Guangzhou, China, 20–22 November 2020; pp. 338–341. [Google Scholar]
- Li, R.; Singh, M. Sex differences in cognitive impairment and Alzheimer’s disease. Front. Neuroendocrinol. 2014, 35, 385–403. [Google Scholar] [CrossRef]
- Lee, B.H.; Eid, R.S.; Hodges, T.E.; Barth, C.; Galea, L.A.M. Leveraging research into sex differences and steroid hormones to improve brain health. Nat. Rev. Endocrinol. 2025, 21, 214–229. [Google Scholar] [CrossRef]
- Bagit, A.; Hayward, G.C.; MacPherson, R.E.K. Exercise and estrogen: Common pathways in Alzheimer’s disease pathology. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E164–E168. [Google Scholar] [CrossRef] [PubMed]
- Rettberg, J.R.; Yao, J.; Brinton, R.D. Estrogen: A master regulator of bioenergetic systems in the brain and body. Front. Neuroendocrinol. 2014, 35, 8–30. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, O.; Rahman, A.; Hristov, H.; Berkowitz, C.; Isaacson, R.S.; Diaz Brinton, R.; Mosconi, L. Female Sex and Alzheimer’s Risk: The Menopause Connection. J. Prev. Alzheimers Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef]
- Rahman, A.; Schelbaum, E.; Hoffman, K.; Diaz, I.; Hristov, H.; Andrews, R.; Jett, S.; Jackson, H.; Lee, A.; Sarva, H.; et al. Sex-driven modifiers of Alzheimer risk: A multimodality brain imaging study. Neurology 2020, 95, e166–e178. [Google Scholar] [CrossRef]
- Lv, W.; Du, N.; Liu, Y.; Fan, X.; Wang, Y.; Jia, X.; Hou, X.; Wang, B. Low Testosterone Level and Risk of Alzheimer’s Disease in the Elderly Men: A Systematic Review and Meta-Analysis. Mol. Neurobiol. 2016, 53, 2679–2684. [Google Scholar] [CrossRef] [PubMed]
- Gouras, G.K.; Xu, H.; Gross, R.S.; Greenfield, J.P.; Hai, B.; Wang, R.; Greengard, P. Testosterone reduces neuronal secretion of Alzheimer’s beta-amyloid peptides. Proc. Natl. Acad. Sci. USA 2000, 97, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, E.; Bandelow, S.; Combrinck, M.; Smith, A.D. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp. Gerontol. 2004, 39, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Cherrier, M.M.; Matsumoto, A.M.; Amory, J.K.; Asthana, S.; Bremner, W.; Peskind, E.R.; Raskind, M.A.; Craft, S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology 2005, 64, 2063–2068. [Google Scholar] [CrossRef]
- Tan, R.S.; Pu, S.J. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male 2003, 6, 13–17. [Google Scholar] [CrossRef]
- Marriott, R.J.; Murray, K.; Flicker, L.; Hankey, G.J.; Matsumoto, A.M.; Dwivedi, G.; Antonio, L.; Almeida, O.P.; Bhasin, S.; Dobs, A.S.; et al. Lower serum testosterone concentrations are associated with a higher incidence of dementia in men: The UK Biobank prospective cohort study. Alzheimers Dement. 2022, 18, 1907–1918. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Panizzon, M.S.; Chen, X.; Andrews, M.; Galasko, D.; Banks, S.J. Sex differences in Alzheimer’s-related Tau biomarkers and a mediating effect of testosterone. Biol. Sex Differ. 2020, 11, 33. [Google Scholar] [CrossRef]
- Zhu, D.; Montagne, A.; Zhao, Z. Alzheimer’s pathogenic mechanisms and underlying sex difference. Cell Mol. Life Sci. 2021, 78, 4907–4920. [Google Scholar] [CrossRef]
- Casaletto, K.B.; Nichols, E.; Aslanyan, V.; Simone, S.M.; Rabin, J.S.; La Joie, R.; Brickman, A.M.; Dams-O’Connor, K.; Palta, P.; Kumar, R.G.; et al. Sex-specific effects of microglial activation on Alzheimer’s disease proteinopathy in older adults. Brain 2022, 145, 3536–3545. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef]
- Biechele, G.; Rauchmann, B.S.; Janowitz, D.; Buerger, K.; Franzmeier, N.; Weidinger, E.; Guersel, S.; Schuster, S.; Finze, A.; Harris, S.; et al. Associations between sex, body mass index and the individual microglial response in Alzheimer’s disease. J. Neuroinflammation 2024, 21, 30. [Google Scholar] [CrossRef]
- Guillot-Sestier, M.V.; Araiz, A.R.; Mela, V.; Gaban, A.S.; O’Neill, E.; Joshi, L.; Chouchani, E.T.; Mills, E.L.; Lynch, M.A. Microglial metabolism is a pivotal factor in sexual dimorphism in Alzheimer’s disease. Commun. Biol. 2021, 4, 711. [Google Scholar] [CrossRef]
- O’Neill, E.; Mela, V.; Gaban, A.S.; Bechet, S.; McGrath, A.; Walsh, A.; McIntosh, A.; Lynch, M.A. Sex-Related Microglial Perturbation Is Related to Mitochondrial Changes in a Model of Alzheimer’s Disease. Front. Cell Neurosci. 2022, 16, 939830. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hong, T.; Chen, F.; Sun, Y.; Wang, Y.; Cui, L. Interplay Between Microglia and Alzheimer’s Disease-Focus on the Most Relevant Risks: APOE Genotype, Sex and Age. Front. Aging Neurosci. 2021, 13, 631827. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, C.; Castrogiovanni, P.; Imbesi, R.; Vecchio, M.; Sortino, M.; Musumeci, G.; Vinciguerra, M.; Di Rosa, M. Exploring SERPINA3 as a neuroinflammatory modulator in Alzheimer’s disease with sex and regional brain variations. Metab. Brain Dis. 2025, 40, 83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, X.; Yu, H.; Xu, X.; Wang, M.; Le, W. Factors Influencing Alzheimer’s Disease Risk: Whether and How They are Related to the APOE Genotype. Neurosci. Bull. 2022, 38, 809–819. [Google Scholar] [CrossRef]
- Belloy, M.E.; Napolioni, V.; Greicius, M.D. A Quarter Century of APOE and Alzheimer’s Disease: Progress to Date and the Path Forward. Neuron 2019, 101, 820–838. [Google Scholar] [CrossRef]
- Sampedro, F.; Vilaplana, E.; de Leon, M.J.; Alcolea, D.; Pegueroles, J.; Montal, V.; Carmona-Iragui, M.; Sala, I.; Sánchez-Saudinos, M.B.; Antón-Aguirre, S.; et al. APOE-by-sex interactions on brain structure and metabolism in healthy elderly controls. Oncotarget 2015, 6, 26663–26674. [Google Scholar] [CrossRef]
- Edmunds, K.J.; Pandos, A.A.; Hoang, I.; Mamlouk, G.M.; Motovylyak, A.; Lose, S.R.; Asthana, S.; Stremlau, M.; Johnson, S.C.; van Praag, H.; et al. BDNF expression mediates verbal learning and memory in women in a cohort enriched with risk for Alzheimer’s disease. Alzheimers Dement. 2025, 17, e70062. [Google Scholar] [CrossRef]
- Ritchie, S.J.; Cox, S.R.; Shen, X.; Lombardo, M.V.; Reus, L.M.; Alloza, C.; Harris, M.A.; Alderson, H.L.; Hunter, S.; Neilson, E.; et al. Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cereb. Cortex 2018, 28, 2959–2975. [Google Scholar] [CrossRef]
- Ingalhalikar, M.; Smith, A.; Parker, D.; Satterthwaite, T.D.; Elliott, M.A.; Ruparel, K.; Hakonarson, H.; Gur, R.E.; Gur, R.C.; Verma, R. Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. USA 2014, 111, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lee, C.; Torres, E.R.S.; Carling, G.; Gan, L. Mechanisms of sex differences in Alzheimer’s disease. Neuron 2024, 112, 1208–1221. [Google Scholar] [CrossRef]
- Dubal, D.B. Sex difference in Alzheimer’s disease: An updated, balanced and emerging perspective on differing vulnerabilities. Handb. Clin. Neurol. 2020, 175, 261–273. [Google Scholar] [CrossRef]
- Sultana, O.F.; Bandaru, M.; Islam, M.A.; Reddy, P.H. Unraveling the complexity of human brain: Structure, function in healthy and disease states. Ageing Res. Rev. 2024, 100, 102414. [Google Scholar] [CrossRef]
- Rahman, A.; Jackson, H.; Hristov, H.; Isaacson, R.S.; Saif, N.; Shetty, T.; Etingin, O.; Henchcliffe, C.; Brinton, R.D.; Mosconi, L. Sex and Gender Driven Modifiers of Alzheimer’s: The Role for Estrogenic Control Across Age, Race, Medical, and Lifestyle Risks. Front. Aging Neurosci. 2019, 11, 315. [Google Scholar] [CrossRef]
- Subramaniapillai, S.; Almey, A.; Natasha Rajah, M.; Einstein, G. Sex and gender differences in cognitive and brain reserve: Implications for Alzheimer’s disease in women. Front. Neuroendocrinol. 2021, 60, 100879. [Google Scholar] [CrossRef] [PubMed]
- Geraets, A.F.J.; Leist, A.K. Sex/gender and socioeconomic differences in modifiable risk factors for dementia. Sci. Rep. 2023, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Sramek, J.J.; Murphy, M.F.; Cutler, N.R. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci. 2016, 18, 447–457. [Google Scholar] [CrossRef]
- Jee, H.J.; Shin, W.; Jung, H.J.; Kim, B.; Lee, B.K.; Jung, Y.S. Impact of Sleep Disorder as a Risk Factor for Dementia in Men and Women. Biomol. Ther. 2020, 28, 58–73. [Google Scholar] [CrossRef]
- Franke, K.; Ristow, M.; Gaser, C. Gender-specific impact of personal health parameters on individual brain aging in cognitively unimpaired elderly subjects. Front. Aging Neurosci. 2014, 6, 94. [Google Scholar] [CrossRef][Green Version]
- Ye, K.X.; Sun, L.; Wang, L.; Khoo, A.L.Y.; Lim, K.X.; Lu, G.; Yu, L.; Li, C.; Maier, A.B.; Feng, L. The role of lifestyle factors in cognitive health and dementia in oldest-old: A systematic review. Neurosci. Biobehav. Rev. 2023, 152, 105286. [Google Scholar] [CrossRef]
- Davuluri, S.; Bajpai, A.K.; Thirumurugan, K.; Acharya, K.K. The molecular basis of gender disparities in smoking lung cancer patients. Life Sci. 2021, 267, 118927. [Google Scholar] [CrossRef]
- Schwarzinger, M.; Pollock, B.G.; Hasan, O.S.M.; Dufouil, C.; Rehm, J. Contribution of alcohol use disorders to the burden of dementia in France 2008-13: A nationwide retrospective cohort study. Lancet Public Health 2018, 3, e124–e132. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.E.; Alicea Pauneto, C.D.M.; Barnett, A.M.; Coleman, L.G., Jr. Chronic Ethanol Causes Persistent Increases in Alzheimer’s Tau Pathology in Female 3xTg-AD Mice: A Potential Role for Lysosomal Impairment. Front. Behav. Neurosci. 2022, 16, 886634. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Hu, H.Y.; Ou, Y.N.; Shen, X.N.; Xu, W.; Wang, Z.T.; Dong, Q.; Tan, L.; Yu, J.T. Association of body mass index with risk of cognitive impairment and dementia: A systematic review and meta-analysis of prospective studies. Neurosci. Biobehav. Rev. 2020, 115, 189–198. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, Y.J.; Jang, W.; Son, K.Y.; Park, H.S.; Kim, Y.S. Body mass index trajectories and the risk for Alzheimer’s disease among older adults. Sci. Rep. 2021, 11, 3087. [Google Scholar] [CrossRef]
- Gustafson, D.; Rothenberg, E.; Blennow, K.; Steen, B.; Skoog, I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch. Intern. Med. 2003, 163, 1524–1528. [Google Scholar] [CrossRef]
- Govindugari, V.L.; Golla, S.; Reddy, S.D.M.; Chunduri, A.; Nunna, L.S.V.; Madasu, J.; Shamshabad, V.; Bandela, M.; Suryadevara, V. Thwarting Alzheimer’s Disease through Healthy Lifestyle Habits: Hope for the Future. Neurol. Int. 2023, 15, 162–187. [Google Scholar] [CrossRef] [PubMed]
- Kiliaan, A.J.; Arnoldussen, I.A.; Gustafson, D.R. Adipokines: A link between obesity and dementia? Lancet Neurol. 2014, 13, 913–923. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Eberstein, J.A. Metabolic syndrome and the role of dietary lifestyles in Alzheimer’s disease. J. Neurochem. 2008, 106, 1503–1514. [Google Scholar] [CrossRef]
- Morris, M.C. Nutrition and risk of dementia: Overview and methodological issues. Ann. N. Y. Acad. Sci. 2016, 1367, 31–37. [Google Scholar] [CrossRef]
- Colizzi, C. The protective effects of polyphenols on Alzheimer’s disease: A systematic review. Alzheimers Dement. 2019, 5, 184–196. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006, 20, 2313–2320. [Google Scholar] [CrossRef]
- Dhana, K.; Evans, D.A.; Rajan, K.B.; Bennett, D.A.; Morris, M.C. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology 2020, 95, e374–e383. [Google Scholar] [CrossRef] [PubMed]
- Dhana, K.; Franco, O.H.; Ritz, E.M.; Ford, C.N.; Desai, P.; Krueger, K.R.; Holland, T.M.; Dhana, A.; Liu, X.; Aggarwal, N.T.; et al. Healthy lifestyle and life expectancy with and without Alzheimer’s dementia: Population based cohort study. BMJ 2022, 377, e068390. [Google Scholar] [CrossRef]
- Toro, C.A.; Zhang, L.; Cao, J.; Cai, D. Sex differences in Alzheimer’s disease: Understanding the molecular impact. Brain Res. 2019, 1719, 194–207. [Google Scholar] [CrossRef]
- Barha, C.K.; Falck, R.S.; Skou, S.T.; Liu-Ambrose, T. Personalising exercise recommendations for healthy cognition and mobility in aging: Time to address sex and gender (Part 1). Br. J. Sports Med. 2021, 55, 300–301. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Srivastava, S.; Muhammad, T. Relationship between physical activity and cognitive functioning among older Indian adults. Sci. Rep. 2022, 12, 2725. [Google Scholar] [CrossRef]
- Barha, C.K.; Davis, J.C.; Falck, R.S.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Front. Neuroendocrinol. 2017, 46, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Starkey, S.Y.; Hsiung, G.Y.R.; Tam, R.; Liu-Ambrose, T. Aerobic exercise improves executive functions in females, but not males, without the BDNF Val66Met polymorphism. Biol. Sex Differ. 2023, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.D.; Falkenstein, M. Lifelong physical activity and executive functions in older age assessed by memory based task switching. Neuropsychologia 2015, 73, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Benini, R.; Nunes, P.R.P.; Orsatti, C.L.; Portari, G.V.; Orsatti, F.L. Influence of sex on cytokines, heat shock protein and oxidative stress markers in response to an acute total body resistance exercise protocol. J. Exerc. Sci. Fit. 2015, 13, 1–7. [Google Scholar] [CrossRef]
- Safdar, B.; Jarman, A.F.; Madsen, T.E.; DeLamielleure, L.E.; Zhou, B.; Axtell, R.; Geirsson, A.; Mangi, A.A. Sex Differences in Response to a 12-Week Resistance Training Exercise Intervention After Cardiac Surgery: A Proof-of-Concept Intervention Trial. Clin. Ther. 2025, 47, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.R.; Chuang, Y.F.; Harris, G.C.; Tan, E.J.; Carlson, M.C. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus 2015, 25, 605–615. [Google Scholar] [CrossRef]
- Moschny, A.; Platen, P.; Klaassen-Mielke, R.; Trampisch, U.; Hinrichs, T. Physical activity patterns in older men and women in Germany: A cross-sectional study. BMC Public Health 2011, 11, 559. [Google Scholar] [CrossRef]
- Keyhani, D.; Tartibian, B.; Dabiri, A.; Teixeira, A.M.B. Effect of High-Intensity Interval Training Versus Moderate-Intensity Aerobic Continuous Training on Galectin-3 Gene Expression in Postmenopausal Women: A Randomized Controlled Trial. J. Aging Phys. Act. 2020, 28, 987–995. [Google Scholar] [CrossRef]
- Barha, C.K.; Best, J.R.; Rosano, C.; Yaffe, K.; Catov, J.M.; Liu-Ambrose, T. Sex-Specific Relationship Between Long-Term Maintenance of Physical Activity and Cognition in the Health ABC Study: Potential Role of Hippocampal and Dorsolateral Prefrontal Cortex Volume. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 764–770. [Google Scholar] [CrossRef]
- van Uffelen, J.G.; Chinapaw, M.J.; van Mechelen, W.; Hopman-Rock, M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br. J. Sports Med. 2008, 42, 344–351. [Google Scholar] [CrossRef]
- Barha, C.K.; Hsu, C.L.; Ten Brinke, L.; Liu-Ambrose, T. Biological Sex: A Potential Moderator of Physical Activity Efficacy on Brain Health. Front. Aging Neurosci. 2019, 11, 329. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, R.; Martínez-Vizcaíno, V.; Reina-Gutiérrez, S.; Bizzozero-Peroni, B.; Torres-Costoso, A.; Rodríguez-Gutiérrez, E.; Díaz-Goñi, V.; Cadenas-Sánchez, C. Sex Differences in Effects of Exercise on Physical Function in Aging: A Systematic Review with Meta-Analysis. World J. Mens. Health 2024, 42, 694–711. [Google Scholar] [CrossRef]
- Barha, C.K.; Falck, R.S.; Davis, J.C.; Nagamatsu, L.S.; Liu-Ambrose, T. Sex differences in aerobic exercise efficacy to improve cognition: A systematic review and meta-analysis of studies in older rodents. Front. Neuroendocrinol. 2017, 46, 86–105. [Google Scholar] [CrossRef]
- White, Z.; Terrill, J.; White, R.B.; McMahon, C.; Sheard, P.; Grounds, M.D.; Shavlakadze, T. Voluntary resistance wheel exercise from mid-life prevents sarcopenia and increases markers of mitochondrial function and autophagy in muscles of old male and female C57BL/6J mice. Skelet. Muscle 2016, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Liu-Ambrose, T. Sex differences in exercise efficacy: Is midlife a critical window for promoting healthy cognitive aging? FASEB J. 2020, 34, 11329–11336. [Google Scholar] [CrossRef] [PubMed]
- Barha, C.K.; Hsiung, G.R.; Best, J.R.; Davis, J.C.; Eng, J.J.; Jacova, C.; Lee, P.E.; Munkacsy, M.; Cheung, W.; Liu-Ambrose, T. Sex Difference in Aerobic Exercise Efficacy to Improve Cognition in Older Adults with Vascular Cognitive Impairment: Secondary Analysis of a Randomized Controlled Trial. J. Alzheimers Dis. 2017, 60, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A.; et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar] [CrossRef]
- Stojanovic, M.; Babulal, G.M.; Head, D. Determinants of physical activity engagement in older adults. J. Behav. Med. 2023, 46, 757–769. [Google Scholar] [CrossRef]
- García-Mesa, Y.; López-Ramos, J.C.; Giménez-Llort, L.; Revilla, S.; Guerra, R.; Gruart, A.; Laferla, F.M.; Cristòfol, R.; Delgado-García, J.M.; Sanfeliu, C. Physical exercise protects against Alzheimer’s disease in 3xTg-AD mice. J. Alzheimers Dis. 2011, 24, 421–454. [Google Scholar] [CrossRef]
- Naghibi, S.; Shariatzadeh Joneydi, M.; Barzegari, A.; Davoodabadi, A.; Ebrahimi, A.; Eghdami, E.; Fahimpour, N.; Ghorbani, M.; Mohammadikia, E.; Rostami, M.; et al. Treadmill exercise sex-dependently alters susceptibility to depression-like behaviour, cytokines and BDNF in the hippocampus and prefrontal cortex of rats with sporadic Alzheimer-like disease. Physiol. Behav. 2021, 241, 113595. [Google Scholar] [CrossRef]
- Cortes, C.J.; De Miguel, Z. Precision Exercise Medicine: Sex Specific Differences in Immune and CNS Responses to Physical Activity. Brain Plast. 2022, 8, 65–77. [Google Scholar] [CrossRef]
- Zhou, C.N.; Chao, F.L.; Zhang, Y.; Jiang, L.; Zhang, L.; Luo, Y.M.; Xiao, Q.; Chen, L.M.; Tang, Y. Sex Differences in the White Matter and Myelinated Fibers of APP/PS1 Mice and the Effects of Running Exercise on the Sex Differences of AD Mice. Front. Aging Neurosci. 2018, 10, 243. [Google Scholar] [CrossRef]
- Kohman, R.A.; Bhattacharya, T.K.; Wojcik, E.; Rhodes, J.S. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J. Neuroinflammation 2013, 10, 114. [Google Scholar] [CrossRef]
- Bretland, K.A.; Lin, L.; Bretland, K.M.; Smith, M.A.; Fleming, S.M.; Dengler-Crish, C.M. Irisin treatment lowers levels of phosphorylated tau in the hippocampus of pre-symptomatic female but not male htau mice. Neuropathol. Appl. Neurobiol. 2021, 47, 967–978. [Google Scholar] [CrossRef]
- Short, A.K.; Bui, V.; Zbukvic, I.C.; Hannan, A.J.; Pang, T.Y.; Kim, J.H. Sex-dependent effects of chronic exercise on cognitive flexibility but not hippocampal Bdnf in aging mice. Neuronal Signal 2022, 6, Ns20210053. [Google Scholar] [CrossRef]
- Herring, A.; Donath, A.; Yarmolenko, M.; Uslar, E.; Conzen, C.; Kanakis, D.; Bosma, C.; Worm, K.; Paulus, W.; Keyvani, K. Exercise during pregnancy mitigates Alzheimer-like pathology in mouse offspring. FASEB J. 2012, 26, 117–128. [Google Scholar] [CrossRef]
- García-Mesa, Y.; Pareja-Galeano, H.; Bonet-Costa, V.; Revilla, S.; Gómez-Cabrera, M.C.; Gambini, J.; Giménez-Llort, L.; Cristòfol, R.; Viña, J.; Sanfeliu, C. Physical exercise neuroprotects ovariectomized 3xTg-AD mice through BDNF mechanisms. Psychoneuroendocrinology 2014, 45, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Chelucci, E.; Scarfò, G.; Piccarducci, R.; Rizza, A.; Fusi, J.; Epifani, F.; Carpi, S.; Polini, B.; Betti, L.; Costa, B.; et al. Sex Differences in Blood Accumulation of Neurodegenerative-Related Proteins and Antioxidant Responses to Regular Physical Exercise. J. Mol. Neurosci. 2024, 74, 105. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; García, Y.; Buccieri, K.; Revilla, S.; Suñol, C.; Cristofol, R.; Sanfeliu, C. Gender-Specific Neuroimmunoendocrine Response to Treadmill Exercise in 3xTg-AD Mice. Int. J. Alzheimers Dis. 2010, 2010, 128354. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaudhari, K.; Wong, J.M.; Vann, P.H.; Como, T.; O’Bryant, S.E.; Sumien, N. ApoE Genotype-Dependent Response to Antioxidant and Exercise Interventions on Brain Function. Antioxidants 2020, 9, 553. [Google Scholar] [CrossRef] [PubMed]
| Experimental Model | Type of Physical Exercise | Protocol | Exercise Intensity | Duration | Number of Days Per Week | Effects | Reference |
|---|---|---|---|---|---|---|---|
| Human (≥55 years) | Aerobic training | Classes were 60 min in duration (10 min warm-up, 40 min walking, 10 min cool down) | Moderate, progressed to 60–70% HRR | 6 months | 3 times per week | Female: ↑ executive function (set-shifting), ↑ BDNF; male: ↑ functional fitness | [115] |
| 33 Participants | Treadmill, stationary bicycle, elliptical trainer | 45 to 60 min per session | Progressive to 75–85% HRR | 6 months | 4 times per week | Female: ↑ executive function, ↑ glucose disposal, ↓ insulin, cortisol, BDNF; male:↑ IGF-1, slight cognitive improvement (Trails B) | [116] |
| Participants (aged 61 to 92 years old) | N/A | Physical Activity Scale for the Elderly | N/A | N/A | N/A | Female: mood was a significant predictor of physical activity engagement; male: physical activity levels are correlated with ptau181 accumulation | [117] |
| 3xTg-AD mice | Wheel running | Experiment I: 1 month (starting at 3 months of age, ending at 4 months) Experiment II: 1 month or 6 months (starting at 6 or 1 month of age, ending at 7 months) Experiment III: 6 months (starting at 1 month of age, ending at 7 months) | voluntary | 6 months | N/A | Female: Greater or comparable benefit in AD-related pathology and behavior; male: higher oxidative stress. Both sexes: ↓ cognitive decline, ↓ anxiety/startle response, ↑ antioxidant defense, partial synaptic protection | [118] |
| 80 adult male and female Wistar rats | Treadmill exercise | a running session (30 min) per day, “warm-up” period of 7 min at 5 m/min, “running” 16 min at 10 m/min (weeks 0–7) or 12 m/min (weeks 7–12), and a “cool-up” period of 7 min at 5 m/min | N/A | 12 weeks | 5 days per week | Female: ↑ BDNF (hippocampus), ↑ IL-10 (prefrontal cortex), ↓ TNF-α (hippocampus and PFC), ↓ depressive-like behaviors (including anhedonia), ↓ body weight and food intake; male: ↓ TNF-α (hippocampus), ↓ anhedonia-like behavior, ↓ body weight and food intake; no significant ↑ in BDNF or other cytokines; limited behavioral improvement | [119] |
| APP/PS1 double transgenic mice | Treadmill running | Adaptation period: One week (5 m/min, 10 min/day), training period: 10 m/min, 20 min/day | N/A | 4 months | 6 days per week | Female: Greater improvements in spatial learning and memory, ↑ white matter volume and myelinated fiber parameters; male: Less pronounced changes | [121] |
| BALB/c mice | Wheel running | N/A | N/A | Experiment 1: 10 weeks; Experiment 2: 8 weeks | N/A | Female: ↓ CD86+ and MHC II+ microglia in hippocampus; male: ↓ CD86+ in brain, ↑ MHC II+ in hippocampus and brain | [122] |
| C57Bl/6J mice | Wheel running | 3 months access from age 8–11 months | Voluntary | 3 months | N/A | Female: Conditional fear has no effect, ↑ BDNF mRNA expression; male: Recovery of conditional fear ability, ↓ decline in cognitive ability, ↑ expression of BDNF mRNA | [124] |
| Offspring of APP transgenic mice | Running exercise | Mice ran an average of 0.63 ± 0.08 km daily | Voluntary | N/A | daily | Female: Exercise reduced Aβ burden and inflammation in offspring; improved vascular function and synaptic plasticity | [125] |
| 3xTg—AD mice | Running wheel exercise | N/A | Voluntary | 3 months | N/A | Female: Exercise protected against memory loss, apathy, BPSD-like behaviors, and frailty; partially restored BDNF-CREB signaling | [126] |
| 120 Participants | Aerobic fitness | ≥10 years of aerobic training, 150 min/week | Moderate (Borg Rating and Perceived Exertion scale) | N/A | N/A | Female: ↓ erythrocyte β-amyloid, ↑ plasma antioxidant capacity; male: ↓ tau levels in erythrocytes; ↑ plasma antioxidant capacity | [127] |
| 3xTg-AD mice | Treadmill training | Gradual treadmill training: 15 → 30 min/day, 5 → 7 cm/s | Low to moderate | 5 weeks | 5 days per week | Female: ↑ sensorimotor function; male: ↓ oxidative stress, ↓ GABA-A receptor dysfunction; Both sexes: ↓ Aβ42/40 ratio | [128] |
| GFAP-ApoE3 and GFAP-ApoE4 mice | Treadmill training | Progressive treadmill training to 1 h/day over 12 days (up to 14 m/min, 40 min) | N/A | 8 weeks | N/A | Female: Exercise improved activity, learning, and memory in GFAP-ApoE3 females; reduced IL-6 in GFAP-ApoE3 females, but increased TNFα in GFAP-ApoE4 females; showed better bridge walking and discrimination task performance under Ex-Aox condition; male: Exercise improved motor coordination, learning (Morris Water Maze), and GABA-related function in GFAP-ApoE3 males; less pronounced cognitive gains in GFAP-ApoE4 males | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Shi, L.; Ma, Y.; Liu, T.; Sun, Y.; Jin, Q. Gender Differences in the Effects of Exercise Interventions on Alzheimer’s Disease. Brain Sci. 2025, 15, 812. https://doi.org/10.3390/brainsci15080812
Dong Y, Shi L, Ma Y, Liu T, Sun Y, Jin Q. Gender Differences in the Effects of Exercise Interventions on Alzheimer’s Disease. Brain Sciences. 2025; 15(8):812. https://doi.org/10.3390/brainsci15080812
Chicago/Turabian StyleDong, Yahong, Lei Shi, Yixiao Ma, Tong Liu, Yingjie Sun, and Qiguan Jin. 2025. "Gender Differences in the Effects of Exercise Interventions on Alzheimer’s Disease" Brain Sciences 15, no. 8: 812. https://doi.org/10.3390/brainsci15080812
APA StyleDong, Y., Shi, L., Ma, Y., Liu, T., Sun, Y., & Jin, Q. (2025). Gender Differences in the Effects of Exercise Interventions on Alzheimer’s Disease. Brain Sciences, 15(8), 812. https://doi.org/10.3390/brainsci15080812







