tDCS and Cognitive Training for Fatigued and Cognitively Impaired People with Multiple Sclerosis: An SCED Study
Abstract
1. Introduction
2. Materials and Methods
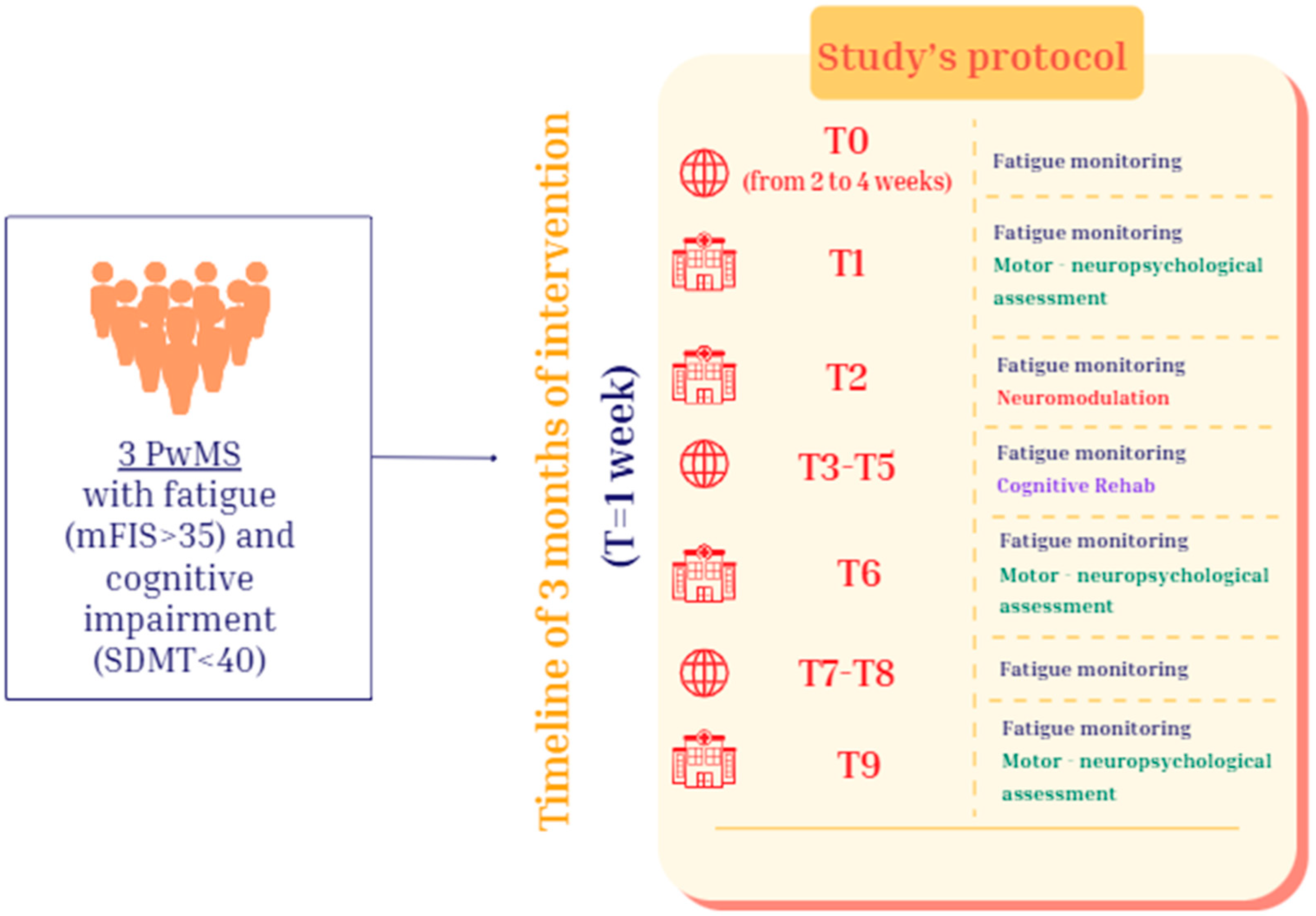
2.1. Study Design
2.2. Participants
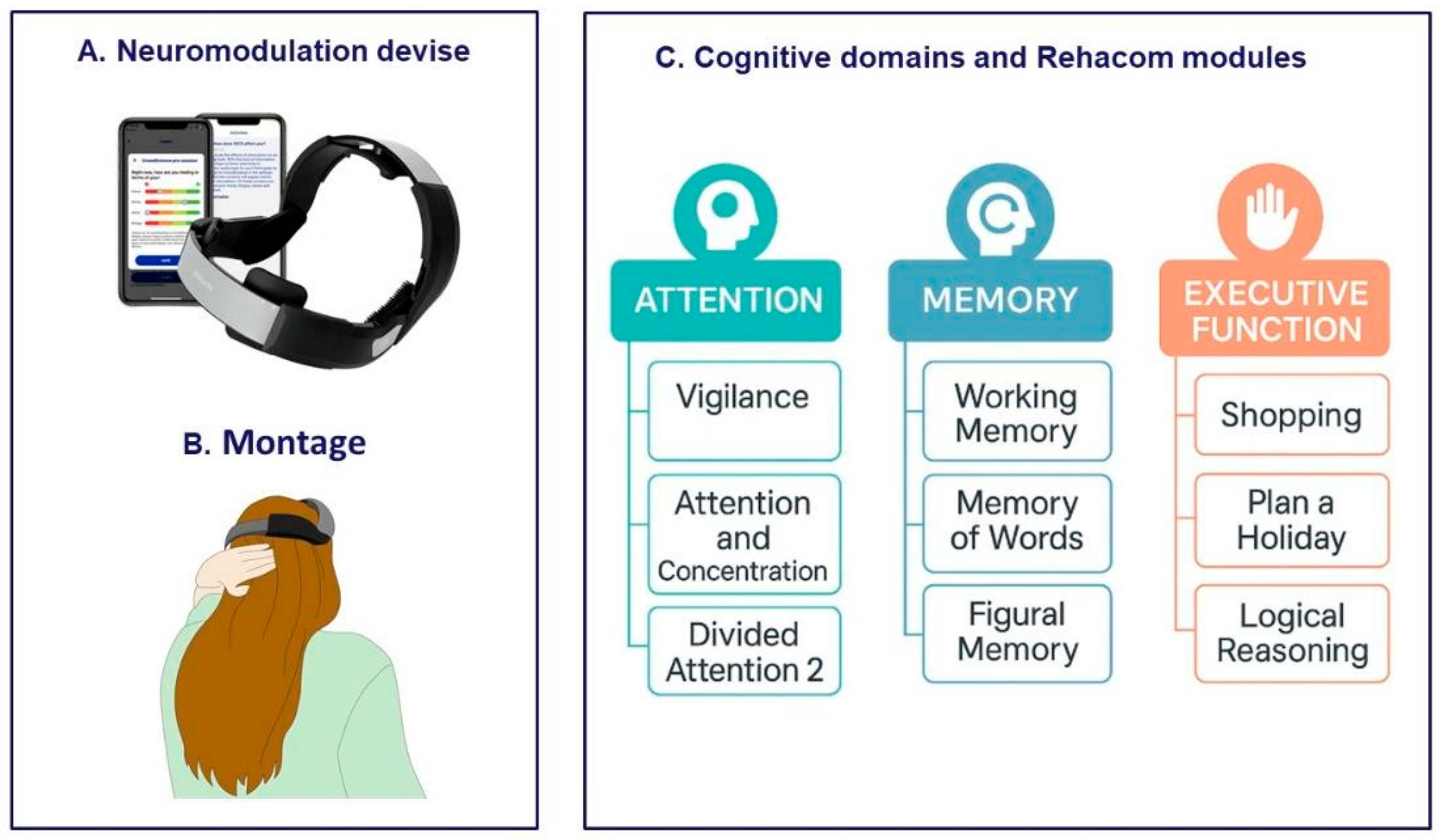
2.3. Procedure
2.4. Statistical Analysis
3. Results
3.1. Feasibility
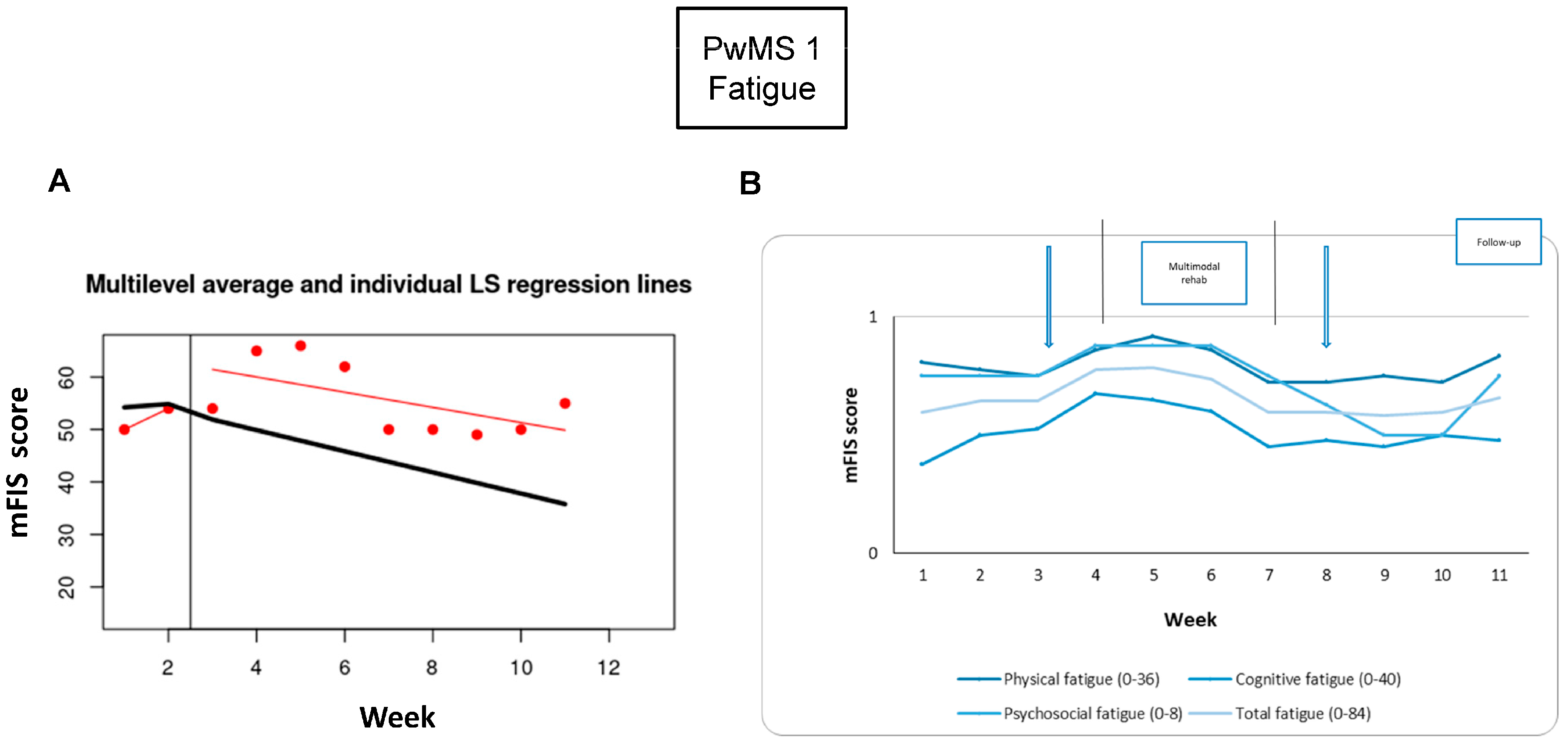
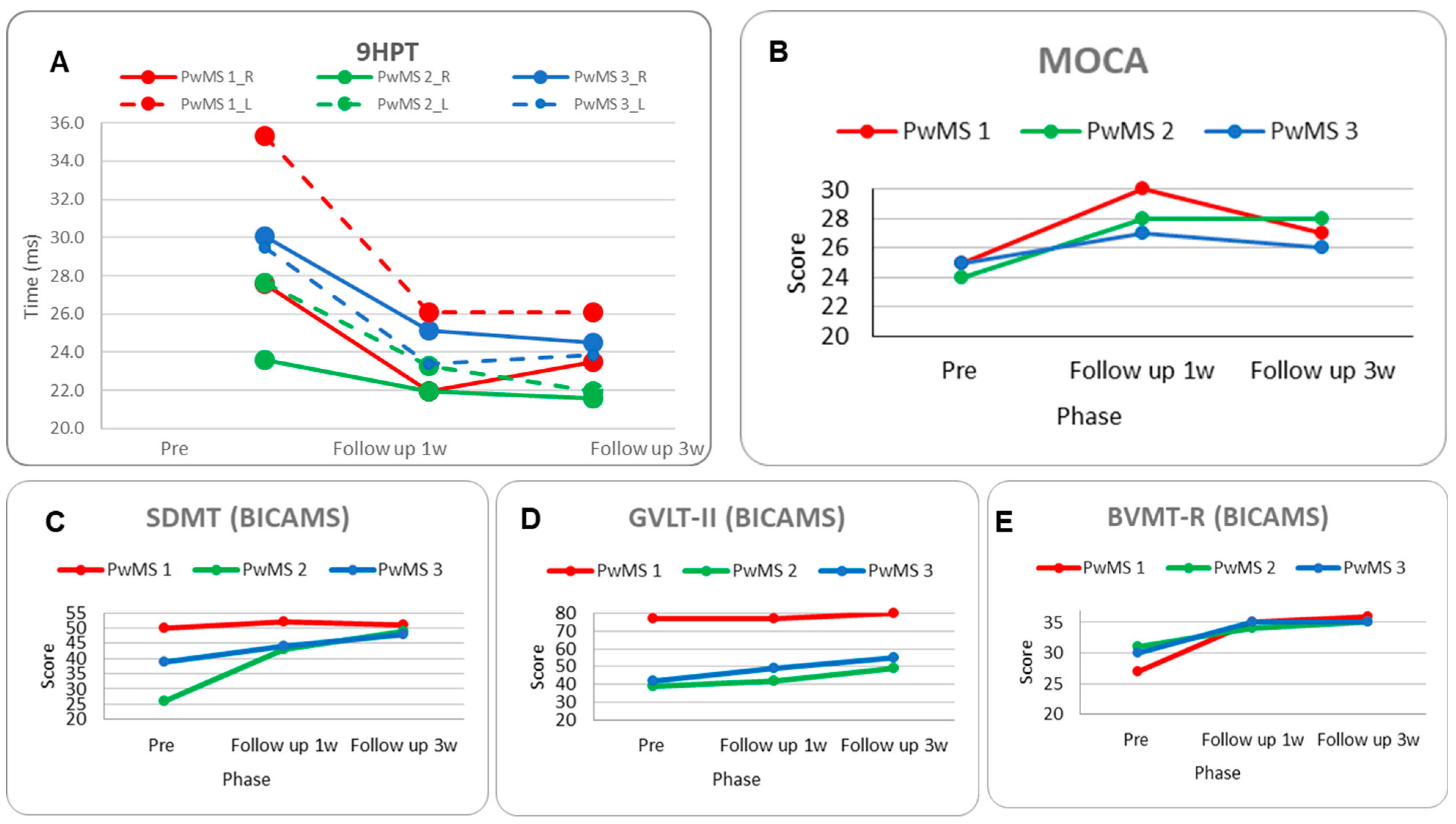
3.2. PwMS n.1
3.2.1. Individual Responsiveness
3.2.2. Acceptance
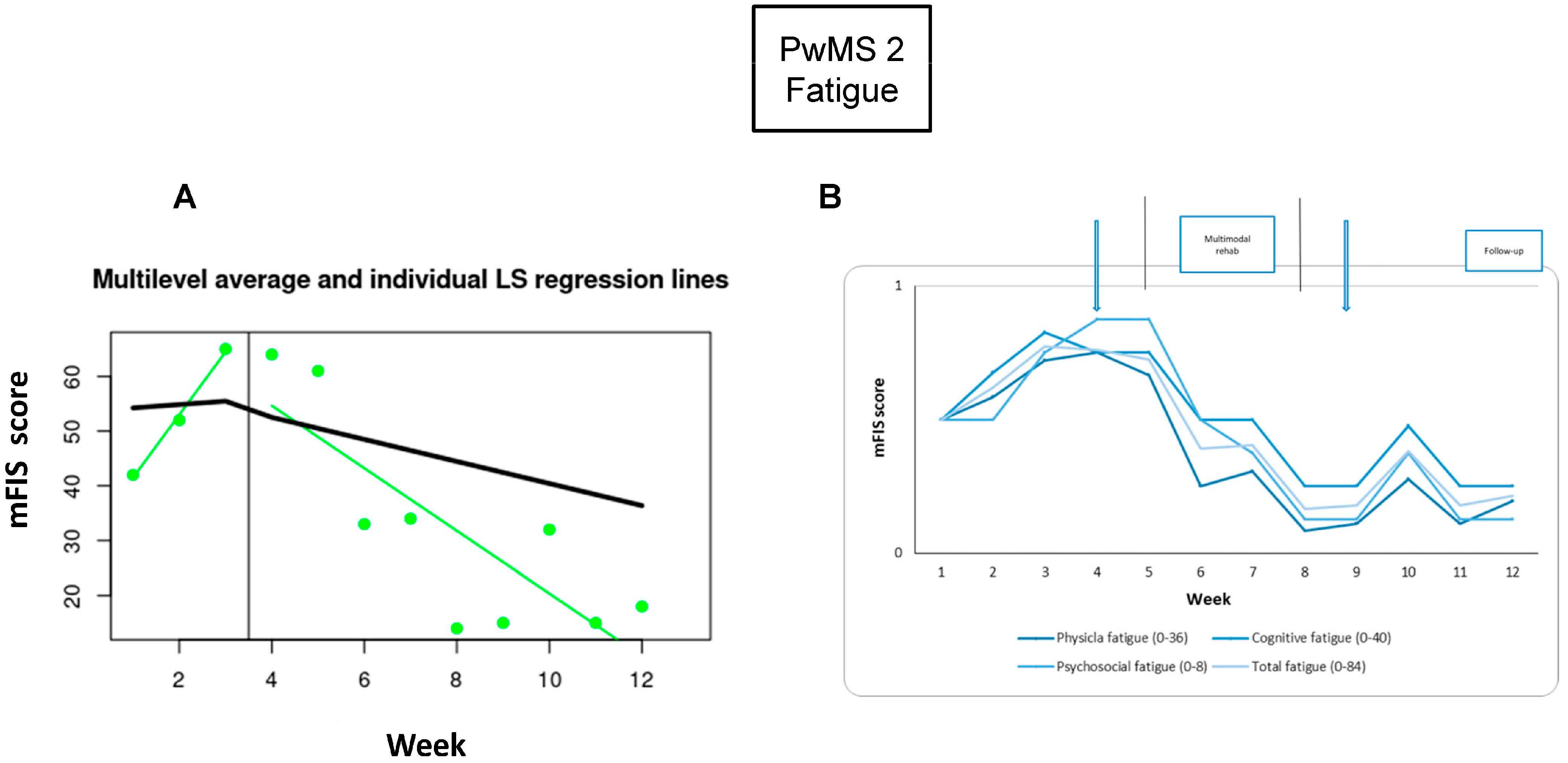
3.3. PwMS n.2
3.3.1. Individual Responsiveness
3.3.2. Acceptance
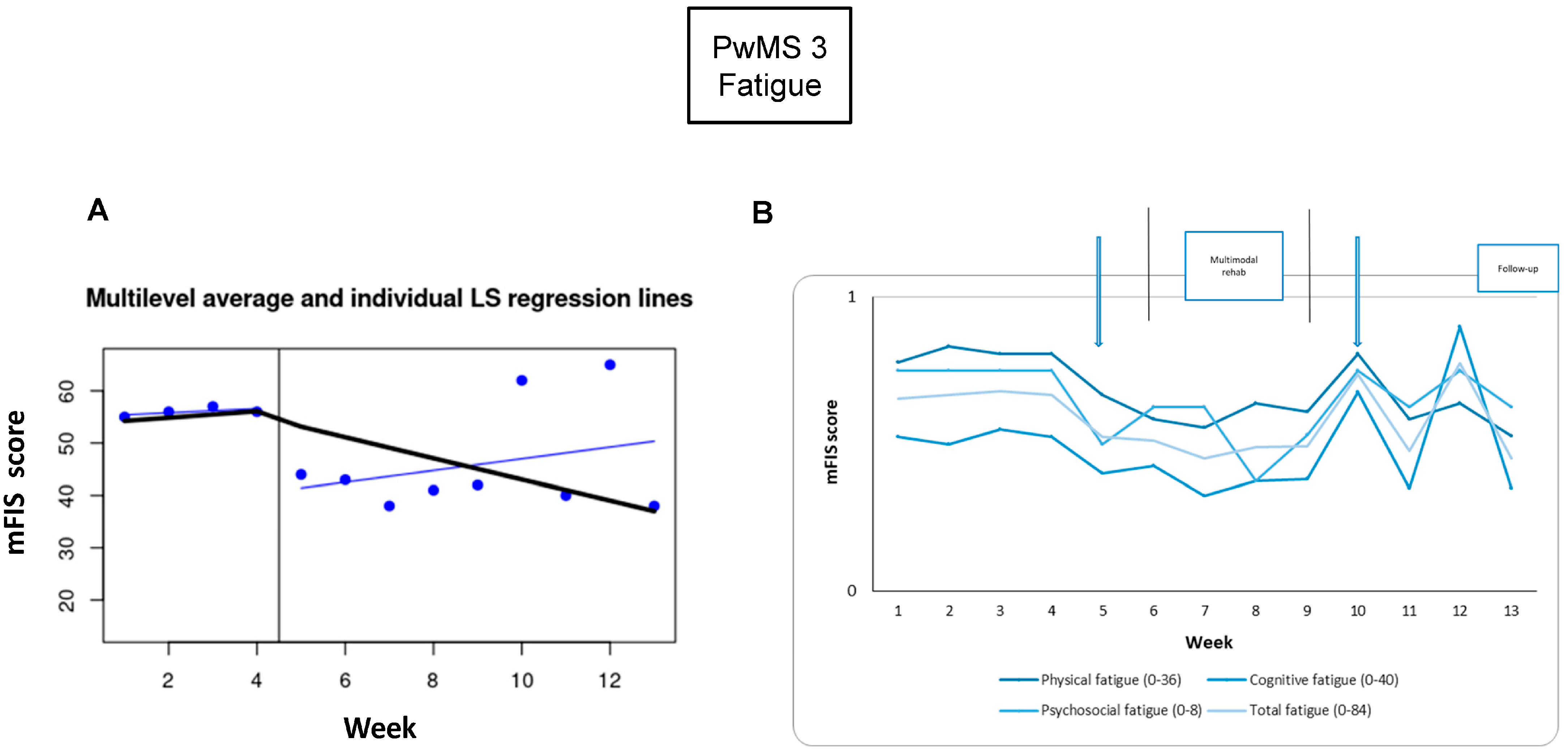
3.4. PwMS n.3
3.4.1. Individual Responsiveness
3.4.2. Acceptance
4. Discussion
4.1. Feasibility
4.2. Individual Responsiveness
4.3. Acceptance
4.4. Multicenter Collaboration Strengthened Personalized Strategies
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PwMS | People with Multiple Sclerosis |
| MS | multiple sclerosis |
| tDCS | transcranial direct current stimulation |
| TMS | transcranial magnetic stimulation |
| mbSCED | Multiple Baseline Single-Case Experimental Design |
| mFIS | modified Fatigue Impact Scale |
| EDSS | Expanded Disability Status Scale |
| SDMT | Symbol Digit Modality Test |
| 9 HPT | 9-Hole Peg Test |
| MoCA | Montreal Cognitive Assessment |
| GVLT | Greek Verbal Learning Test |
| BVMT-R | Brief Visuospatial Memory Test-Revised |
| BICAMS | Brief International Cognitive Assessment for MS |
| BDI | Beck Depression Inventory |
| MSQoL-54 | MS Quality of Life Questionnaire-54 |
| UEQ | User Experience Questionnaire |
| mA | milliAmpere |
| PCS | Physical Composite Score |
| MCS | Mental Composite Score |
Appendix A. RehaCom Protocol
Appendix A.1. RehaCom Adaptive Algorithm Details
Appendix A.2. Rehacom Protocol Details
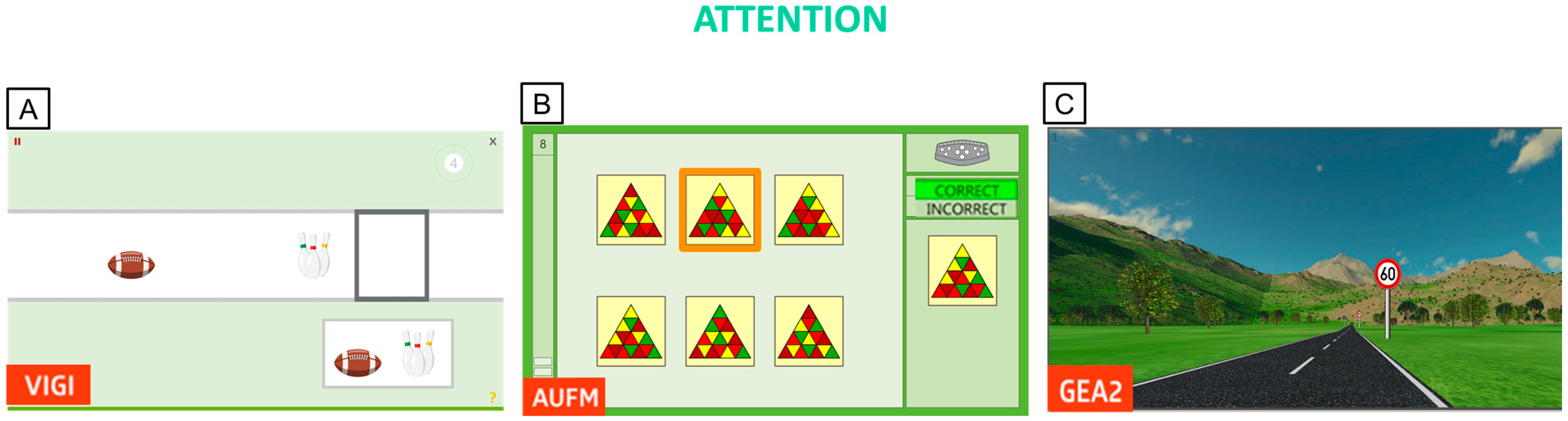
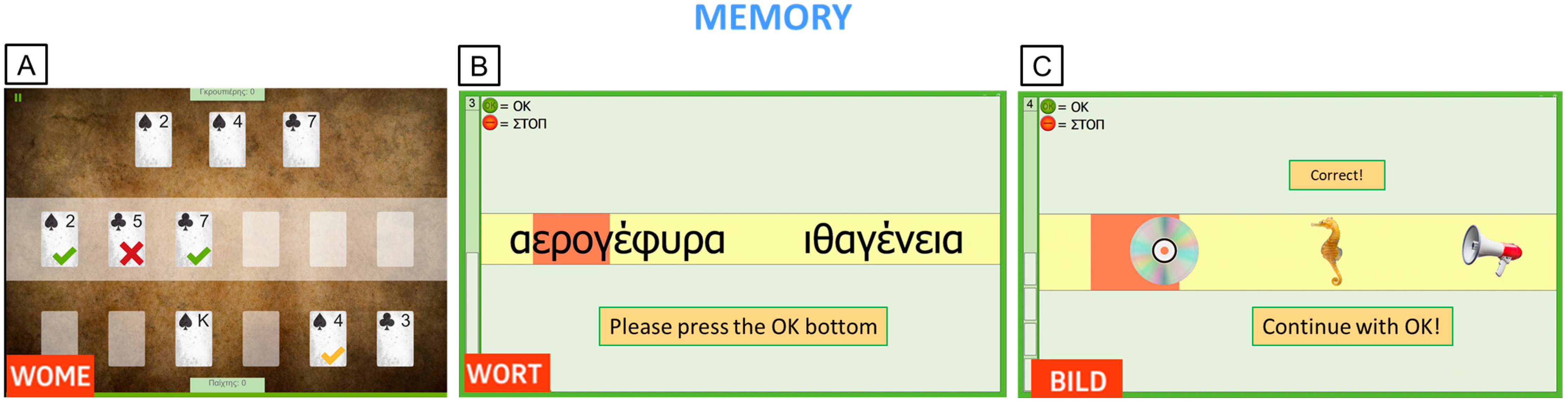
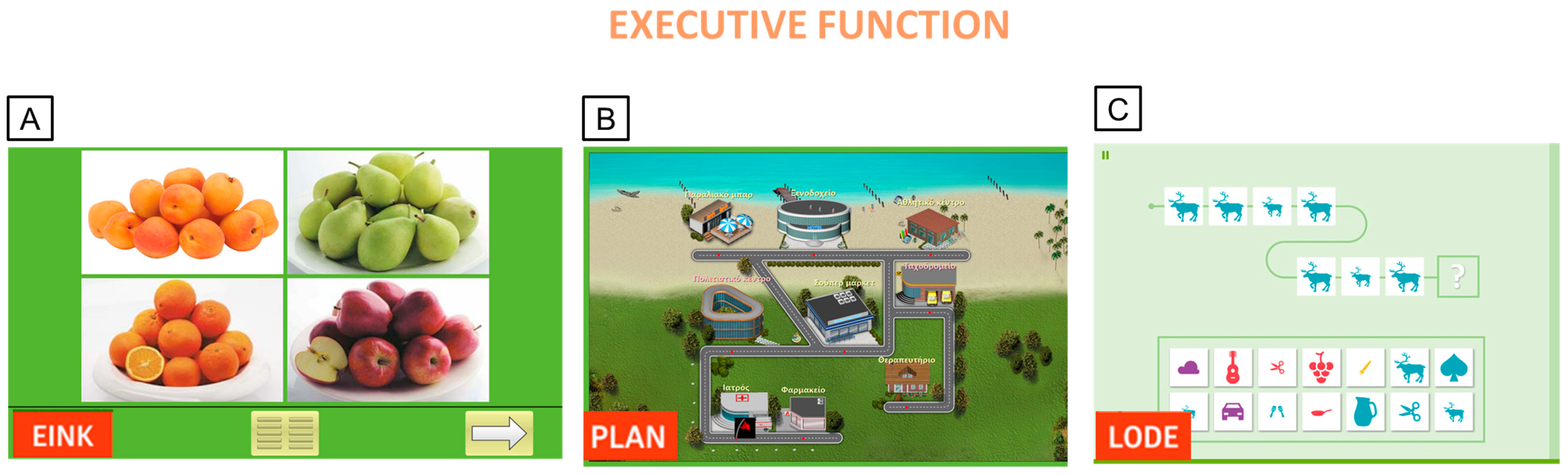
Appendix B. Ad Hoc Questionnaire for PwMS Experience
| Partecipant | Neurostimulation | Cognitive rehabilitation | Fatigue monitoration | ||||||||||||||
| Did it bother you? | Did it weigh on you to do this? | Was it difficult to use the Rehacom program this week? | Can you explain what difficulties you encountered? | Does knowing that there is a questionnaire to monitor fatigue make you feel more understood? | |||||||||||||
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Week1 | Week2 | Week3 | Week1 | Week2 | Week3 | Follow up w3 | |
| PwMS 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 6 | 5 | 5 | Concentration | Physical weakness, which affects concentration and mental activities. | No difficulties | 9 |
| PwMS 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 7 | 1 | No difficulties | Frustration | No difficulties | 8 |
| PwMS 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 | No difficulties | No difficulties | Long working hours shift affected concentration | 9 |
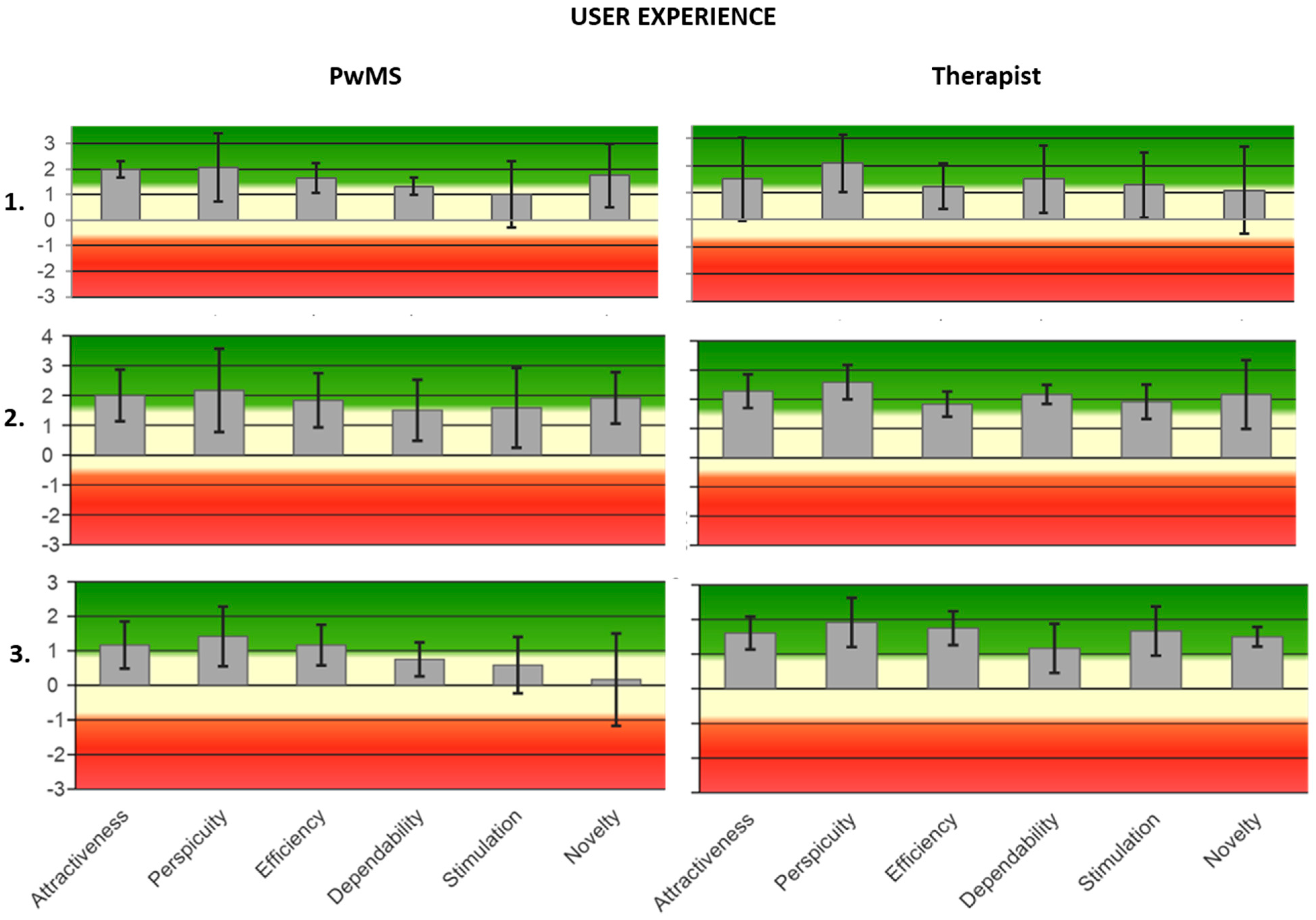
Appendix C. Graphs of User Experience Satisfaction
References
- Giovannoni, G.; Butzkueven, H.; Dhib-Jalbut, S.; Hobart, J.; Kobelt, G.; Pepper, G.; Sormani, M.P.; Thalheim, C.; Traboulsee, A.; Vollmer, T. Brain Health: Time Matters in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2016, 9 (Suppl. S1), S5–S48. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive Impairment in Multiple Sclerosis: Clinical Management, MRI, and Therapeutic Avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Galea, M. Management of Fatigue in Persons with Multiple Sclerosis. Front. Neurol. 2014, 5, 110403. [Google Scholar] [CrossRef]
- Messinis, L.; Kosmidis, M.H.; Lyros, E.; Papathanasopoulos, P. Assessment and Rehabilitation of Cognitive Impairment in Multiple Sclerosis. Int. Rev. Psychiatry 2010, 22, 22–34. [Google Scholar] [CrossRef]
- Engel, C.; Greim, B.; Zettl, U.K. Diagnostics of Cognitive Dysfunctions in Multiple Sclerosis. J. Neurol. 2007, 254 (Suppl. S2), II30–II34. [Google Scholar] [CrossRef]
- Rocca, M.A.; Amato, M.P.; De Stefano, N.; Enzinger, C.; Geurts, J.J.; Penner, I.K.; Rovira, A.; Sumowski, J.F.; Valsasina, P.; Filippi, M. Clinical and Imaging Assessment of Cognitive Dysfunction in Multiple Sclerosis. Lancet Neurol. 2015, 14, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, C.; Lommers, E.; Delrue, G.; Gester, E.; Maquet, P.; Collette, F. The Complex Interplay Between Trait Fatigue and Cognition in Multiple Sclerosis. Psychol. Belg. 2022, 62, 108. [Google Scholar] [CrossRef] [PubMed]
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J. New Insights into the Burden and Costs of Multiple Sclerosis in Europe. Mult. Scler. J. 2017, 23, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple Sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Chiaravalloti, N.D.; DeLuca, J. Cognitive Impairment in Multiple Sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar] [CrossRef]
- Tur, C. Fatigue Management in Multiple Sclerosis. Curr. Treat. Options Neurol. 2016, 18, 26. [Google Scholar] [CrossRef]
- Nourbakhsh, B.; Revirajan, N.; Morris, B.; Cordano, C.; Creasman, J.; Manguinao, M.; Krysko, K.; Rutatangwa, A.; Auvray, C.; Aljarallah, S.; et al. Safety and Efficacy of Amantadine, Modafinil, and Methylphenidate for Fatigue in Multiple Sclerosis: A Randomised, Placebo-Controlled, Crossover, Double-Blind Trial. Lancet Neurol. 2021, 20, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.L.; Genova, H.M.; Deluca, J.; Chiaravalloti, N.D. Information Processing Speed in Multiple Sclerosis: Past, Present, and Future. Mult. Scler. J. 2017, 23, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Mitolo, M.; Venneri, A.; Wilkinson, I.D.; Sharrack, B. Cognitive Rehabilitation in Multiple Sclerosis: A Systematic Review. J. Neurol. Sci. 2015, 354, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leocani, L.; Chieffo, R.; Gentile, A.; Centonze, D. Beyond Rehabilitation in MS: Insights from Non-Invasive Brain Stimulation. Mult. Scler. J. 2019, 25, 1363–1371. [Google Scholar] [CrossRef]
- Bertoli, M.; Tecchio, F. Fatigue in Multiple Sclerosis: Does the Functional or Structural Damage Prevail? SAGE Publications Ltd.: Washington, DC, USA, 2020; Volume 26, pp. 1809–1815. [Google Scholar]
- Uygur-Kucukseymen, E.; Pacheco-Barrios, K.; Yuksel, B.; Gonzalez-Mego, P.; Soysal, A.; Fregni, F. Non-Invasive Brain Stimulation on Clinical Symptoms in Multiple Sclerosis Patients: A Systematic Review and Meta-Analysis. Mult. Scler. Relat. Disord. 2023, 78, 104927. [Google Scholar] [CrossRef]
- Nasios, G.; Bakirtzis, C.; Messinis, L. Cognitive Impairment and Brain Reorganization in MS: Underlying Mechanisms and the Role of Neurorehabilitation. Front. Neurol. 2020, 11, 524745. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Cheng, C.H.; Zanto, T.P.; Gazzaley, A.; Bove, R.M. Effects of Transcranial Direct Current Stimulation on Cognition, Mood, Pain, and Fatigue in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 626113. [Google Scholar] [CrossRef]
- Krasny-Pacini, A.; Evans, J. Single-Case Experimental Designs to Assess Intervention Effectiveness in Rehabilitation: A Practical Guide. Ann. Phys. Rehabil. Med. 2018, 61, 164–179. [Google Scholar] [CrossRef]
- Lillie, E.O.; Patay, B.; Diamant, J.; Issell, B.; Topol, E.J.; Schork, N.J. The N-of-1 Clinical Trial: The Ultimate Strategy for Individualizing Medicine? Pers. Med. 2011, 8, 161–173. [Google Scholar] [CrossRef]
- Doshi, A.; Chataway, J. Multiple Sclerosis, a Treatable Disease. Clin. Med. 2016, 16, s53–s59. [Google Scholar] [CrossRef]
- Charvet, L.; Shaw, M.; Dobbs, B.; Frontario, A.; Sherman, K.; Bikson, M.; Datta, A.; Krupp, L.; Zeinapour, E.; Kasschau, M. Remotely Supervised Transcranial Direct Current Stimulation Increases the Benefit of At-Home Cognitive Training in Multiple Sclerosis. Neuromodulation 2018, 21, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.; Machado, L. Using TDCS Priming to Improve Brain Function: Can Metaplasticity Provide the Key to Boosting Outcomes? Neurosci. Biobehav. Rev. 2017, 83, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended Diagnostic Criteria for Multiple Sclerosis: Guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef]
- Urbaniak, G.C.; Plous, S. Research Randomizer [Computer Software], Version 4.0. 2013. Available online: https://www.randomizer.org/ (accessed on 21 July 2025).
- Strober, L.B.; Bruce, J.M.; Arnett, P.A.; Alschuler, K.N.; DeLuca, J.; Chiaravalloti, N.; Lebkuecher, A.; Di Benedetto, M.; Cozart, J.; Thelen, J.; et al. Tired of Not Knowing What That Fatigue Score Means? Normative Data of the Modified Fatigue Impact Scale (MFIS). Mult. Scler. Relat. Disord. 2020, 46, 102576. [Google Scholar] [CrossRef]
- Van Schependom, J.; D’hooghe, M.B.; Cleynhens, K.; D’hooge, M.; Haelewyck, M.C.; De Keyser, J.; Nagels, G. The Symbol Digit Modalities Test as Sentinel Test for Cognitive Impairment in Multiple Sclerosis. Eur. J. Neurol. 2014, 21, 1219-e72. [Google Scholar] [CrossRef]
- Kellor, M.; Frost, J.; Silberberg, N.; Iversen, I.; Cummings, R. Hand Strength and Dexterity. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 1971, 25, 77–83. [Google Scholar]
- Konstantopoulos, K.; Vogazianos, P. Montreal Cognitive Assessment in a Greek Sample of Patients with Multiple Sclerosis: A Validation Study. Appl. Neuropsychol. Adult 2021, 28, 48–52. [Google Scholar] [CrossRef]
- Messinis, L.; Bakirtzis, C.; Kosmidis, M.H.; Economou, A.; Nasios, G.; Anyfantis, E.; Konitsiotis, S.; Ntoskou, A.; Peristeri, E.; Dardiotis, E.; et al. Symbol Digit Modalities Test: Greek Normative Data for the Oral and Written Version and Discriminative Validity in Patients with Multiple Sclerosis. Arch. Clin. Neuropsychol. 2021, 36, 117–125. [Google Scholar] [CrossRef]
- Vlahou, C.H.; Kosmidis, M.H.; Dardagani, A.; Tsotsi, S.; Giannakou, M.; Giazkoulidou, A.; Zervoudakis, E.; Pontikakis, N. Development of the Greek Verbal Learning Test: Reliability, Construct Validity, and Normative Standards. Arch. Clin. Neuropsychol. 2013, 28, 52–64. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Groninger, L.; Schretlen, D.; Dobraski, M.; Shpritz, B. Revision of the Brief Visuospatial Memory Test: Studies of Normal Performance, Reliability, and, Validity. Psychol. Assess. 1996, 8, 145–153. [Google Scholar] [CrossRef]
- Bakalidou, D.; Voumvourakis, K.; Tsourti, Z.; Papageorgiou, E.; Poulios, A.; Giannopoulos, S. Validity and Reliability of the Greek Version of the Modified Fatigue Impact Scale in Multiple Sclerosis Patients. Int. J. Rehabil. Res. 2014, 37, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, D.; Peritogiannis, V. Depressive Symptoms and Quality of Life in Elderly People Undergoing Physical Therapy. Int. J. Caring Sci. 2021, 14, 854. [Google Scholar]
- Kapina, V. The Working Ability of Patients Suffering from Muliple Sclerosis: Correlation of Clinical, Psychological and Neuroradiological Parameters. Doctoral Dissertation, Aristotle University of Thessaloniki, Thessaloniki, Greece, 2009. [Google Scholar]
- Vickrey, B.G.; Hays, R.D.; Harooni, R.; Myers, L.W.; Ellison, G.W. A Health-Related Quality of Life Measure for Multiple Sclerosis. Qual. Life Res. 1995, 4, 187–206. [Google Scholar] [CrossRef]
- Laugwitz, B.; Held, T.; Schrepp, M. Construction and Evaluation of a User Experience Questionnaire. In HCI and Usability for Education and Work, Proceedings of the 4th Symposium of the Workgroup Human-Computer Interaction and Usability Engineering of the Austrian Computer Society, USAB 2008, Graz, Austria, 20–21 November 2008; Lecture Notes in Computer Science Series (Including Subseries Programming and Software Engineering); Springer: Berlin/Heidelberg, Germany, 2008; pp. 63–76. [Google Scholar] [CrossRef]
- Cancelli, A.; Cottone, C.; Giordani, A.; Migliore, S.; Lupoi, D.; Porcaro, C.; Mirabella, M.; Rossini, P.M.; Filippi, M.M.; Tecchio, F. Personalized, Bilateral Whole-Body Somatosensory Cortex Stimulation to Relieve Fatigue in Multiple Sclerosis. Mult. Scler. J. 2018, 24, 1366–1374. [Google Scholar] [CrossRef]
- Tecchio, F.; Cancelli, A.; Cottone, C.; Zito, G.; Pasqualetti, P.; Ghazaryan, A.; Rossini, P.M.; Filippi, M.M. Multiple Sclerosis Fatigue Relief by Bilateral Somatosensory Cortex Neuromodulation. J. Neurol. 2014, 261, 1552–1558. [Google Scholar] [CrossRef]
- Tecchio, F.; Cancelli, A.; Pizzichino, A.; L’Abbate, T.; Gianni, E.; Bertoli, M.; Paulon, L.; Zannino, S.; Giordani, A.; Lupoi, D.; et al. Home Treatment against Fatigue in Multiple Sclerosis by a Personalized, Bilateral Whole-Body Somatosensory Cortex Stimulation. Mult. Scler. Relat. Disord. 2022, 63, 103813. [Google Scholar] [CrossRef]
- Tecchio, F.; Bertoli, M.; Sbragia, E.; Stara, S.; Pasqualetti, P.; L’Abbate, T.; Croce, P.; Pizzichino, A.; Cancelli, A.; Armonaite, K.; et al. Fatigue Relief in Multiple Sclerosis by Personalized Neuromodulation: A Multicenter Pilot Study [FaremusGE]. Mult. Scler. Relat. Disord. 2025, 94, 106276. [Google Scholar] [CrossRef]
- Naeeni Davarani, M.; Arian Darestani, A.; Hassani-Abharian, P.; Vaseghi, S.; Zarrindast, M.R.; Nasehi, M. RehaCom Rehabilitation Training Improves a Wide-Range of Cognitive Functions in Multiple Sclerosis Patients. Appl. Neuropsychol. Adult 2022, 29, 262–272. [Google Scholar] [CrossRef]
- Kratochwill, T.R.; Hitchcock, J.H.; Horner, R.H.; Levin, J.R.; Odom, S.L.; Rindskopf, D.M.; Shadish, W.R. Single-Case Intervention Research Design Standards. Remedial Spec. Educ. 2012, 34, 26–38. [Google Scholar] [CrossRef]
- Bloom, D.A.; Kaplan, D.J.; Mojica, E.; Strauss, E.J.; Gonzalez-Lomas, G.; Campbell, K.A.; Alaia, M.J.; Jazrawi, L.M. The Minimal Clinically Important Difference: A Review of Clinical Significance. Am. J. Sports Med. 2023, 51, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Feys, P.; Lamers, I.; Francis, G.; Benedict, R.; Phillips, G.; Larocca, N.; Hudson, L.D.; Rudick, R. The Nine-Hole Peg Test as a Manual Dexterity Performance Measure for Multiple Sclerosis. Mult. Scler. J. 2017, 23, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.B.; Amato, M.P.; Boringa, J.; Brochet, B.; Foley, F.; Fredrikson, S.; Hamalainen, P.; Hartung, H.; Krupp, L.; Penner, I.; et al. Brief International Cognitive Assessment for MS (BICAMS): International Standards for Validation. BMC Neurol. 2012, 12, 55. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Kendall, P.C.; Hollon, S.D.; Beck, A.T.; Hammen, C.L.; Ingram, R.E. Issues and Recommendations Regarding Use of the Beck Depression Inventory. Cognit. Ther. Res. 1987, 11, 289–299. [Google Scholar] [CrossRef]
- Heredia-Callejón, A.; García-Pérez, P.; Armenta-Peinado, J.A.; Infantes-Rosales, M.Á.; Rodríguez-Martínez, M.C. Influence of the Therapeutic Alliance on the Rehabilitation of Stroke: A Systematic Review of Qualitative Studies. J. Clin. Med. 2023, 12, 4266. [Google Scholar] [CrossRef]
- Gopal, A.; Bonanno, V.; Block, V.J.; Bove, R.M. Accessibility to Telerehabilitation Services for People With Multiple Sclerosis: Analysis of Barriers and Limitations. Int. J. MS Care 2022, 24, 260–265. [Google Scholar] [CrossRef]
- Nissim, N.R.; O’Shea, A.; Indahlastari, A.; Kraft, J.N.; von Mering, O.; Aksu, S.; Porges, E.; Cohen, R.; Woods, A.J. Effects of Transcranial Direct Current Stimulation Paired With Cognitive Training on Functional Connectivity of the Working Memory Network in Older Adults. Front. Aging Neurosci. 2019, 11, 340. [Google Scholar] [CrossRef]
- Simani, L.; Roozbeh, M.; Shojaei, M.; Ramezani, M.; Roozbeh, M.; Gharehgozli, K.; Rostami, M. The Effectiveness of Anodal TDCS and Cognitive Training on Cognitive Functions in Multiple Sclerosis; a Randomized, Double-Blind, Parallel-Group Study. Mult. Scler. Relat. Disord. 2022, 68, 104392. [Google Scholar] [CrossRef]
- David Ruban, S.; Christina Hilt, C.; Petersen, T. Quality of Life in Multiple Sclerosis: The Differential Impact ofmotor and Cognitive Fatigue. Mult. Scler. J.-Exp. Transl. Clin. 2021, 7, 2055217321996040. [Google Scholar] [CrossRef]
- Riemenschneider, M.; Trénel, P.; Nørgaard, M.; Boesen, F. Multimethodological Validation of the Modified Fatigue Impact Scale in a Danish Population of People with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 65, 104012. [Google Scholar] [CrossRef] [PubMed]
- Traut, H.J.; Guild, R.M.; Munakata, Y. Why Does Cognitive Training Yield Inconsistent Benefits? A Meta-Analysis of Individual Differences in Baseline Cognitive Abilities and Training Outcomes. Front. Psychol. 2021, 12, 662139. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Liang, J.; Xue, J.; Zhu, T.; Jiang, Y.; Wang, J.; Chen, S. The Transfer Effects of Cognitive Training on Working Memory among Chinese Older Adults with Mild Cognitive Impairment: A Randomized Controlled Trial. Front. Aging Neurosci. 2019, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, F.; Stampatori, C.; Scarpazza, C.; Parrinello, G.; Capra, R. Persistence of the Effects of Attention and Executive Functions Intensive Rehabilitation in Relapsing Remitting Multiple Sclerosis. Mult. Scler. Relat. Disord. 2012, 1, 168–173. [Google Scholar] [CrossRef]
- Pappalardo, A. Neurorehabilitation in Persons with Multiple Sclerosis: Scientific Basis and Options of Treatment. Phys. Med. Rehabil. Res. 2016, 1, 71–79. [Google Scholar] [CrossRef][Green Version]
- Filippi, M.; Riccitelli, G.; Mattioli, F.; Capra, R.; Stampatori, C.; Pagani, E.; Valsasina, P.; Copetti, M.; Falini, A.; Comi, G.; et al. Multiple Sclerosis: Effects of Cognitive Rehabilitation on Structural and Functional MR Imaging Measures—An Explorative Study. Radiology 2012, 262, 932–940. [Google Scholar] [CrossRef]
- Chiaravalloti, N.D.; Genova, H.M.; DeLuca, J. Cognitive Rehabilitation in Multiple Sclerosis: The Role of Plasticity. Front. Neurol. 2015, 6, 136401. [Google Scholar] [CrossRef]
- Eschweiler, M.; Bohr, L.; Kessler, J.; Fink, G.R.; Kalbe, E.; Onur, O.A. Combined Cognitive and Motor Training Improves the Outcome in the Early Phase after Stroke and Prevents a Decline of Executive Functions: A Pilot Study. NeuroRehabilitation 2021, 48, 97–108. [Google Scholar] [CrossRef]
- Philip, B.A.; Frey, S.H. Increased Functional Connectivity between Cortical Hand Areas and Praxis Network Associated with Training-Related Improvements in Non-Dominant Hand Precision Drawing. Neuropsychologia 2016, 87, 157. [Google Scholar] [CrossRef]
- Boroujeni, T.S.; Abbasnia, A.; Doosti, M. Training with Non-Dominant Limb: A Helpful Strategy for Motor Function and Dual-Task Cost in Multiple Sclerosis Patients. Int. J. Mot. Control. Learn. 2023, 5, 24–29. [Google Scholar] [CrossRef]
- Gidon, A.; Zolnik, T.A.; Fidzinski, P.; Bolduan, F.; Papoutsi, A.; Poirazi, P.; Holtkamp, M.; Vida, I.; Larkum, M.E. Dendritic Action Potentials and Computation in Human Layer 2/3 Cortical Neurons. Science 2020, 367, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.C.; Martinez, A.P.; Hyland, M.H. The Impact of Cognitive Rehabilitation on Quality of Life in Multiple Sclerosis: A Pilot Study. Mult. Scler. J.-Exp. Transl. Clin. 2021, 7, 20552173211040239. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.M.; Lev-Ari, S. Resilience, Stress, Well-Being, and Sleep Quality in Multiple Sclerosis. J. Clin. Med. 2023, 12, 716. [Google Scholar] [CrossRef]
- Bikson, M.; Hanlon, C.A.; Woods, A.J.; Gillick, B.T.; Charvet, L.; Lamm, C.; Madeo, G.; Holczer, A.; Almeida, J.; Antal, A.; et al. Guidelines for TMS/TES Clinical Services and Research through the COVID-19 Pandemic. Brain Stimul. 2020, 13, 1124–1149. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, H.; Yu, S.; Lee, M.; Kim, H.J.; Kwon, R.; Kim, S.; Fond, G.; Boyer, L.; Rahmati, M.; et al. Effects and Safety of Transcranial Direct Current Stimulation on Multiple Health Outcomes: An Umbrella Review of Randomized Clinical Trials. Mol. Psychiatry 2024, 29, 3789–3801. [Google Scholar] [CrossRef]
- Gianni, E.; Bertoli, M.; Simonelli, I.; Paulon, L.; Tecchio, F.; Pasqualetti, P. TDCS Randomized Controlled Trials in No-Structural Diseases: A Quantitative Review. Sci. Rep. 2021, 11, 16311. [Google Scholar] [CrossRef]
- Fager, S.K.; Burnfield, J.M. Patients’ Experiences with Technology during Inpatient Rehabilitation: Opportunities to Support Independence and Therapeutic Engagement. Disabil. Rehabil. Assist. Technol. 2014, 9, 121–127. [Google Scholar] [CrossRef]
| PwMS1 | PwMS2 | PwMS3 | ||
|---|---|---|---|---|
| Demographic data | Age | 60 | 47 | 42 |
| Sex | F | M | M | |
| Education | 16 | 14 | 12 | |
| Working status | unemployed | employee | freelancer | |
| Social status | single | married | engaged | |
| Clinical data | Years of disease | 31 | 18 | 1 |
| EDSS | 4 | 2.5 | 3 | |
| Relapsing rate | 0 | 0 | 0.67 | |
| Disease Treatment | Glatiramer acetate | Dimethyl fumarate | Ofatumumab |
| Phased Compared | PwMS 1 | PwMS 2 | PwMS 3 |
|---|---|---|---|
| Baseline vs. Post tDCS | 23 | 9 | −20 |
| Pre vs. Post RehaCom | −23 | −77 | −3 |
| Baseline vs. Follow-up at 1 week | −5 | −73 | 16 |
| Baseline vs. Follow-up at 3 weeks | 4 | −68 | −29 |
| Assesment | Test | PwMS1 | PwMS2 | PwMS3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Follow-Up 1 w | Follow-Up 3 w | Pre | Follow-Up 1 w | Follow-Up 3 w | Pre | Follow-Up 1 w | Follow-Up 3 w | |||
| Motor performance | 9HPT | Dominant-hand | 27.6 | 21.9 | 23.5 | 23.6 | 21.9 | 21.6 | 30.1 | 25.1 | 24.5 |
| Non- dominant hand | 35.3 | 26.1 | 26.1 | 27.6 | 23.3 | 22.0 | 29.5 | 23.4 | 23.9 | ||
| Cognitive performance | MOCA | 25 | 30 | 27 | 24 | 28 | 28 | 25 | 27 | 26 | |
| SDMT (BICAMS) | 50 | 52 | 51 | 26 | 43 | 49 | 39 | 44 | 48 | ||
| GVLT-II (BICAMS) | 77 | 77 | 80 | 39 | 42 | 49 | 42 | 49 | 55 | ||
| BVMT-R (BICAMS) | 27 | 35 | 36 | 31 | 34 | 35 | 30 | 35 | 35 | ||
| Quality of life | MSQoL-54 | PCS | 25.0 | 32.8 | 26.3 | 68.5 | 82.2 | 75.6 | 47.2 | 39.7 | 67.1 |
| MCS | 22.1 | 33.7 | 35.7 | 31.4 | 66.1 | 51.7 | 48.6 | 30.6 | 78.8 | ||
| Energy subscale | 0 | 0.48 | 0.48 | 2.4 | 3.84 | 3.84 | 2.88 | 1.92 | 5.28 | ||
| Cognitive subscale | 9 | 10.5 | 11.25 | 4.50 | 11.25 | 12 | 11.25 | 9 | 12 | ||
| Mood | BDI | 17 | 15 | 22 | 23 | 8 | 19 | 9 | 11 | 10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
L’Abbate, T.; Dimitriou, N.K.; Dimakopoulos, G.; Tecchio, F.; Nasios, G. tDCS and Cognitive Training for Fatigued and Cognitively Impaired People with Multiple Sclerosis: An SCED Study. Brain Sci. 2025, 15, 807. https://doi.org/10.3390/brainsci15080807
L’Abbate T, Dimitriou NK, Dimakopoulos G, Tecchio F, Nasios G. tDCS and Cognitive Training for Fatigued and Cognitively Impaired People with Multiple Sclerosis: An SCED Study. Brain Sciences. 2025; 15(8):807. https://doi.org/10.3390/brainsci15080807
Chicago/Turabian StyleL’Abbate, Teresa, Nefeli K. Dimitriou, George Dimakopoulos, Franca Tecchio, and Grigorios Nasios. 2025. "tDCS and Cognitive Training for Fatigued and Cognitively Impaired People with Multiple Sclerosis: An SCED Study" Brain Sciences 15, no. 8: 807. https://doi.org/10.3390/brainsci15080807
APA StyleL’Abbate, T., Dimitriou, N. K., Dimakopoulos, G., Tecchio, F., & Nasios, G. (2025). tDCS and Cognitive Training for Fatigued and Cognitively Impaired People with Multiple Sclerosis: An SCED Study. Brain Sciences, 15(8), 807. https://doi.org/10.3390/brainsci15080807









