Considering Litter Effects in Preclinical Research: Evidence from E17.5 Acid-Sensing Ion Channel 2a Knockout Mice Exposed to Acute Seizures
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. ASIC2a Genotyping
2.3. Breeding and Establishment of Timed Pregnant ASIC2a Mice
2.4. Seizure Induction
2.5. Euthanasia and Tissue Processing
2.6. Statistics and Data Analysis
- Individual embryo/placenta treated as an independent sample. In this analysis model, individual embryos and placentas were grouped based on genotype, sex, and/or seizure exposure and analyzed.
- Averaged data per litter: To calculate embryo and placental weights, measurements were averaged to obtain mean data per sex per genotype for each litter. These data were then reported per dam in each figure.
- Averaged data per litter grouped by maternal genotype. Averaged data were further grouped based on maternal genotype, resulting in 5 groups: ASIC2a+/+ embryos from ASIC2a+/+ dams, ASIC2a+/+ embryos from ASIC2a+/− dams, ASIC2a+/− embryos from ASIC2a+/− dams, ASIC2a−/− embryos from ASIC2a−/− dams, or ASIC2a−/− embryos from ASIC2a+/− dams.
- Individual embryo/placenta as independent samples grouped by genotype and position on the uterine horn.
3. Results
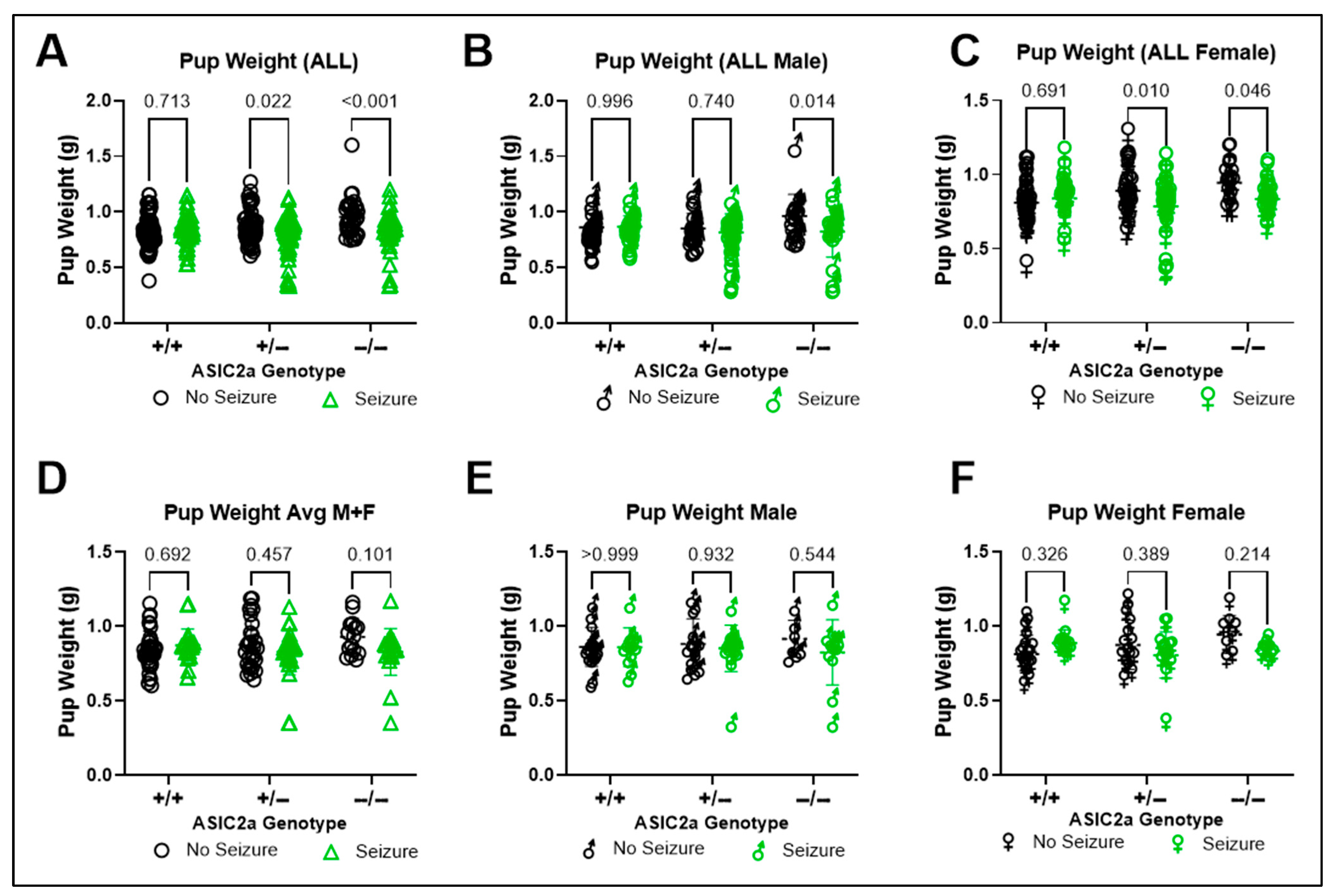
3.1. Effect of ASIC2a Genotype and Acute Seizure Exposure on Embryo Weight
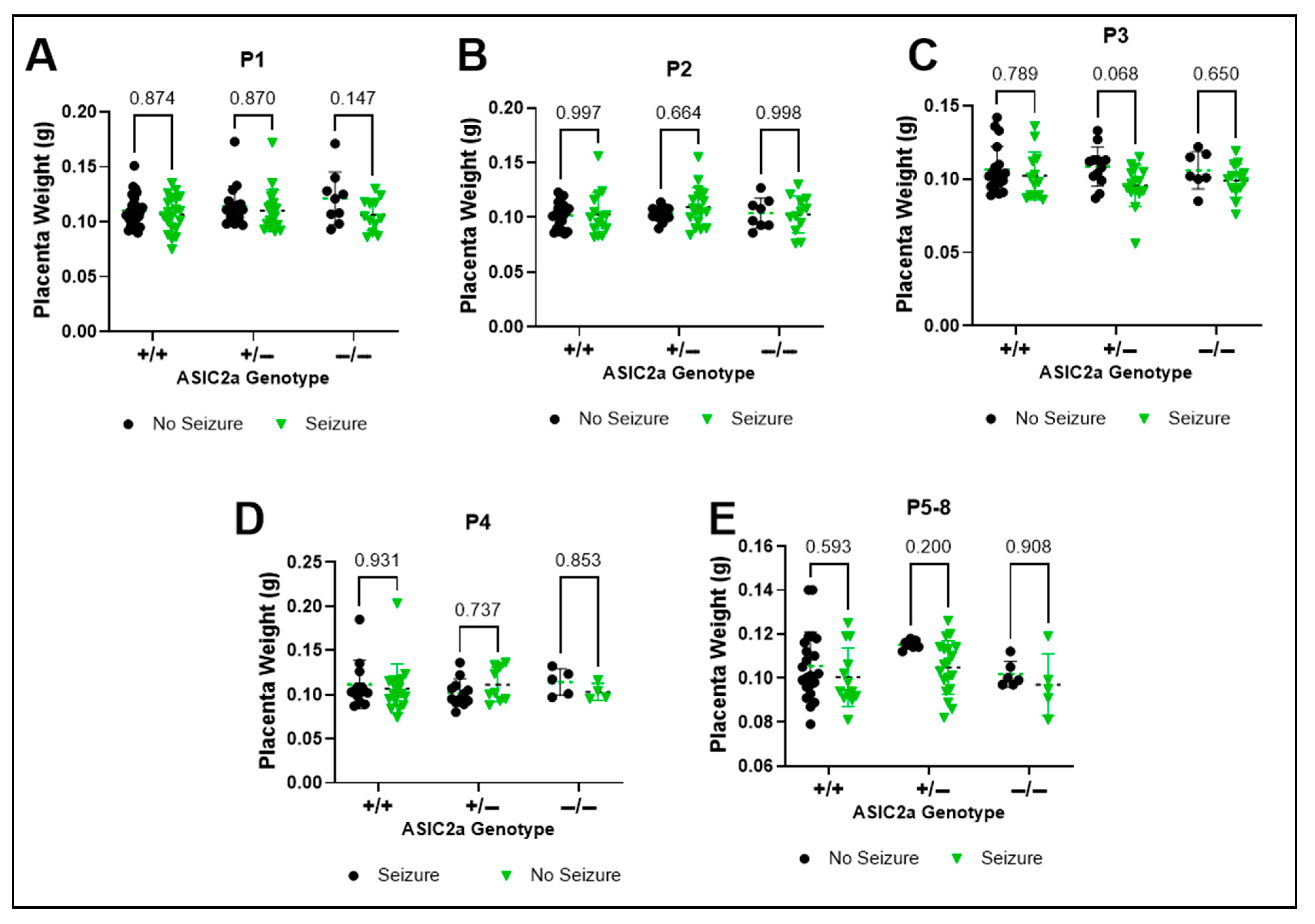
3.2. Effect of ASIC2a Genotype and Acute Seizure Exposure on Placenta Weight
3.3. Influence of Maternal Genotype on Embryo and Placenta Weight
3.4. Influence of Embryo and Placenta Location on the Uterine Horn on Embryo and Placenta Weight
3.5. Effect of Seizure Exposure on Embryo Weight Within Uterine Positions
3.6. Effect of Seizure Exposure on Placenta Weight Within Uterine Positions
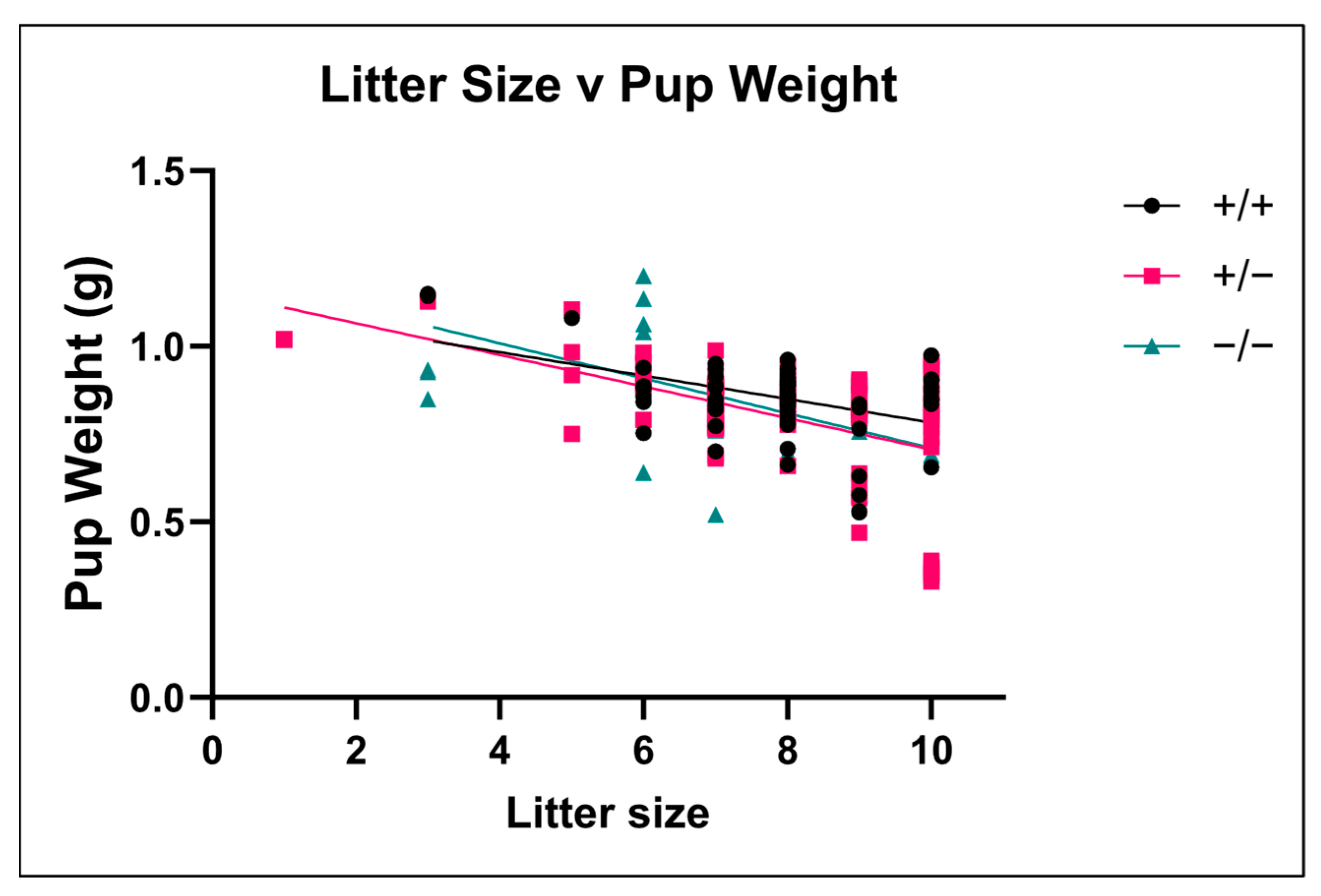
3.7. Effect of Litter Size on Fetal Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASIC2a | Acid-sensing ion channels |
| GD | Gestational day |
| PTZ | Pentylenetetrazol |
| IUGR | Intrauterine Growth Restriction |
References
- Shansky, R.M.; Murphy, A.Z. Considering sex as a biological variable will require a global shift in science culture. Nat. Neurosci. 2021, 24, 457–464. [Google Scholar] [CrossRef]
- Clayton, J.A. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol. Behav. 2018, 187, 2–5. [Google Scholar] [CrossRef]
- Denfeld, Q.E.; Lee, C.S.; Habecker, B.A. A primer on incorporating sex as a biological variable into the conduct and reporting of basic and clinical research studies. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H350–H354. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Jiang, B.Y.; Chen, C.C. Acid-sensing ion channels: Dual function proteins for chemo-sensing and mechano-sensing. J. Biomed. Sci. 2018, 25, 46. [Google Scholar] [CrossRef] [PubMed]
- Deval, E.; Noël, J.; Lay, N.; Alloui, A.; Diochot, S.; Friend, V.; Jodar, M.; Lazdunski, M.; Lingueglia, E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008, 27, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Yermolaieva, O.; Leonard, A.S.; Schnizler, M.K.; Abboud, F.M.; Welsh, M.J. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 2004, 101, 6752–6757. [Google Scholar] [CrossRef]
- Jones-Muhammad, M.; Shao, Q.; Cain-Shields, L.; Shaffery, J.P.; Warrington, J.P. Acid Sensing Ion Channel 2a Is Reduced in the Reduced Uterine Perfusion Pressure Mouse Model and Increases Seizure Susceptibility in Pregnant Mice. Cells 2021, 10, 1135. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, F.; Sha, L.; Lin, W.; Ma, Y.; Yan, H.; Wang, P.; Fang, J.; Huang, Q.; Chen, F.; et al. Association of seizure control during pregnancy with adverse offspring outcomes in women with epilepsy. J. Neurol. Neurosurg. Psychiatry 2025, 96, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Li, Y.; Lu, Y.; Zhang, L.; Wei, L.; Wu, Y. Reproductive and fetal outcomes in women with epilepsy: A systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 2024, 37, 2351196. [Google Scholar] [CrossRef]
- Katsi, V.; Svigkou, A.; Dima, I.; Tsioufis, K. Diagnosis and Treatment of Eclampsia. J. Cardiovasc. Dev. Dis. 2024, 11, 257. [Google Scholar] [CrossRef]
- Naseer, M.I.; Shupeng, L.; Kim, M.O. Maternal epileptic seizure induced by pentylenetetrazol: Apoptotic neurodegeneration and decreased GABAB1 receptor expression in prenatal rat brain. Mol. Brain 2009, 2, 20. [Google Scholar] [CrossRef]
- Rajabzadeh, A.; Bideskan, A.E.; Fazel, A.; Sankian, M.; Rafatpanah, H.; Haghir, H. The effect of PTZ-induced epileptic seizures on hippocampal expression of PSA-NCAM in offspring born to kindled rats. J. Biomed. Sci. 2012, 19, 56. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, L.; Hu, B.; Di, X.; Brennecke, S.P.; Liu, H. Decreased seizure threshold in an eclampsia-like model induced in pregnant rats with lipopolysaccharide and pentylenetetrazol treatments. PLoS ONE 2014, 9, e89333. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Tremble, S.M.; Chan, S.L.; Moseley, J.; LaMarca, B.; Nagle, K.J.; Cipolla, M.J. Magnesium sulfate treatment reverses seizure susceptibility and decreases neuroinflammation in a rat model of severe preeclampsia. PLoS ONE 2014, 9, e113670. [Google Scholar] [CrossRef] [PubMed]
- Warrington, J.P. Placental ischemia increases seizure susceptibility and cerebrospinal fluid cytokines. Physiol. Rep. 2015, 3, e12634. [Google Scholar] [CrossRef]
- Jones-Muhammad, M.; Shao, Q.; Warrington, J.P. Increased seizure sensitivity in pregnant mice with genetic knockdown of acid sensing ion channel 2a is associated with impaired hippocampal inflammatory response. Front. Physiol. 2022, 13, 983506. [Google Scholar] [CrossRef]
- Wise, T.; Roberts, A.J.; Christenson, R.K. Relationships of light and heavy fetuses to uterine position, placental weight, gestational age, and fetal cholesterol concentrations. J. Anim. Sci. 1997, 75, 2197–2207. [Google Scholar] [CrossRef]
- D’Errico, J.N.; Fournier, S.B.; Stapleton, P.A. Considering intrauterine location in a model of fetal growth restriction after maternal titanium dioxide nanoparticle inhalation. Front. Toxicol. 2021, 3, 643804. [Google Scholar] [CrossRef]
- Zha, X.M.; Costa, V.; Harding, A.M.; Reznikov, L.; Benson, C.J.; Welsh, M.J. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J. Neurosci. 2009, 29, 8438–8446. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, L.; Truong, V.; Palmer, J.S.; Wilhelm, D. Novel PCR assay for determining the genetic sex of mice. Sex. Dev. 2013, 7, 207–211. [Google Scholar] [CrossRef]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Barker, D.J.; Godfrey, K.M.; Osmond, C.; Bull, A. The relation of fetal length, ponderal index and head circumference to blood pressure and the risk of hypertension in adult life. Paediatr. Perinat. Epidemiol. 1992, 6, 35–44. [Google Scholar] [CrossRef]
- Reading, A.J. Effects of parity and litter size on the birth weight of inbred mice. J. Mammal. 1966, 47, 111–114. [Google Scholar] [CrossRef]
- Alexander, B.T. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 2003, 41, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, N.B.; Grigore, D.; Yanes, L.L.; Iliescu, R.; Robertson, E.B.; Zhang, H.; Alexander, B.T. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R758–R763. [Google Scholar] [CrossRef]
- Payne, J.A.; Alexander, B.T.; Khalil, R.A. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension 2003, 42, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, P.; Bo, T.; Luo, K. Cognitive and Behavioral Outcomes of Intrauterine Growth Restriction School-Age Children. Pediatrics 2016, 137, e20153868. [Google Scholar] [CrossRef]
- Morsing, E.; Asard, M.; Ley, D.; Stjernqvist, K.; Marsál, K. Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics 2011, 127, e874–e882. [Google Scholar] [CrossRef]
- Raikkonen, K.; Kajantie, E.; Pesonen, A.K.; Heinonen, K.; Alastalo, H.; Leskinen, J.T.; Nyman, K.; Henriksson, M.; Lahti, J.; Lahti, M.; et al. Early life origins cognitive decline: Findings in elderly men in the Helsinki Birth Cohort Study. PLoS ONE 2013, 8, e54707. [Google Scholar] [CrossRef]
- Tuovinen, S.; Aalto-Viljakainen, T.; Eriksson, J.G.; Kajantie, E.; Lahti, J.; Pesonen, A.K.; Heinonen, K.; Lahti, M.; Osmond, C.; Barker, D.J.; et al. Maternal hypertensive disorders during pregnancy: Adaptive functioning and psychiatric and psychological problems of the older offspring. BJOG 2014, 121, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chiou, H.Y.; Lin, H.C.; Lin, H.L. Affect of seizures during gestation on pregnancy outcomes in women with epilepsy. Arch. Neurol. 2009, 66, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Henriksen, T.B.; Ostergaard, J.R. Birth weight in offspring of women with epilepsy. Epidemiol. Rev. 2000, 22, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Henriksen, T.B.; Ostergaard, J.R.; Dam, M. Epilepsy and pregnancy: Effect of antiepileptic drugs and lifestyle on birthweight. BJOG 2000, 107, 896–902. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warrington, J.P.; Pryor, T.; Jones-Muhammad, M.; Shao, Q. Considering Litter Effects in Preclinical Research: Evidence from E17.5 Acid-Sensing Ion Channel 2a Knockout Mice Exposed to Acute Seizures. Brain Sci. 2025, 15, 802. https://doi.org/10.3390/brainsci15080802
Warrington JP, Pryor T, Jones-Muhammad M, Shao Q. Considering Litter Effects in Preclinical Research: Evidence from E17.5 Acid-Sensing Ion Channel 2a Knockout Mice Exposed to Acute Seizures. Brain Sciences. 2025; 15(8):802. https://doi.org/10.3390/brainsci15080802
Chicago/Turabian StyleWarrington, Junie P., Tyranny Pryor, Maria Jones-Muhammad, and Qingmei Shao. 2025. "Considering Litter Effects in Preclinical Research: Evidence from E17.5 Acid-Sensing Ion Channel 2a Knockout Mice Exposed to Acute Seizures" Brain Sciences 15, no. 8: 802. https://doi.org/10.3390/brainsci15080802
APA StyleWarrington, J. P., Pryor, T., Jones-Muhammad, M., & Shao, Q. (2025). Considering Litter Effects in Preclinical Research: Evidence from E17.5 Acid-Sensing Ion Channel 2a Knockout Mice Exposed to Acute Seizures. Brain Sciences, 15(8), 802. https://doi.org/10.3390/brainsci15080802






