The Olfactory Bulbectomy Model of Depression: Brief History, Current Status and Critique
Abstract
1. Introduction
| Type | Description |
|---|---|
| Stress Induced | |
| Learned helplessness [13] | Unavoidable shock leading to failure to escape avoidable shock. |
| Restraint [14] | Chronic exposure to escape-proof confining space. |
| Chronic mild stress [6] | Unpredictable, mildly stressful experiences, e.g., tilted cage, wet bedding. |
| Social defeat [15] | Placement in home cage of dominant resident followed by chronic sensory exposure to resident. |
| Early life adversity [16] | Enforced maternal separation for extended periods. |
| Biologically Induced | |
| Bulbectomy [11] | Surgical ablation of olfactory bulbs bilaterally. |
| Neuroinflammation [17] | Injection of lipopolysaccharide, triggering immune response. |
| Corticosterone admin [18] | Injection or imbibition of “stress hormone.” |
| Neurocircuit manipulation [19] | Optogenetic manipulation of neural circuits, e.g., BNST. |
| Genetic manipulation [20] | Molecular knockout of neurotransmitter, receptor, or reuptake pump. |
2. The Birth of a Model
3. The Procedure
4. Behavioral Sequelae of OBX
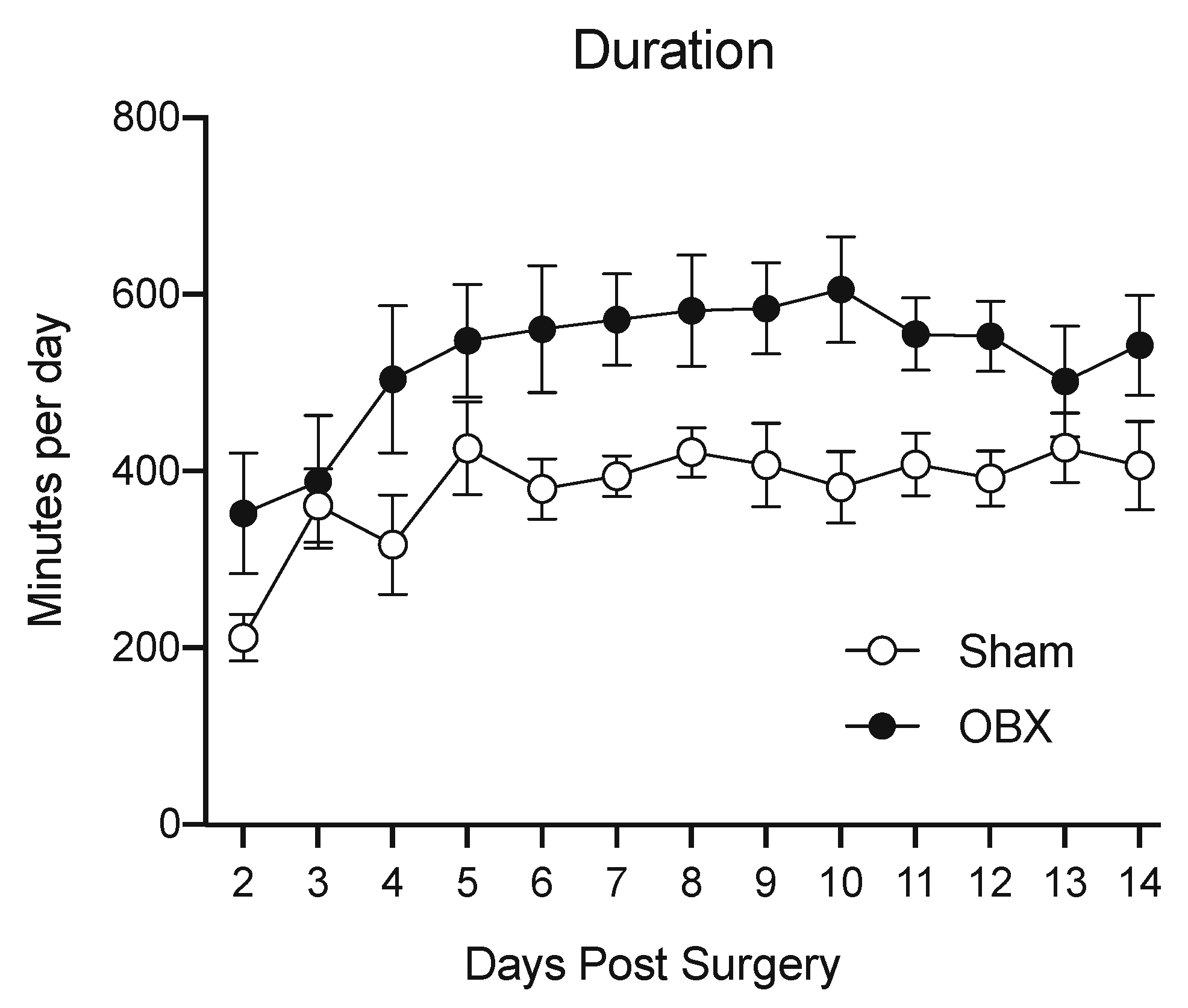
4.1. Locomotion
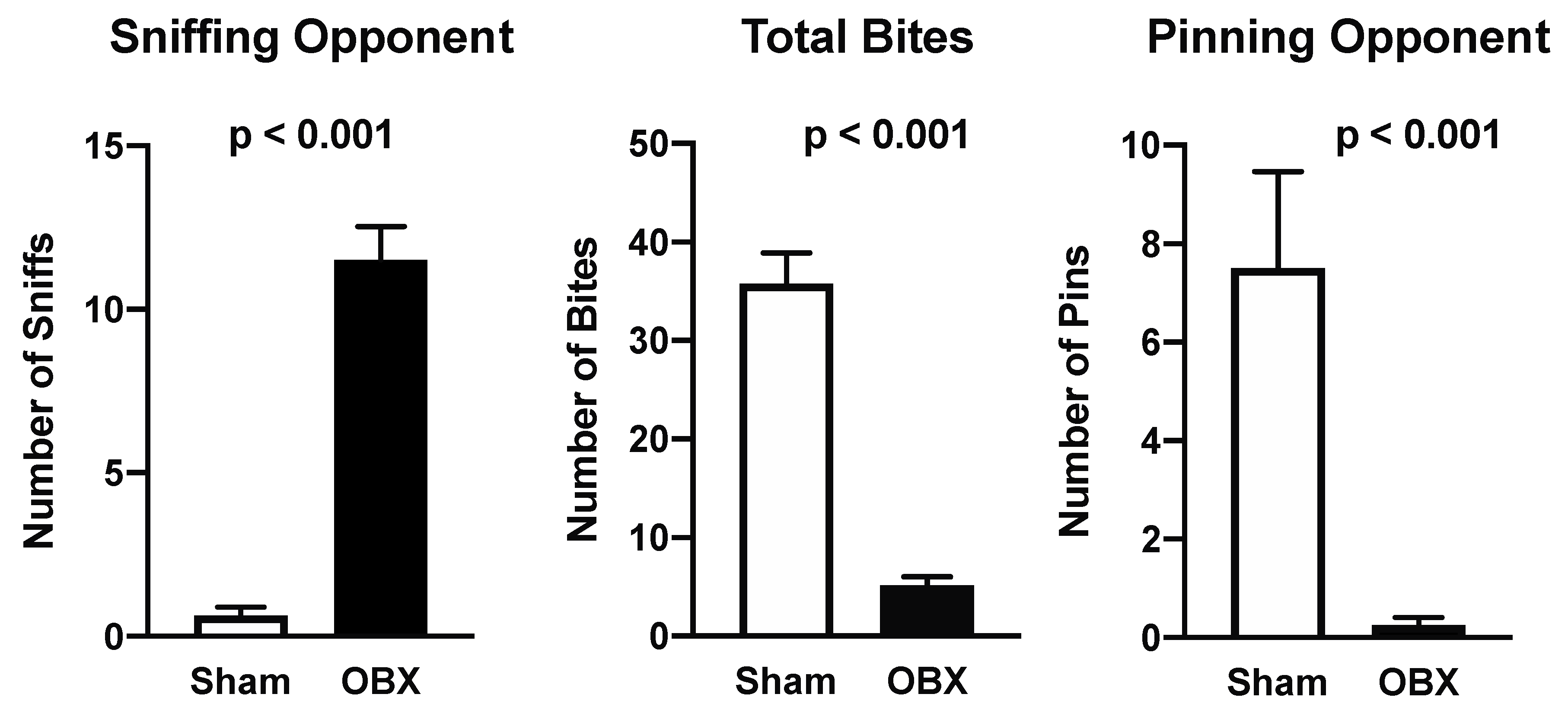
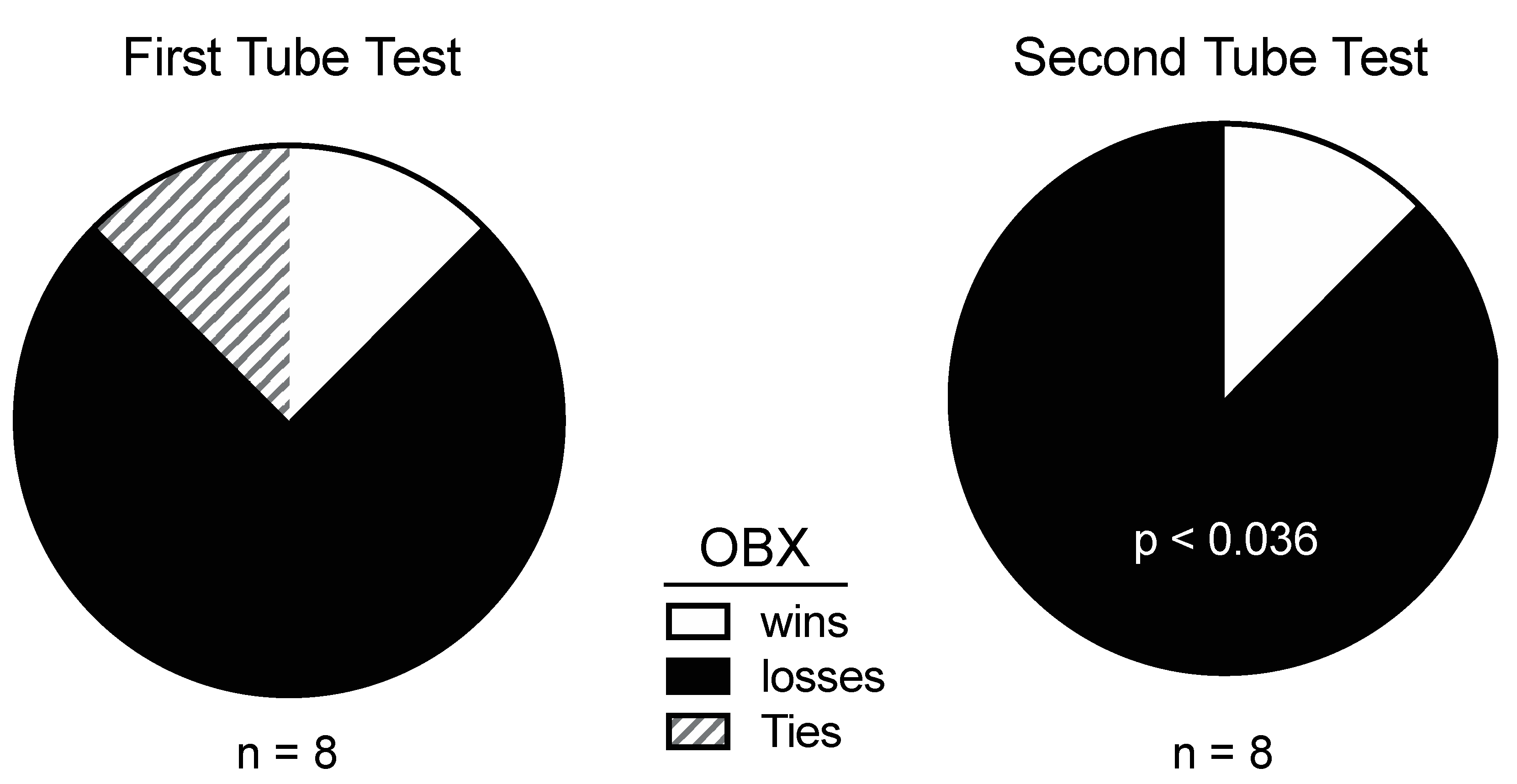
4.2. Aggression
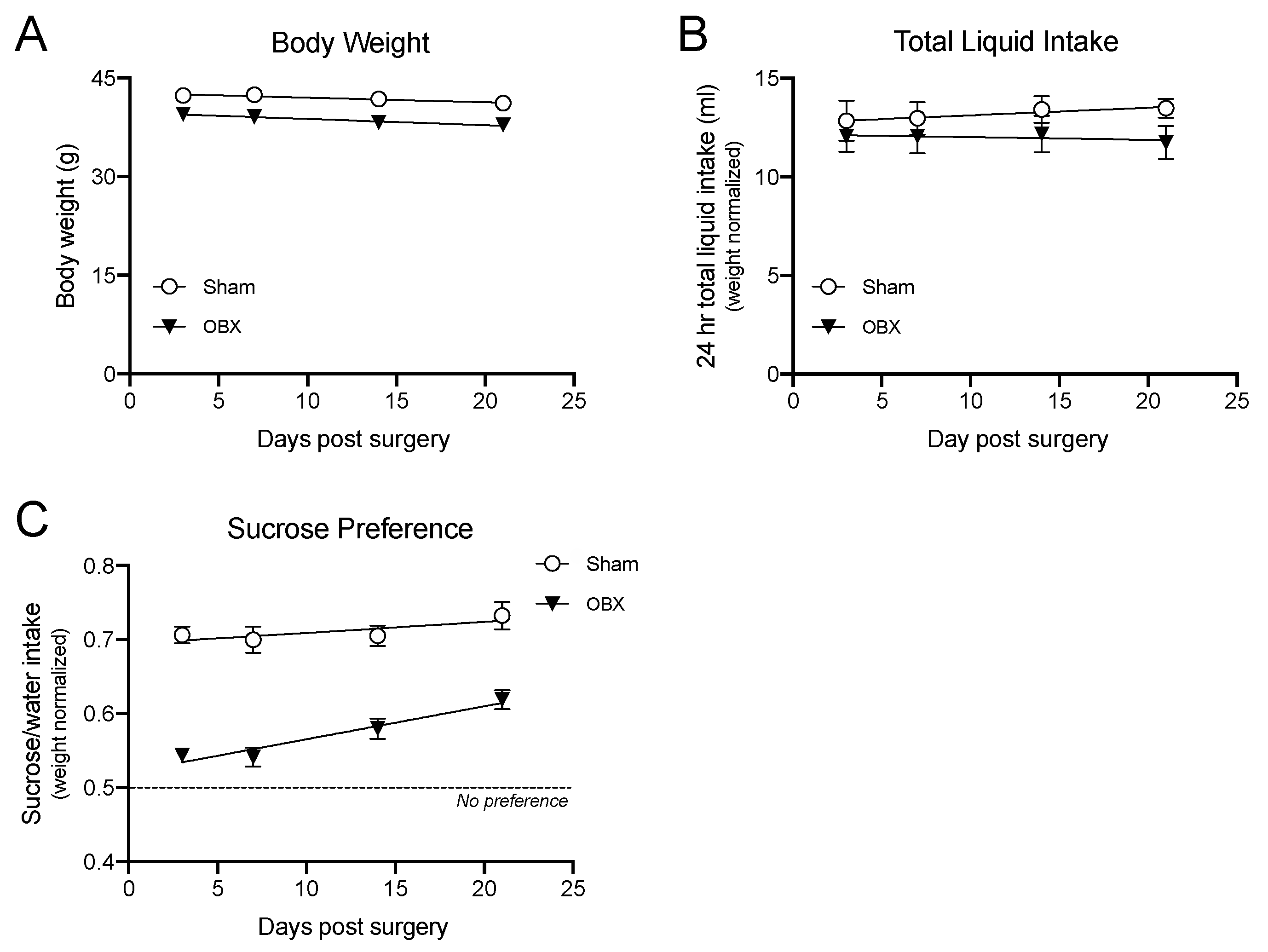
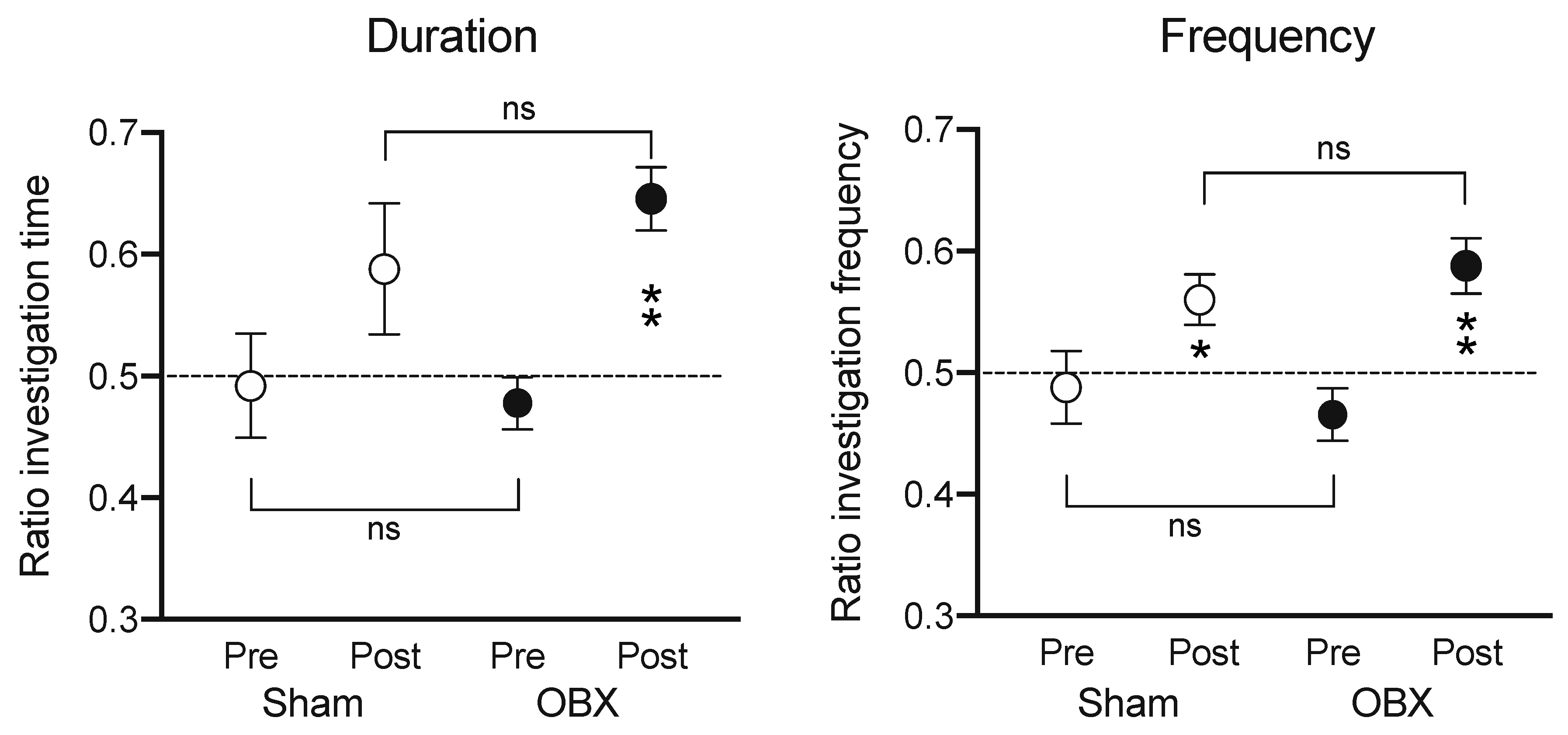
4.3. Pleasure Taking
4.4. Learning and Memory
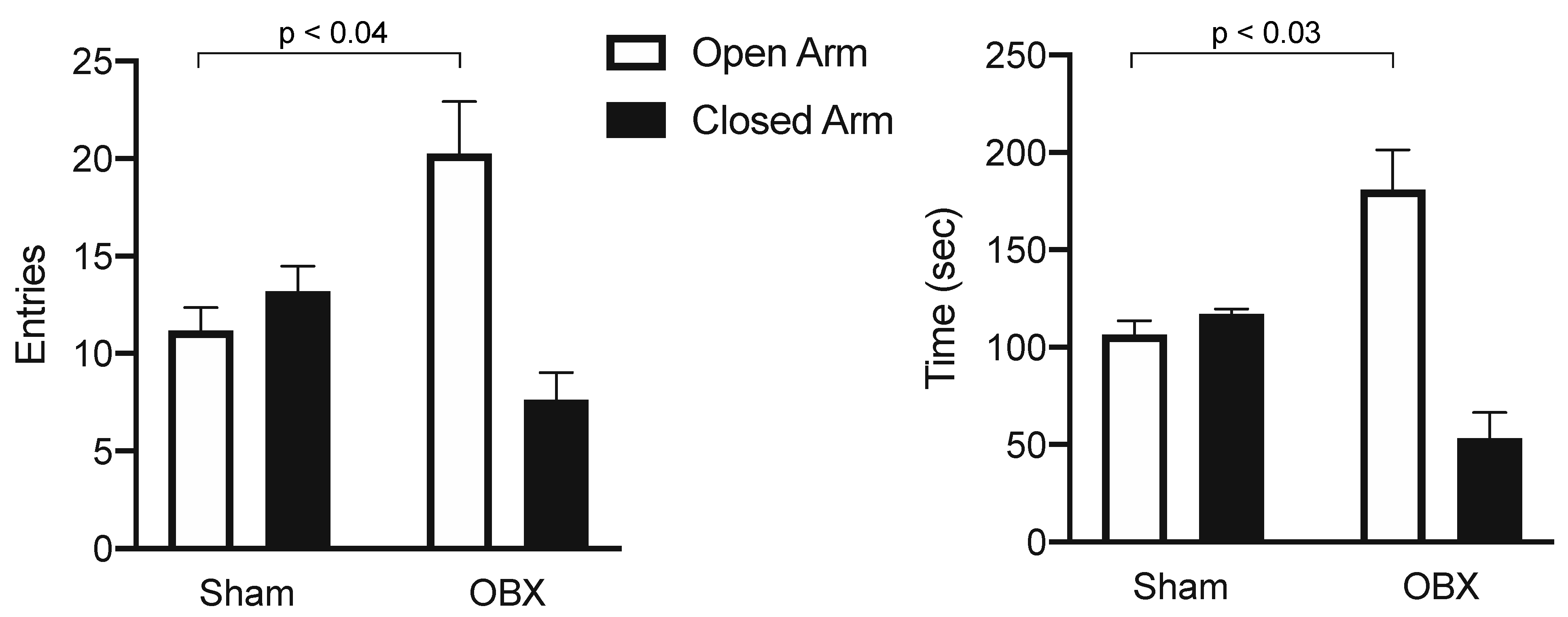
4.5. Fearfulness
5. Physiology
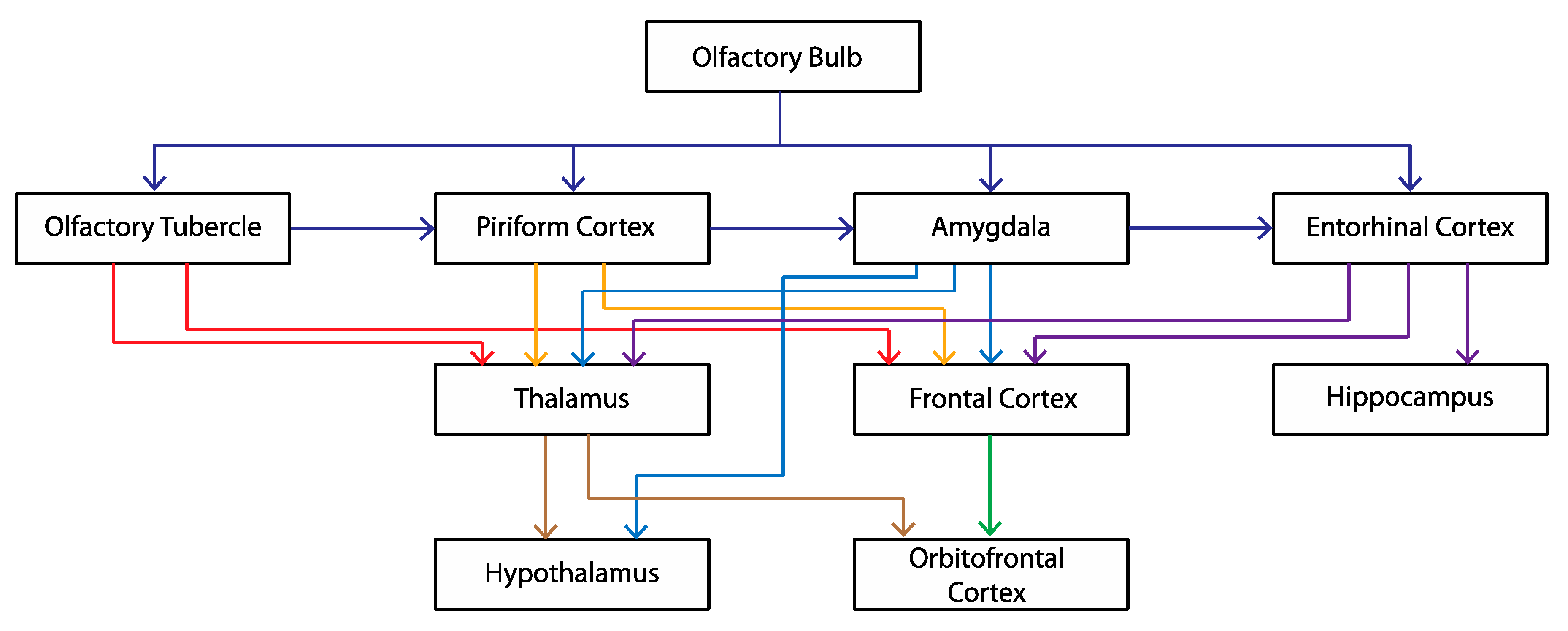
6. Why Should OBX Cause a Depression-like State?
7. Validity of the OBX Model of Depression
7.1. Face Validity
7.2. Construct Validity
7.3. Predictive Validity
8. OBX Model of Depression: Remaining Questions
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: www.nimh.nih.gov (accessed on 17 July 2025).
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2017, 72, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Takeuchi, Y.; Wang, J.; Gellért, L.; Barcsai, L.; Pedraza, L.K.; Nagy, A.J.; Kozák, G.; Nakai, S.; Kato, S.; et al. Reinstating olfactory bulb-derived limbic gamma oscillations alleviates depression-like behavioral deficits in rodents. Neuron 2023, 111, 2065–2075.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chesselet, M.F.; Carmichael, S.T. Animal models of neurological disorders. Neurotherapeutics 2012, 9, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Anisman, H.; Matheson, K. Stress depression and anhedonia: Caveats concerning animal models. Neursci. Biobehav. Rev. 2005, 29, 525–546. [Google Scholar] [CrossRef] [PubMed]
- Willner, P. Validity, reliability and utility of the chronic mild stress model of depression: A 10 year review and evaluation. Psychopharmacology 1997, 134, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Ramaker, M.J.; Dulawa, S.C. Identifying fast-onset antidepressants using rodent models. Mol. Psychiatry 2017, 22, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, R.; Dawe, G.S. OBscure but not OBsolete: Perturbations of the frontal cortex in common between rodent olfactory bulbectomy model and major depression. J. Chem. Neuroant. 2018, 91, 63–100. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.M.; Waters, P.R. The olfactory bulbectomy disease model: A Re-evaluation. Physiol. Behav. 2021, 240, 113548. [Google Scholar] [CrossRef] [PubMed]
- Nekhoroshev, E.V.; Kleshchev, M.A.; Volgin, A.D.; Shevlyakov, A.D.; Bao, X.; Wang, S.; de Abreu, M.S.; Amstislavskaya, T.G.; Kalueff, A.V. Laser-Induced olfactory bulbectomy in adult zebrafish as a novel putative model for Affective Syndrome: A Research Tribute to Brian Leonard. Eur. J. Neurosci. 2025, 61, e16660, Erratum in Eur. J. Neurosci. 2025, 61, e70029. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; Wrynn, A.S.; Leonard, B.E. The olfactory bulbectomized rat as a model of depression: An update. Pharmcol. Ther. 1997, 74, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Leonard, B.E. The olfactory bulbectomised rat as a model of depression. Neurosci. Biobehav. Rev. 2005, 29, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Seligman, M.E.; Rosellini, R.A.; Kozak, M.J. Learned helplessness in the rat: Time course, immunization, and reversibility. J. Comp. Physiol. Psychol. 1975, 88, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Kennett, G.A.; Dickinson, S.L.; Curzon, G. Central serotonergic responses and behavioural adaptation to repeated immobilisation: The effect of the corticosterone synthesis inhibitor metyrapone. Eur. J. Pharmacol. 1985, 119, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.A.; Covington, H.E.; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Vetulani, J. Early maternal separation: A rodent model of depression and a prevailing human condition. Pharmacol. Rep. 2013, 65, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Gregus, A.; Wintink, A.J.; Davis, A.C.; Kalynchuk, L.E. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav. Brain Res. 2005, 156, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.B.; Emmons, E.B.; Anderson, R.M.; Glanz, R.M.; Romig-Martin, S.A.; Narayanan, N.S.; LaLumiere, R.T.; Radley, J.J. A basal forebrain site coordinates the modulation of endocrine and behavioral stress responses via divergent neural pathways. J. Neurosci. 2016, 36, 8687–8699. [Google Scholar] [CrossRef] [PubMed]
- Vinkers, C.H.; Groenink, L.; Pattij, T.; Olivier, B.; Bouwknecht, J.A. 5-HT1A receptor sensitivity in 5-HT1B receptor KO mice is unaffected by chronic fluvoxamine treatment. Eur. J. Pharmacol. 2011, 667, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.B. Kinaesthetic and organic sensations: Their role in the reactions of the white rat to the maze. Psychol. Rev. 1907, 8, 1–100. [Google Scholar] [CrossRef]
- van Riezen, H.; Schnieden, H.; Wren, A. Behavioral changes following olfactory bulbectomy in rats: A possible model for the detection of antidepressant drugs. Br. J. Pharmacol. 1976, 57, 426–427. [Google Scholar]
- Kumadaki, N.; Hitomi, M.; Kumada, S. Effect of psychotherapeutic drugs on hyperemotionality of rats in which the olfactory bulb was removed. Jpn. J. Pharmacol. 1967, 17, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Nurimoto, S.; Ogawa, N. Effects of psychotropic drugs on emotional behavior in rats with limbic lesions with special reference to olfactory bulb ablations. Folia Psychiatr. Neurol. Jpn. 1972, 26, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Brunjes, P. Lessons from lesions: The effects of olfactory bulbectomy. Chem. Senses 1992, 17, 729–763. [Google Scholar] [CrossRef]
- Slotnick, B.; Coppola, D.M. Odor-cued taste avoidance: A simple and robust test of mouse olfaction. Chem. Senses 2015, 40, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.C.; Slotnick, B.M. Olfaction in rats with extensive lesions of the olfactory bulbs: Implications for odor coding. Neuroscience 1998, 84, 849–866. [Google Scholar] [CrossRef] [PubMed]
- Slotnick, B. Olfactory performance of rats after selective deafferentation of the olfactory bulb by 3-methyl indole. Chem. Senses 2007, 32, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Reardon, S. Depression researchers rethink mouse swim test. Nature 2019, 571, 456–457. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Holmes, A. The ascent of mouse: Advances in modelling human depression and anxiety. Nat. Rev. Drug Disc. 2005, 4, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.; Brown, T.S. Exploratory behavior and spontaneous alternation in blind and ansomic rats. J. Comp. Physiol. Psychol. 1969, 68, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Giardina, W.J.; Radek, R.J. Effects of imipramine on the nocturnal behavior of bilateral olfactory bulbectomized rats. Biol. Psychiatry. 1991, 29, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Vinkers, C.H.; Breuer, M.E.; Westphal, K.G.; Korte, S.M.; Oosting, R.S.; Olivier, B.; Groenink, L. Olfactory bulbectomy induces rapid and stable changes in basal and stress-induced locomotor activity heart rate and body temperature responses in the home cage. Neuroscience 2009, 159, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sieck, M.H.; Baumbach, H.D.; Gordon, B.L.; Turner, J.F. Changes in spontaneous odor modulated and shock induced behavior patterns following discrete olfactory system lesions. Physiol. Behav. 1974, 13, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Cairncross, K.D. Olfactory bulbectomy as a model of depression. In Animal Models in Psychopathology; Bond, N.W., Ed.; Academic Press: New York, NY, USA, 1976; pp. 99–128. [Google Scholar]
- Calcagnetti, D.J.; Quatrella, L.A. Schechter MD. Olfactory bulbectomy disrupts the expression of cocaine-induced conditioned place preference. Physiol. Behav. 1996, 59, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.P. The role of the olfactory bulb in limbic mechanisms. Psychol. Bull. 1974, 81, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.S.; Rosenblatt, J.S. Olfactory regulation of maternal behavior in rats. I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J. Comp. Physiol. Psychol. 1974, 86, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhu, H.; Zhou, T.; Wang, S.; Wu, Y.; Hu, H. Using the tube test to measure social hierarchy in mice. Nature Prot. 2019, 14, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Bandler, R.J., Jr.; Chi, C.C. Effects of olfactory bulb removal on aggression: A reevaluation. Physiol. Behav. 1972, 8, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Mucignat-Caretta, C.; Bondi, M.; Caretta, A. Animal models of depression: Olfactory lesions affect amygdala subventricular zone and aggression. Neurobiol. Dis. 2004, 16, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, C.J.; Preti, G. Pheromones in mammals. In Encyclopedia of Neuroscience; Elsevier: Amsterdam, The Netherlands, 2009; pp. 625–632. [Google Scholar]
- Yang, T.; Yang, C.F.; Chizari, M.D.; Maheswaranathan, N.; Burke, K.J., Jr.; Borius, M.; Inoue, S.; Chiang, M.C.; Bender, K.J.; Ganguli, S.; et al. Social Control of Hypothalamus-Mediated Male Aggression. Neuron 2017, 95, 955–970.e4, Erratum in Neuron 2024, 112, 1892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strekalova, T.; Spanagel, R.; Bartsch, D.; Henn, F.A.; Gass, P. Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 2004, 29, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Clepce, M.; Gossler, A.; Reich, K.; Kornhuber, J.; Thuerauf, N. The relation between depression anhedonia and olfactory hedonic estimates—A pilot study in major depression. Neurosci. Lett. 2010, 471, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Rinwa, P.; Kumar, A. Panax quinquefolium involves nitric oxide pathway in olfactory bulbectomy rat model. Physiol. Behav. 2014, 129, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Park, S.S.; Lee, J.M.; Kim, T.W.; Kim, Y.P. Treadmill exercise improves depression-like symptoms by enhancing serotonergic function through upregulation of 5-HT1A expression in the olfactory bulbectomized rats. J. Exerc. Rehabil. 2017, 13, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; Norman, T.R.; Leonard, B.E. Behavioral responses to olfactory bulbectomy in rats maintained in reversed cycle lighting. Eur. Neuropsychopharm. 1993, 3, 363. [Google Scholar] [CrossRef]
- Redmond, A.M.; Kelly, J.P.; Leonard, B.E. Effects of paroxetine and fluvoxamine on behavioral changes in a number of paradigms in the olfactory bulbectomized rat model of depression. J. Serotonin Res. 1994, 1, 199–205. [Google Scholar]
- Jancsár, S.M.; Leonard, B.E. The effects of antidepressant drugs on conditioned taste aversion learning of the olfactory bulbectomized rat. Neuropharmacology 1981, 20, 1341–1345. [Google Scholar] [PubMed]
- Joly, D.; Sanger, D.J. The effects of fluoxetine and zimeldine on the behavior of olfactory bulbectomized rats. Pharmacol. Biochem. Behav. 1986, 24, 199–204. [Google Scholar] [CrossRef] [PubMed]
- King, M.E.; Cairncross, K.D. Effects of olfactory bulb projections to the para hippocampal area of the rat. J. Comp. Neural. 1974, 68, 467–482. [Google Scholar]
- Ennaceur, A.; Meliani, K. A new one-trial test for neurobiological studies of memory in rats III. Spatial vs. non-spatial working memory. Behav. Brain Res. 1988, 51, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Stackman, R.W. Assessing rodent hippocampal involvement in Novel Object Recognition Task. A Review. Behav. Brain Res. 2015, 285, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Han, F.; Shioda, N.; Yamamoto, Y.; Nakajima, T.; Nakagawasai, O.; Tadano, T.; Yeh, J.Z.; Narahashi, T.; Fukunaga, K. Nefiracetam activation of CaM kinase II and protein kinase C mediated by NMDA and metabotropic glutamate receptors in olfactory bulbectomized mice. J. Neurochem. 2009, 110, 170–181. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Mar, A.; Spreekmeester, E.; Rochford, J. Antidepressants preferentially enhance habituation to novelty in the olfactory bulbectomized rat. Psychopharmacology 2000, 150, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Holubova, K.; Kleteckova, L.; Skurlova, M.; Ricny, J.; Stuchlik, A.; Vales, K. Rapamycin blocks the antidepressant effect of ketamine in task-dependent manner. Psychopharmacology 2016, 233, 2077–2097. [Google Scholar] [CrossRef] [PubMed]
- Pause, B.M.; Miranda, A.; Göder, R.; Aldenhoff, J.B.; Ferstl, R. Reduced olfactory performance in patients with major depression. J. Psychiatr. Res. 2001, 35, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Lombion-Pouthier, S.; Vandel, P.; Nezelof, S.; Haffen, E.; Millot, J.L. Odor perception in patients with mood disorders. J. Affect. Disord. 2006, 90, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Pollatos, O.; Albrecht, J.; Kopietz, R.; Linn, J.; Schoepf, V.; Kleemann, A.M.; Schreder, T.; Schandry, R.; Wiesmann, M. Reduced olfactory sensitivity in subjects with depressive symptoms. J. Affect. Disord. 2007, 102, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Negoias, S.; Croy, I.; Gerber, J.; Puschmann, S.; Petrowski, K.; Joraschky, P.; Hummel, T. Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience 2010, 169, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Symmank, A.; Schellong, J.; Hummel, C.; Gerber, J.; Joraschky, P.; Hummel, T. Olfaction as a marker for depression in humans. J. Affect. Disord. 2014, 160, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, B.; El-Hage, W.; Chabanet, C.; Gaillard, P.; Belzung, C.; Camus, V. Olfactory anhedonia and negative olfactory alliesthesia in depressed patients. Psychiatry Res. 2010, 176, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zucco, G.M.; Bollini, F. Odour recognitionmemory and odour identification in patients with mild and severe major depressive disorders. Psychiatry Res. 2011, 190, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Hummel, T. Olfaction as a marker for depression. J. Neurol. 2017, 264, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Czéh, B.; Fuchs, E.; Wiborg, O.; Simon, M. Animal models of major depression and their clinical implications. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 293–310. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, B.; O’Conner, W.T.; Leonard, B.E. Depressed neutrophil phagocytosis in the rat following olfactory bulbectomy reversed by chronic desipramine treatment. Med. Sci. Res. 1987, 15, 276–278. [Google Scholar]
- Song, C.; Leonard, B.E. Serotonin reuptake inhibitors reverse the impairments of behaviour neurotransmitter and immune functions in the olfactory bulbectomized rat. Human Psychopharmacol. 1994, 9, 135–146. [Google Scholar] [CrossRef]
- Cairncross, K.D.; Wren, A.; Cox, B.; Schnieden, H. Effects of olfactory bulbectomy and domicile on stress-induced corticosterone release in the rat. Physiol. Behav. 1977, 19, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J. The dexamethasone suppression test for melancholia. Br. J. Psychiatry. 1982, 140, 292–304. [Google Scholar] [CrossRef] [PubMed]
- van Riezen, H.; Leonard, B.E. Effects of psychotropic drugs on the behavior and neurochemistry of olfactory bulbectomized rats. Pharmacol. Ther. 1990, 47, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Healy, D.; Carney, P.A.; Leonard, B.E. Monoamine-related markers of depression: Changes following treatment. J. Psychiatr. Res. 1982–1983, 17, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Jancsàr, S.M.; Leonard, B.E. Changes in neurotransmitter metabolism following olfactory bulbectomy in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 1984, 8, 263–269. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US); Institute of Medicine (US). Committee on Depression, Parenting Practices, and the Healthy Development of Children. In Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention; England, M.J., Sim, L.J., Eds.; National Academies Press: Washington, DC, USA, 2009. [Google Scholar] [PubMed]
- Hendrie, C.; Pickles, A.; Stanford, S.C.; Robinson, E. The failure of the antidepressant drug discovery process is systemic. J. Psychopharmacol. 2013, 27, 407–413; discussion 413–416. [Google Scholar] [CrossRef] [PubMed]
- Alberts, J.R. Producing and interpreting experimental olfactory deficits. Physiol. Behav. 1974, 12, 657–670. [Google Scholar] [CrossRef] [PubMed]
- McBride, K.; Slotnick, B.; Margolis, F.L. Does intranasal application of zinc sulfate produce anosmia in the mouse? An olfactometric and anatomical study. Chem. Senses 2003, 28, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Slotnick, B.M.; Gutman, L.A. Evaluation of intranasal zinc sulfate treatment on olfactory discrimination in rats. J. Comp. Physiol. Psychol. 1977, 91, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Yurttas, C.; Schmitz, C.; Turgut, M.; Strekalova, T.; Steinbusch, H.W.M. The olfactory bulbectomized rat model is not an appropriate model for studying depression based on morphological/stereological studies of the hippocampus. Brain. Res. Bull. 2017, 134, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, N.A.; Potenza, M.N.; Chamberlain, S.R.; Berlin, H.A.; Menzies, L.; Bechara, A.; Sahakian, B.J.; Robbins, T.W.; Bullmore, E.T.; Hollander, E. Probing compulsive and impulsive behaviors from animal models to endophenotypes: A narrative review. Neuropsychopharmacology 2010, 35, 591–604. [Google Scholar] [CrossRef] [PubMed]
| Symptoms | Analogue in OBX Rodent |
|---|---|
| Depressed mood | No analog |
| Diminished interest/pleasure in activities | Decrease in sucrose preference |
| Significant increase or decrease in weight | Little or none |
| Change in sleep | Little or no change in circadian rhythm |
| Change in activity level (locomotion) | Increased in open field and home cage |
| Decreased energy | None |
| Feelings of worthlessness or guilt | No analog |
| Decreased ability to concentrate | Unknown |
| Suicidality | No analog |
| Strengths | Limitations | Open Questions |
|---|---|---|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, D. The Olfactory Bulbectomy Model of Depression: Brief History, Current Status and Critique. Brain Sci. 2025, 15, 775. https://doi.org/10.3390/brainsci15080775
Coppola D. The Olfactory Bulbectomy Model of Depression: Brief History, Current Status and Critique. Brain Sciences. 2025; 15(8):775. https://doi.org/10.3390/brainsci15080775
Chicago/Turabian StyleCoppola, David. 2025. "The Olfactory Bulbectomy Model of Depression: Brief History, Current Status and Critique" Brain Sciences 15, no. 8: 775. https://doi.org/10.3390/brainsci15080775
APA StyleCoppola, D. (2025). The Olfactory Bulbectomy Model of Depression: Brief History, Current Status and Critique. Brain Sciences, 15(8), 775. https://doi.org/10.3390/brainsci15080775




