Differential Cortical Activations Among Young Adults Who Fall Versus Those Who Recover Successfully Following an Unexpected Slip During Walking
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup
2.3. Experimental Protocol
2.4. Data Collection, Processing, and Analysis
2.5. Outcome Measures
2.5.1. Perturbation Outcome
2.5.2. Cortical Outcomes
2.5.3. Biomechanical Outcomes
2.6. Statistical Analysis
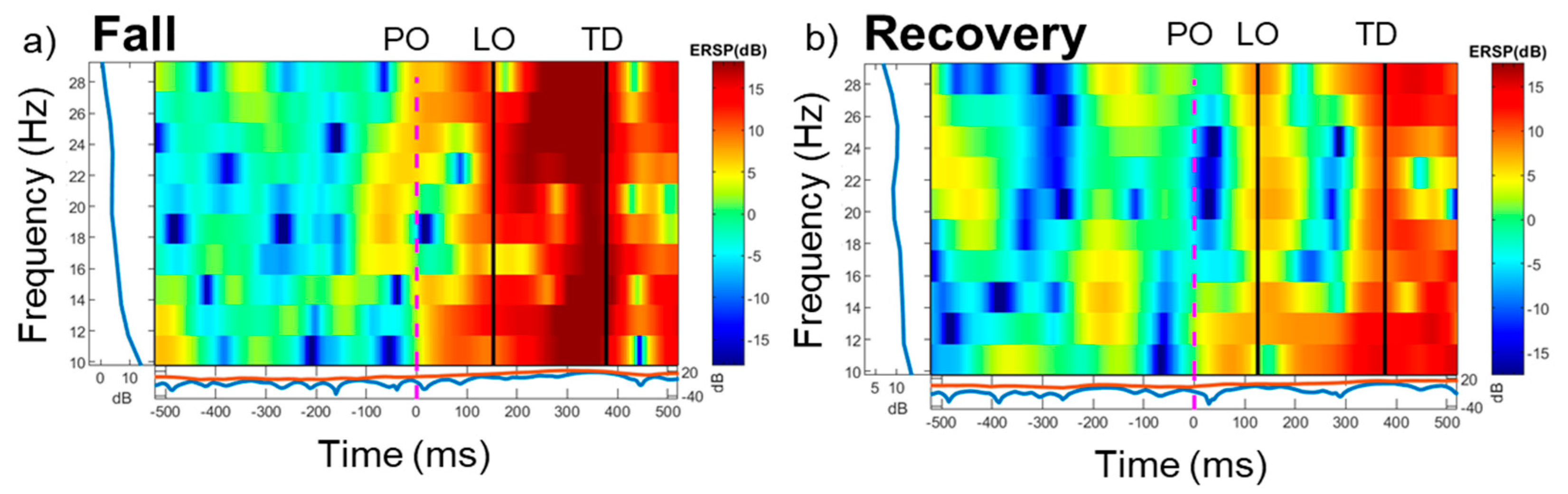
3. Results
4. Discussion
- Pre-perturbation cortical activity might not influence the perturbation outcome (fall vs. recovery).
- A significant increase in sensorimotor beta power during the early post-perturbation period was seen in individuals who fell.
- Potential role of beta frequency oscillations in the context of reactive balance responses and falls.
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tuunainen, E.; Rasku, J.; Jantti, P.; Moisio-Vilenius, P.; Hämäläinen, H.; Viljanen, V. Risk factors of falls in community dwelling active elderly. Auris Nasus Larynx 2014, 41, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Filer, W.; Harris, M. Falls and traumatic brain injury among older adults. N. C. Med. J. 2015, 76, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Kenny, R.A.; Romero-Ortuno, R.; Kumar, P. Falls in older adults. Medicine 2017, 45, 28–33. [Google Scholar] [CrossRef]
- Lukaszyk, C.; Coombes, J.; Sherrington, C.; Tiedemann, A.; Mikolaizak, A.S.; Clemson, L.; Close, J.C. Risk factors, incidence, consequences and prevention strategies for falls and fall-injury within older indigenous populations: A systematic review. Aust. N. Z. J. Public Health 2016, 40, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Vaishya, R.; Vaish, A. Falls in older adults are serious. Indian J. Orthop. 2020, 54, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Boyé, N.D.; Mattace-Raso, F.U.; Van Lieshout, E.M.; Hartholt, K.A.; Van Beeck, E.F.; Van der Cammen, T.J. The impact of falls in the elderly. Trauma 2013, 15, 29–35. [Google Scholar] [CrossRef]
- Okubo, Y.; Schoene, D.; Lord, S.R. Stepping impairment and falls in older adults: A systematic review and meta-analysis of volitional and reactive step tests. Ageing Res. Rev. 2021, 66, 101238. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.; Stanhope, S.J.; Morton, S.M. Impaired reactive stepping adjustments in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Van Dieën, J.H.; Pijnappels, M. Balance control in older adults. In Locomotion and Posture in Older Adults: The Role of Aging and Movement Disorders; Springer: Cham, Switzerland, 2017; pp. 237–262. [Google Scholar]
- Carty, C.P.; Cronin, N.J.; Lichtwark, G.A.; Mills, P.M.; Barrett, R.S. Reactive stepping behaviour in response to forward loss of balance predicts future falls in community-dwelling older adults. Age Ageing 2015, 44, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Nashner, L.M. Central programming of postural movements: Adaptation to altered support-surface configurations. J. Neurophysiol. 1986, 55, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; McIlroy, W.E.; Fernie, G.R. Change-in-support reactions for balance recovery. IEEE Eng. Med. Biol. Mag. 2003, 22, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Hsiao-Wecksler, E.T. Biomechanical and age-related differences in balance recovery using the tether-release method. J. Electromyogr. Kinesiol. 2008, 18, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; McIlroy, W.E. Control of rapid limb movements for balance recovery: Age-related changes and implications for fall prevention. Age Ageing 2006, 35 (Suppl. S2), ii12–ii18. [Google Scholar] [CrossRef] [PubMed]
- Purohit, R.; Wang, S.; Bhatt, T. Effect of Aging and Cortical Stroke on Motor Adaptation to Overground Gait-Slips: Quantifying Differences in Adaptation Rate and Adaptation Plateau. Biomechanics 2023, 3, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Salot, P.; Patel, P.; Bhatt, T. Reactive balance in individuals with chronic stroke: Biomechanical factors related to perturbation-induced backward falling. Phys. Ther. 2016, 96, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Tokuno, C.D.; Eng, J.J.; Horak, F.B. Age-related changes in postural responses revealed by support-surface translations with a long acceleration-deceleration interval. Clin. Neurophysiol. 2010, 121, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Pavol, M.J.; Pai, Y.C. Deficient limb support is a major contributor to age differences in falling. J. Biomech. 2007, 40, 1318–1325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shulman, D.; Spencer, A.; Vallis, L.A. Older adults exhibit variable responses in stepping behaviour following unexpected forward perturbations during gait initiation. Hum. Mov. Sci. 2019, 63, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Shulman, D.; Spencer, A.; Vallis, L.A. Age-related alterations in reactive stepping following unexpected mediolateral perturbations during gait initiation. Gait Posture 2018, 64, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.C.; Prentice, S.D.; McIlroy, W.E. Age-related challenges in reactive control of mediolateral stability during compensatory stepping: A focus on the dynamics of restabilisation. J. Biomech. 2016, 49, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, T.; Wening, J.; Pai, Y.C. Adaptive control of gait stability in reducing slip-related backward loss of balance. Exp. Brain Res. 2006, 170, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Espy, D.D.; Yang, F.; Bhatt, T.; Pai, Y.C. Independent influence of gait speed and step length on stability and fall risk. Gait Posture 2010, 32, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Bhatt, T.; Pai, Y.C. Role of stability and limb support in recovery against a fall following a novel slip induced in different daily activities. J. Biomech. 2009, 42, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Sawers, A.; Bhatt, T. Neuromuscular determinants of slip-induced falls and recoveries in older adults. J. Neurophysiol. 2018, 120, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Sawers, A.; Allen, J.L.; Ting, L.H.; Bhatt, T. Neuromuscular responses differ between slip-induced falls and recoveries in older adults. J. Neurophysiol. 2017, 117, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Varas-Diaz, G.; Bhatt, T. Muscle synergy differences between voluntary and reactive backward stepping. Sci. Rep. 2021, 11, 15462. [Google Scholar] [CrossRef] [PubMed]
- Barrera Curiel, A. Structural, Functional, and Behavioral Plasticity of Sensorimotor Integration. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 2020. [Google Scholar]
- Ozdemir, R.A.; Contreras-Vidal, J.L.; Paloski, W.H. Cortical control of upright stance in elderly. Mech. Ageing Dev. 2018, 169, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Solis-Escalante, T.; Stokkermans, M.; Cohen, M.X.; Weerdesteyn, V.; Schouten, A.C. Cortical dynamics during preparation and execution of reactive balance responses with distinct postural demands. Neuroimage 2019, 188, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Ghosn, N.J.; Palmer, J.A.; Wright, R.A.; Ting, L.H. Cortical Beta Oscillatory Activity Evoked during Reactive Balance Recovery Scales with Perturbation Difficulty and Individual Balance Ability. Brain Sci. 2020, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Mezzina, G.; Palmer, J.A.; Frechette, M.L.; Layer, J.K.; Ting, L.H. Cortical reactive balance responses to unexpected slippages while walking: A pilot study. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6868–6871. [Google Scholar]
- Peterson, S.M.; Ferris, D.P. Differentiation in theta and beta electrocortical activity between visual and physical perturbations to walking and standing balance. eNeuro 2018, 5, ENEURO.0207-18.2018. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.P.; Staines, W.R.; McIlroy, W.E. Frequency characteristics of cortical activity associated with perturbations to upright stability. Neurosci. Lett. 2014, 578, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.P.; Staines, W.R.; McIlroy, W.E. Activity in Functional Cortical Networks Temporally Associated with Postural Instability. Neuroscience 2019, 401, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Nakajima, K.; Ozdemir, R.A.; Paloski, W.H.; Contreras-Vidal, J.L. Effects of speed and direction of perturbation on electroencephalographic and balance responses. Exp. Brain Res. 2018, 236, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.M.; Ting, L.H. Balance perturbation-evoked cortical N1 responses are larger when stepping and not influenced by motor planning. J. Neurophysiol. 2020, 124, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.M.; Ting, L.H. Worse balance is associated with larger perturbation-evoked cortical responses in healthy young adults. Gait Posture 2020, 80, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Petruzzello, S.J.; Hernandez, M.E. Beta cortical oscillatory activities and their relationship to postural control in a standing balance demanding test: Influence of aging. Front. Aging Neurosci. 2023, 15, 1126002. [Google Scholar] [CrossRef] [PubMed]
- Adkin, A.L.; Quant, S.; Maki, B.E.; McIlroy, W.E. The influence of postural threat on the cortical response to unpredictable and predictable postural perturbations. Neurosci. Lett. 2008, 435, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Sibley, K.M.; Carpenter, M.G.; Perry, J.C.; Frank, J.S. The relationship between physiological arousal and cortical and autonomic responses to postural instability. Exp. Brain Res. 2010, 203, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.A.; Ghosn, N.J.; Payne, A.M.; Ting, L.H. Cortical Engagement Metrics During Reactive Balance Are Associated With Distinct Aspects of Balance Behavior in Older Adults. Front. Aging Neurosci. 2021, 13, 684743. [Google Scholar] [CrossRef] [PubMed]
- McCrum, C.; Gerards, M.H.G.; Karamanidis, K.; Zijlstra, W.; Meijer, K. A systematic review of gait perturbation paradigms for improving reactive stepping responses and falls risk among healthy older adults. Eur. Rev. Aging Phys. Act. 2017, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Yoo, D.; Lee, B.-C. Electrocortical activity changes in response to unpredictable trip perturbations induced by a split-belt treadmill. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6863–6866. [Google Scholar]
- Solis-Escalante, T.; de Kam, D.; Cohen, M.X.; Weerdesteyn, V.; Schouten, A.C. Cortical responses to whole-body balance perturbations index perturbation magnitude and predict reactive stepping behavior. Eur. J. Neurosci. 2021, 54, 8120–8138. [Google Scholar] [CrossRef] [PubMed]
- Kajrolkar, T.; Bhatt, T. Falls-risk post-stroke: Examining contributions from paretic versus non-paretic limbs to unexpected forward gait slips. J. Biomech. 2016, 49, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Kajrolkar, T.; Yang, F.; Pai, Y.C.; Bhatt, T. Dynamic stability and compensatory stepping responses during anterior gait-slip perturbations in people with chronic hemiparetic stroke. J. Biomech. 2014, 47, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Pai, Y.C.; Bhatt, T.; Wang, E.; Espy, D.; Pavol, M.J. Inoculation against falls: Rapid adaptation by young and older adults to slips during daily activities. Arch. Phys. Med. Rehabil. 2010, 91, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, T.; Wang, E.; Pai, Y.C. Retention of adaptive control over varying intervals: Prevention of slip-induced backward balance loss during gait. J. Neurophysiol. 2006, 95, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Purohit, R.; Bhatt, T. Near-Fall Detection in Unexpected Slips during Over-Ground Locomotion with Body-Worn Sensors among Older Adults. Sensors 2022, 22, 2869. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, X.; Purohit, R.; Bhatt, T. Slip-induced fall-risk assessment based on regular gait pattern in older adults. J. Biomech. 2019, 96, 109334. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Bhatt, T.; Pai, Y.C. Limits of recovery against slip-induced falls while walking. J. Biomech. 2011, 44, 2607–2613. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dusane, S.; Bhatt, T. Can prior exposure to repeated non-paretic slips improve reactive responses on novel paretic slips among people with chronic stroke? Exp. Brain Res. 2022, 240, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Boebinger, S.; Gonzalez, M.A.; Ting, L.H. Precise cortical contributions to sensorimotor feedback control during reactive balance. PLoS Comput. Biol. 2024, 20, e1011562. [Google Scholar] [CrossRef] [PubMed]
- Borich, M.R.; Kimberley, T.J.; Venkatesh, P.; Dromerick, A.W.; Ward, N.S. Applications of electroencephalography to characterize brain activity: Perspectives in stroke. J. Neurol. Phys. Ther. 2015, 39, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Blankertz, B.; Lemm, S.; Treder, M.; Haufe, S.; Müller, K.R. Single-trial analysis and classification of ERP components—A tutorial. NeuroImage 2011, 56, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Blankertz, B.; Tomioka, R.; Lemm, S.; Kawanabe, M.; Müller, K.R. Optimizing spatial filters for robust EEG single-trial analysis. IEEE Signal Process. Mag. 2007, 25, 41–56. [Google Scholar] [CrossRef]
- Poolman, P.; Frank, L.; Luu, P.; Pederson, S.; Tucker, D.M. A single-trial analytic framework for EEG analysis and its application to target detection and classification. NeuroImage 2008, 42, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Matsumoto, Y.; Suzuki, K.; Okamoto, H.; Nishimura, H.; Kanayama, R.; Yamamoto, S. Long-lasting event-related beta synchronizations of electroencephalographic activity in response to support-surface perturbations during upright stance: A pilot study associating beta rebound and active monitoring in the intermittent postural control. Front. Syst. Neurosci. 2021, 15, 660434. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.M.; Ferris, D.P. Group-level cortical and muscular connectivity during perturbations to walking and standing balance. NeuroImage 2019, 198, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Pai, Y.C. Automatic recognition of falls in gait-slip training: Harness load cell based criteria. J. Biomech. 2011, 44, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Purohit, R.; Varas-Diaz, G.; Bhatt, T. Which are the key kinematic and kinetic components to distinguish recovery strategies for overground slips among community-dwelling older adults? J. Appl. Biomech. 2020, 36, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Bolton, D.A.E.; Ghosn, N.J.; Boehm, R.L.; Ting, L.H. Suppressing a Blocked Balance Recovery Step: A Novel Method to Assess an Inhibitory Postural Response. Brain Sci. 2023, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Weerdesteyn, V.; Laing, A.C.; Robinovitch, S.N. The body configuration at step contact critically determines the successfulness of balance recovery in response to large backward perturbations. Gait Posture 2012, 35, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Bhatt, T.; Pai, Y.C. Generalization of treadmill-slip training to prevent a fall following a sudden (novel) slip in over-ground walking. J. Biomech. 2013, 46, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pai, Y.C.; Iqbal, K. Simulated movement termination for balance recovery: Can movement strategies be sought to maintain stability in the presence of slipping or forced sliding? J. Biomech. 1999, 32, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Purohit, R.; Varas-Diaz, G.; Bhatt, T. Functional electrical stimulation to enhance reactive balance among people with hemiparetic stroke. Exp. Brain Res. 2024, 242, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Diener, H.C.; Nashner, L.M. Influence of central set on human postural responses. J. Neurophysiol. 1989, 62, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Horak, F.B. Cortical control of postural responses. J. Neural Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Bolton, D.A. The role of the cerebral cortex in postural responses to externally induced perturbations. Neurosci. Biobehav. Rev. 2015, 57, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Maki, B.E.; McIlroy, W.E. Cognitive demands and cortical control of human balance-recovery reactions. J. Neural Transm. 2007, 114, 1279–1296. [Google Scholar] [CrossRef] [PubMed]
- Purohit, R.; Bhatt, T. Mobile Brain Imaging to Examine Task-Related Cortical Correlates of Reactive Balance: A Systematic Review. Brain Sci. 2022, 12, 1487. [Google Scholar] [CrossRef] [PubMed]
- Allum, J.H.J.; Carpenter, M.G.; Honegger, F.; Adkin, A.L.; Bloem, B.R. Proprioceptive control of posture: A review of new concepts. Gait Posture 1998, 8, 214–242. [Google Scholar] [CrossRef] [PubMed]
- Goar, M. Characterizing the Dynamics of Vestibular Reflex Gain Modulation Using Balance-Relevant Sensory Conflict. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2023. [Google Scholar]
- Saradjian, A.H.; Teasdale, N.; Blouin, J. Sensory modulation of movement, posture and locomotion. Neurophysiol. Clin. 2015, 45, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Borich, M.; Brodie, S.; Gray, W.; Ionta, S.; Boyd, L. Understanding the role of the primary somatosensory cortex: Opportunities for rehabilitation. Neuropsychologia 2015, 79, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Gaerlan, M.G.; Alpert, P.T.; Cross, C.; Louis, M.; Kowalski, S. Postural balance in young adults: The role of visual, vestibular and somatosensory systems. J. Am. Assoc. Nurse Pract. 2012, 24, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Peterka, R.J. Sensorimotor integration in human postural control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, I.; van den Bosch, T.L.H.M.; Weerdesteyn, V.; Schouten, A.C. Sensorimotor recalibration of postural control strategies occurs after whole body vibration. Sci. Rep. 2023, 13, 522. [Google Scholar] [CrossRef] [PubMed]
- Seidler, R.D.; Noll, D.C.; Choe, A.S. Neurocognitive mechanisms of error-based motor learning. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2013; Volume 782, pp. 39–60. [Google Scholar]
- Shadmehr, R.; Mussa-Ivaldi, F.A. Adaptive representation of dynamics during learning of a motor task. J. Neurosci. 1994, 14, 3208–3224. [Google Scholar] [CrossRef] [PubMed]
- Horak, F.B.; Macpherson, J.M. Postural orientation and equilibrium. In Handbook of Physiology; Rowell, L.B., Shepherd, J.T., Eds.; Oxford University Press: New York, NY, USA, 1996; pp. 255–292. [Google Scholar]
- Shumway-Cook, A. Motor Control: Translating Research into Clinical Practice, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Maki, B.E.; McIlroy, W.E. Postural control in the older adult. Clin. Geriatr. Med. 1996, 12, 635–658. [Google Scholar] [CrossRef] [PubMed]
- Borel, L.; Alescio-Lautier, B. Posture and cognition in the elderly: Interaction and contribution to the rehabilitation strategies. Neurophysiol. Clin. 2014, 44, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.J.; Frey, P.W.; Akiduki, H.; Slobounov, S.; Ting, L.H. Voluntary motor command release coincides with restricted sensorimotor beta rhythm phases. J. Neurosci. 2022, 42, 5771–5781. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, B.; Haegens, S. Beyond the status quo: A role for beta oscillations in endogenous content (re)activation. eNeuro 2017, 4, ENEURO.0170-17.2017. [Google Scholar] [CrossRef] [PubMed]
- Tzagarakis, C.; West, S.; Pellizzer, G. Beta-band activity during motor planning reflects response uncertainty. J. Neurosci. 2010, 30, 11270–11277. [Google Scholar] [CrossRef] [PubMed]
- Tzagarakis, C.; West, S.; Pellizzer, G. Brain oscillatory activity during motor preparation: Effect of directional uncertainty on beta, but not alpha, frequency band. Front. Neurosci. 2015, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.G.; Tingley, M.; Palmer, J.A.; Ting, L.H. Cortical beta oscillations help synchronise muscles during static posture holding in healthy motor control. NeuroImage 2024, 298, 120774. [Google Scholar] [CrossRef] [PubMed]
- Tallon-Baudry, C.; Bertrand, O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 1999, 3, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Pai, Y.C.; Bhatt, T.S. Repeated-slip training: An emerging paradigm for prevention of slip-related falls among older adults. Phys. Ther. 2007, 87, 1478–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Pai, Y.C.; Bhatt, T. Is There an Optimal Recovery Step Landing Zone Against Slip-Induced Backward Falls During Walking? Ann. Biomed. Eng. 2020, 48, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Pai, Y.C. Role of individual lower limb joints in reactive stability control following a novel slip in gait. J. Biomech. 2010, 43, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Pai, Y.C.; Yang, F.; Bhatt, T.; Wang, E. Mechanisms of limb collapse following a slip among young and older adults. J. Biomech. 2006, 39, 2194–2204. [Google Scholar] [CrossRef] [PubMed]
- Papegaaij, S.; Taube, W.; Baudry, S.; Otten, E.; Hortobágyi, T. Aging causes a reorganization of cortical and spinal control of posture. Front. Aging Neurosci. 2014, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Sipp, A.R.; Gwin, J.T.; Makeig, S.; Ferris, D.P. Loss of balance during balance beam walking elicits a multifocal theta band electrocortical response. J. Neurophysiol. 2013, 110, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.P.; Marlin, A.; Beyer, K.B.; McIlroy, W.E. Age-related changes in the modulation of cortical activity during walking: A dual-task study. Front. Aging Neurosci. 2016, 8, 112. [Google Scholar]
- Gwin, J.T.; Gramann, K.; Makeig, S.; Ferris, D.P. Electrocortical activity is coupled to gait cycle phase during treadmill walking. NeuroImage 2011, 54, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Parkkonen, E.; Laaksonen, K.; Piitulainen, H.; Parkkonen, L.; Forss, N. Strength of beta rebound predicts motor outcome after stroke. Ann. Clin. Transl. Neurol. 2015, 2, 902–909. [Google Scholar]
- Pfurtscheller, G.; Graimann, B.; Huggins, J.E.; Levine, S.P.; Schuh, L.A. Spatiotemporal patterns of beta desynchronization and EEG single-trial classification of type 1 diabetic patients with neuropathy. Clin. Neurophysiol. 2003, 114, 2393–2405. [Google Scholar]
- Pichiorri, F.; Morone, G.; Petti, M.; Toppi, J.; Pisotta, I.; Molinari, M.; Inghilleri, M.; Giannantoni, N.M.; Tartaglione, B.; Paolucci, S.; et al. Brain–computer interface boosts motor imagery practice during stroke recovery. Ann. Neurol. 2015, 77, 851–865. [Google Scholar] [CrossRef] [PubMed]
- Rossiter, H.E.; Boudrias, M.H.; Ward, N.S. Do movement-related beta oscillations change after stroke? J. Neurophysiol. 2014, 112, 2053–2058. [Google Scholar] [CrossRef] [PubMed]

| Variables | Falls (n = 7) Mean (SD) | Recoveries (n =12) Mean (SD) | t | p-Value |
|---|---|---|---|---|
| COM stability (LO) | −0.31 (0.09) | −0.17(0.10) | −3.69 | 0.002 * |
| COM stability (TD) | −0.55 (0.20) | −0.37 (0.14) | −2.41 | 0.029 * |
| Hip height (LO) | 0.52 (0.03) | 0.50 (0.01) | 1.66 | 0.06 |
| Hip height (TD) | 0.51 (0.03) | 0.49 (0.01) | 1.22 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purohit, R.; Wang, S.; Bhatt, T. Differential Cortical Activations Among Young Adults Who Fall Versus Those Who Recover Successfully Following an Unexpected Slip During Walking. Brain Sci. 2025, 15, 765. https://doi.org/10.3390/brainsci15070765
Purohit R, Wang S, Bhatt T. Differential Cortical Activations Among Young Adults Who Fall Versus Those Who Recover Successfully Following an Unexpected Slip During Walking. Brain Sciences. 2025; 15(7):765. https://doi.org/10.3390/brainsci15070765
Chicago/Turabian StylePurohit, Rudri, Shuaijie Wang, and Tanvi Bhatt. 2025. "Differential Cortical Activations Among Young Adults Who Fall Versus Those Who Recover Successfully Following an Unexpected Slip During Walking" Brain Sciences 15, no. 7: 765. https://doi.org/10.3390/brainsci15070765
APA StylePurohit, R., Wang, S., & Bhatt, T. (2025). Differential Cortical Activations Among Young Adults Who Fall Versus Those Who Recover Successfully Following an Unexpected Slip During Walking. Brain Sciences, 15(7), 765. https://doi.org/10.3390/brainsci15070765






