1. Introduction
Vagal nerve stimulation is the first neurostimulation therapy approved for the treatment of drug-resistant epilepsy. Blinded randomized controlled studies have shown that 23–57% of patients with intractable epilepsy achieve at least a 50% seizure reduction with VNS therapy in the short term [
1,
2,
3,
4,
5]. Longer-term seizure outcome studies suggest that response rates to vagal nerve stimulation improve with chronic stimulation in responding subjects [
1,
4,
6,
7].
Although the response rate to VNS therapy is relatively high, many patients achieve little or no improvement in their epilepsy. Many of these patients request VNS removal. Furthermore, VNSs may be removed because it interferes with subsequent epilepsy evaluations, including magnetoencephalography and MRI, as well as treatments that rely on intra-procedure MRI. While the removal of the VNS generator is straightforward, the removal of the VNS lead from the neck carries risk of injuring the recurrent laryngeal nerve and the vessels of the carotid sheath. This dissection is considerably more challenging than during implantation due to scarring at the site and the loss of anatomical landmarks and planes. A meta-analysis of pediatric VNS explantation revealed that when a two-incision VNS removal is performed, vascular injury occurs in 1% of cases, transient vocal cord paresis/paralysis, dysphonia, and dysphagia occur in 3.6% of cases, and permanent injury occurs in 1.5% of cases. These complications are attributed to the neck dissection [
8].
The VNS device consists of a generator implanted through a chest wall incision and a lead wire inserted via a neck incision. The lead wire has helical stimulating coils that are placed circumferentially around the vagal nerve, and the lead wire is then tunneled to reach the generator [
1]. As per the Liva Nova Physicians Manual (2022), MRI should not be performed if more than 2 cm of lead wire remains in the patient’s chest wall or neck due to the risk of it heating and causing thermal injury. The helical coils, however, are not a contraindication against MRI. During standard two-incision VNS removal procedures, we noted that the VNS wire sometimes fractured with less than 2 cm of lead wire remaining after the application of moderate traction without resulting vascular injury or postoperative dysphagia, dysphonia, or hoarseness. We sought to explore if this finding could be exploited in the operating room to avoid neck incision, and we therefore tested whether the entire lead wire could be removed by fracturing the lead wire/helical coil connection via the application of traction on the exposed electrode at the generator/chest wall incision, along with countertraction at the site of lead placement in the neck.
2. Materials and Methods
2.1. Study Design
After obtaining permission from the Advent Health IRB, the charts of all patients who underwent attempts to remove the VNS lead through a single incision from 2012 to 2024 were retrospectively reviewed. All patients were evaluated by our epilepsy neurology team and the decision for VNS explant was reviewed and approved through our Comprehensive Epilepsy Case Conference. The chart review included but was not limited to surgical technique, duration of surgery, the presence or absence of vocal cord weakness, need for transfusion, injury to the internal jugular vein, and duration of vocal cord weakness postoperatively. The duration of surgery data was only available for patients who underwent VNS removal. This is because when the VNS removal was performed and then followed by a second procedure during the same OR visit, no distinction was made between the OR time due to the VNS removal versus the second procedure, and thus this data was unavailable.
2.2. Surgical Technique and Management
All operations were performed by the senior author (J.E.B.). The surgical technique involves first opening the chest wall incision, followed by the removal of the VNS generator. Next, adhesions are removed from the lead wire in order to ensure the free movement of the wire. Gentle pressure is applied to the anterior neck at the level of the helical coils while traction is applied inferiorly to the lead wire at the chest wall incision (
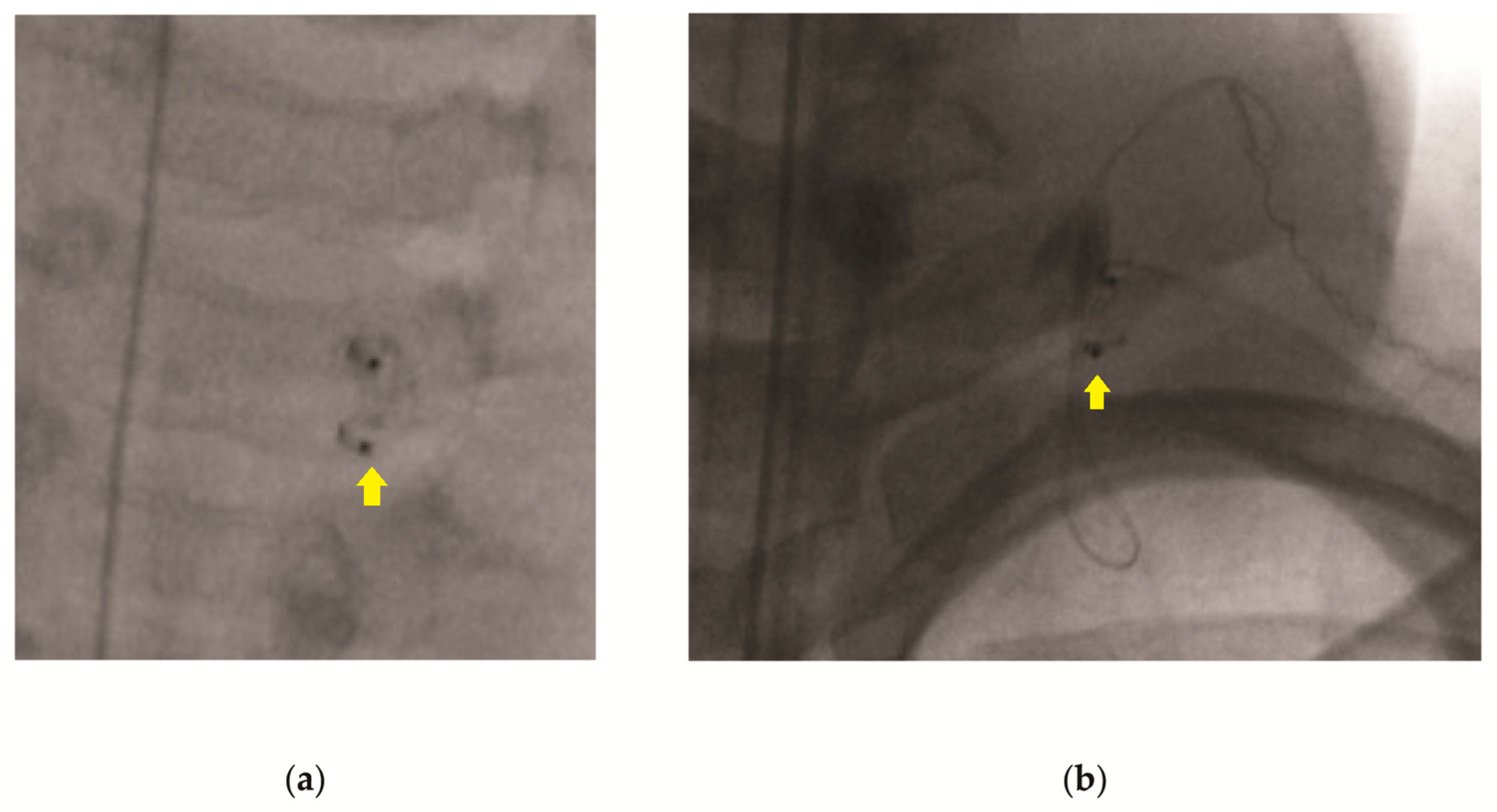
Video S1). Once the lead wire is removed, the neck is evaluated using intraoperative fluoroscopy, looking for residual wire (
Figure 1). If the lead wire is completely removed, the chest incision is closed and the patient is extubated and transported to the recovery room. If fluoroscopy reveals that retained lead wire is present (
Figure 1b), the neck incision is explored, and the residual lead wire removed. Lead wire removal is confirmed by intra-operative fluoroscopy. Both incisions are then closed, and the patient is extubated and transported to the recovery room.
Once the patient has cleared anesthesia, they are evaluated for vocal cord weakness. If present, they are kept NPO until a Speech Language Pathology-supervised swallow study is completed. If the swallow study is abnormal, dietary modifications, including a thickened diet, are initiated. Patients’ vocal cord weakness is followed until cleared by Speech Language Pathology.
2.3. Statistical Analysis
All statistical tests were performed in GraphPad Prism software (version 9.5.1). For binary data, means testing was performed using a two-tailed Fisher’s exact test based on a 2 × 2 contingency table. For continuous data, means testing was achieved via a two-tailed Student’s t-test, with assumptions of the homogeneity of variances and normality achieved via an F-test and Shapiro–Wilk test, respectively.
This study design has inherent limitations due to its retrospective nature, limited study population, lack of a prospective control, and the fact that the decision was made to attempt removal via single incision first in all cases. This strategy, however, is adequate for assessing basic questions pertaining to the safety, viability, and potential time reduction with this approach.
3. Results
3.1. Surgical Outcomes and Patient Characteristics
A total of 73 patients met the inclusion criteria. A single-incision removal was achieved in 48 cases (65.8%), whereas conversion to a two-incision approach was performed in 25 cases (32.9%). The VNS leads were successfully removed without residual lead wire in 71 cases (97.3%). Of these two remaining cases, one was a single-incision approach where residual wire was identified postoperatively, and the patient was returned to the OR and the remaining wire removed via a second incision. In the other case, the wire was left, due to an internal jugular vein injury that was unable to be repaired. There was no statistically significant difference (p = 0.8650, two-tailed Mann–Whitney U test) between the ages of the patients in the single-incision group (24.6 years, range: 3–55) and two-incision group (26.0, range: 8–61).
The time in the operating room for VNS removal was available for 35 patients (24 single-incision cases and 11 two-incision cases). Time in the operating room was 29.4 min (range 11–84) for the single-incision group, as opposed to 74.2 min (range 33–203) for the two-incision group.
3.2. Complications
Overall, 7 out of 48 (14.6%) patients in the single-incision group developed transient hoarseness, dysphagia, or cough, compared to 3 out of 25 (12.0%) patients in the two-incision group. This difference was not significant according to a two-tailed Fisher’s exact test (p = 0.7368). There were no cases of permanent deficits. For the single-incision group, there were no cases of internal jugular vein injury (0%), compared to two cases in the two-incision group (8.0%). Both injuries occurred as a consequence of the neck dissection. This difference did not achieve statistical significance (p = 0.1265, two-tailed Fisher’s exact test).
4. Discussion
While vagal nerve stimulation is often an effective palliative intervention for seizure management, VNS removal may be necessary due to lack of efficacy, interference with MRI-based procedures, and/or cosmetic concerns. This surgery is typically achieved via a two-incision approach, including a neck dissection for the removal of the lead wires. This approach carries a small but non-negligible risk of injury to vascular structures and to the recurrent laryngeal nerve, which may be permanent. This dissection is particularly challenging due to the often heavy scarring and loss of anatomic landmarks.
Here, we report our experience of a single-incision approach to VNS removal. The successful removal of the lead wires was achieved in the majority of cases (65.8%), thereby bypassing the need for neck dissection and possibly reducing the OR time. No cases of vascular injury or permanent recurrent laryngeal nerve injury occurred. The most common complications in this patient population were transient hoarseness, cough, or dysphagia, which likely reflect neurapraxia of the vagal nerve or recurrent laryngeal nerve. Notably, all patients underwent traction on the lead wires. The rates in this study are equivalent in the two groups. Thus, the equivalent rates of hoarseness, cough, or dysphagia may indicate that these side effects are primarily a result of this maneuver. The overall rate of dysfunction in this cohort (13.7%) is higher than that reported in the meta-analysis by Saba et al. [
8]. (5.1%); however, there were no cases of permanent dysfunction in this cohort, as opposed to 1.6% in the meta-analysis.
A possible advantage of this approach is the reduced OR time, with the single-incision approach taking roughly half as long on average as the two-incision method. While there is an artificial component to this result, given that the single-incision approach was attempted first in all cases, both surgical approaches start equivalently, with the dissection of the generator. The attempted traction procedure to fracture the coils takes seconds and, if successful, allows one to skip the neck dissection. Thus, this result is highly likely to be consistent and reproducible. This approach further abrogates the need for a neck incision given the associated risk of neck dissection, wound healing issues, and surgical site infection. This approach may also carry a reduced risk of permanent laryngeal nerve injury and internal jugular injury, though more data are needed to validate this claim.
Furthermore, the viability of this approach may warrant changes in the design of the VNS wire, and it is worthwhile to consider the potential advantages of a wire that has a mechanism in place to be detached, which may reduce the rates of transient nerve injury in this population and increase the rates of successful single-incision removal.
5. Conclusions
VNS removal can be safely achieved via a single-incision method in the majority of cases. This approach results in a possibly reduced OR time, and no patients undergoing single-incision removal experienced permanent vocal cord dysfunction or vascular injury. This approach may increase the risk of transient hoarseness, dysphagia, and/or cough.
Author Contributions
Conceptualization, J.E.B.; methodology, J.E.B.; formal analysis, J.E.B. and M.B.; investigation, J.E.B., M.B. and M.D.; data curation, M.D.; writing—original draft preparation, J.E.B. and M.B.; writing—review and editing, J.E.B. and M.B.; supervision, M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived by the Advent Health IRB for this study due to the retrospective nature of this study, without identifiable information.
Informed Consent Statement
Patient consent was waived due to the retrospective chart review nature of the study, without identifiable information.
Data Availability Statement
The study data is available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IJ | Internal jugular vein |
| LITT | Laser interstitial thermal therapy |
| OR | Operating room |
| VNS | Vagus nerve stimulator |
References
- Gonzalez, H.F.J.; Yengo-Khan, A.; Englot, D.J. Vagus nerve stimulation for the treatment of epilepsy. Neurosurg. Clin. N. Am. 2019, 30, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, E.; Manon-Espaillat, R.; Ristanovic, R.; Wilder, B.J.; Stefan, H.; Mirza, W.; Tarver, W.B.; Wernicke, J.F. Vagus nerve stimulation for treatment of partial seizures: A controlled study of effect on seizures. Epilepsia 1994, 35, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Handforth, A.; Degiorgio, C.M.; Schachter, S.; Uthman, B.M.; Naritoku, D.K.; Tecoma, E.S.; Henry, T.R.; Collins, S.D.; Vaughn, B.V.; Gilmartin, R.C.; et al. Vagus nerve stimulation therapy for partial onset seizures: A randomized active-control trial. Neurology 1985, 51, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Chang, E.F.; Auguste, K.I. Vagus nerve stimulation for epilepsy: A metaanalysis of efficacy and predictors of response. J. Neurosurg. 2011, 115, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, S.; Aalbers, M.W.; Vles, J.S.; Cornips, E.M.; Rijkers, K.; Leenen, L.; Kessels, F.G.; Aldenkamp, A.P.; Majoie, M. Vagus nerve stimulation in children with intractable epilepsy: A randomized controlled trial. Dev. Med. Child. Neurol. 2012, 54, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Rolston, J.D.; Wright, C.W.; Hassnain, K.H.; Chang, E.F. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilspy. Neurosurgery 2015, 79, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Chang, E.F.; Auguste, K.I. Efficacy of vagus nerve stimulation for epilepsy by patient age, epilepsy duration and seizure type. Neurosurg. Clin. N. Am. 2011, 22, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Saba, E.S.; Rivero, A. Pediatric vagal nerve stimulator explantation: A comprehensive literature review and tertiary care experience. Int. J. Pediatr. Otorhionlaryngol. 2023, 170, 111603. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).