Acute Stroke Severity Assessment: The Impact of Lesion Size and Functional Connectivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Clinical Appraisal
2.3. MRI Acquisition
2.4. Image Processing and Data Evaluation
2.4.1. Structural MRI Preprocessing
2.4.2. Preprocessing of rs-fMRI
2.4.3. Functional Connectivity Network Analysis
- Motor Network I: Precentral Gyrus (PreCG), Postcentral Gyrus (PoCG, contributes to sensorimotor integration), and Paracentral Lobule (PCL).
- Motor Network II: Supplementary Motor Area (SMA), Superior Frontal Gyrus—Dorsolateral (SFG), Middle Frontal Gyrus (MFG), and Inferior Frontal Gyrus—Opercular Part (IFGoperc), Rolandic Operculum (ROL), and Superior Frontal Gyrus—Medial (SFGmedial).
- Motor Network III: Insula (INS), Supracallosal Anterior Cingulate Cortex (ACCsup), and Middle Cingulate Cortex (MCC).
- Motor Network IV: Putamen (PUT), Pallidum (PAL), and Caudate Nucleus (CAU).
- Default Mode Network (DMN): Medial Superior Frontal Gyrus (SFGmedial), Posterior Cingulate Cortex (PCC), Subgenual Anterior Cingulate Cortex (ACCsub), Pregenual Anterior Cingulate Cortex (ACCpre), Supragenual Anterior Cingulate Cortex (ACCsup), Angular Gyrus (ANG), and Precuneus (PCUN).
- Frontoparietal Network: Middle Frontal Gyrus (MFG), Inferior Frontal Gyrus—Opercular Part (IFGoperc), Inferior Frontal Gyrus—Triangular Part (IFGtriang), Angular Gyrus (ANG), and Inferior Parietal Gyrus (IPG).
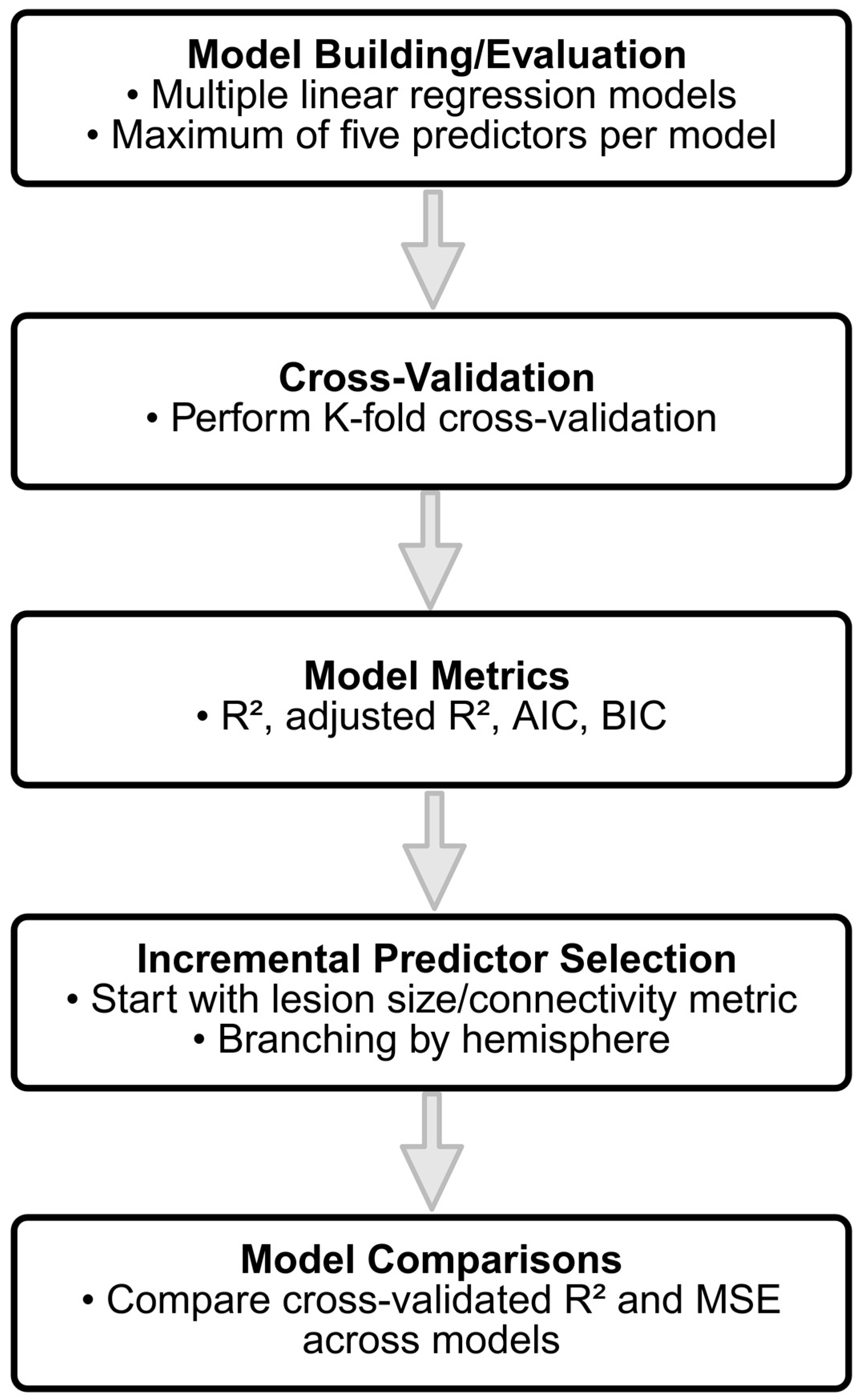
2.5. Statistical Analysis
- Model Building and Evaluation: To evaluate the predictive capabilities of the different connectivity features, multiple linear regression models were constructed incrementally, with a maximum of five predictors per model to avoid overfitting. The initial model was built using a single predictor, and additional predictors were added based on the increment in R-squared, representing the proportion of the variance explained by the model. Predictors that contributed the most significant increase in R-squared were selected iteratively until the limit of five predictors was reached. The restriction to five predictors was guided by the relatively small sample size (n = 44) and the widely recommended rule of thumb requiring a minimum ratio of 10–15 observations per predictor to ensure model stability and generalizability [47]. To ensure validity of the linear regression assumptions, we additionally examined multicollinearity using the Variance Inflation Factor (VIF), ensuring all VIF values remained below two. Normality of residuals was checked visually using Q–Q plots and tested via the Shapiro–Wilk test, confirming approximate normality across models.
- Cross-Validation: To ensure that the model generalizes well to new data, a K-fold cross-validation approach was employed. Specifically, a 5-fold cross-validation was used, where the data was randomly partitioned into 5 equal-sized folds. For each fold, the model was trained on four of the folds and tested on the remaining one. This process was repeated five times, with each fold serving as the test set once, and the results were averaged to obtain the cross-validated R-squared and mean squared error (MSE). The cross-validated R-squared provides an estimate of how well the model is expected to perform on unseen data, mitigating the risks of overfitting.
- Model Metrics: For each model, key statistical metrics were computed, including the R-squared, adjusted R-squared, Akaike Information Criterion (AIC), and Bayesian Information Criterion (BIC). R-squared represents the proportion of variance in the outcome variable explained by the model. Adjusted R-squared accounts for the number of predictors in the model, providing a more conservative estimate compared to R-squared, especially for models with multiple predictors. AIC and BIC are measures of model quality, with penalties for the number of predictors, used to compare models and prevent overfitting.
- Incremental Predictor Selection: To identify the best set of predictors, two incremental model selection approaches were employed: (1) models starting with lesion size as the initial predictor, and (2) models without lesion size, focusing on connectivity metrics. In the incremental approach, the predictor that led to the largest increase in the model R-squared value was added iteratively until a total of five predictors were included. Additionally, models were built separately for different hemispheres, including left hemisphere predictors, right hemisphere predictors, and predictors from both hemispheres, to explore the contributions of region-specific connectivity metrics.
- Model Comparisons: The performance of each model was assessed by comparing cross-validated R-squared and MSE across different models. Cross-validation allowed for an unbiased estimation of model performance on new data, while the use of different subsets of predictors allowed for detailed insights into the relative importance of lesion size versus connectivity-based predictors in explaining clinical outcome. In particular, separate models were built to explore the predictive power of left hemisphere, right hemisphere, and bilateral (both hemispheres) connectivity metrics, providing insights into the specific contributions of different brain regions.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| BIC | Bayesian Information Criterion |
| BOLD | Blood Oxygenation Level Dependent |
| CAT | Computational Anatomy Toolbox |
| CSF | Cerebrospinal fluid |
| DMN | Default Mode Network |
| DTI | Diffusion tensor imaging |
| DWI | Diffusion-weighted imaging |
| EEG | Electroencephalography |
| EP | Evoked potential |
| EPI | Echo-planar imaging |
| FA | Flip angle |
| FLAIR | Fluid-attenuated inversion recovery |
| FOV | Field Of View |
| FWHM | Full-width at half-maximum |
| GM | Gray matter |
| ICA | Independent Component Analysis |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| LST | Lesion Segmentation Toolbox |
| MEG | Magnetoencephalography |
| ML | Machine learning |
| MM | Millimeter |
| MNI | Montreal Neurological Institute |
| MPRAGE | Magnetization-prepared rapid acquisition gradient-echo |
| MRI | Magnetic Resonance Imaging |
| MRS | Modified Rankin Scale |
| MS | Milliseconds |
| MSE | Mean squared error |
| NIHSS | National Institutes of Health Stroke Scale |
| ROI | Region of interest |
| RS-FMRI | Resting-state functional Magnetic Resonance Imaging |
| RSN | Resting state networks |
| SD | Standard Deviation |
| SPM | Statistical Parametric Mapping |
| TE | Echo time |
| TI | Inversion time |
| TMS | Transcranial Magnetic Stimulation |
| TOAST | Trial of Org 10172 in Acute Stroke Treatment |
| TR | Repetition time |
| USDG | Ultrasound dopplerography |
| VBG | Virtual Brain Grafting |
| VBT | Virtual Brain Transplantation |
| VIF | Variance Inflation Factor |
| WM | White matter |
References
- GBD 2019 Stroke Collaborators. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Wafa, H.A.; Wolfe, C.D.A.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe: Thirty-Year Projections of Incidence, Prevalence, Deaths, and Disability-Adjusted Life Years. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef]
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Herpich, F.; Rincon, F. Management of Acute Ischemic Stroke. Crit. Care Med. 2020, 48, 1654–1663. [Google Scholar] [CrossRef]
- Vogt, G.; Laage, R.; Shuaib, A.; Schneider, A.; VISTA Collaboration. Initial Lesion Volume Is an Independent Predictor of Clinical Stroke Outcome at Day 90: An Analysis of the Virtual International Stroke Trials Archive (VISTA) Database. Stroke 2012, 43, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Pei, C.; He, C.; Li, H.; Li, X.; Huang, W.; Liu, J.; Yin, J. Clinical and Imaging Markers for the Prognosis of Acute Ischemic Stroke. Front. Neurol. 2024, 15, 1345914. [Google Scholar] [CrossRef]
- Schiemanck, S.K.; Post, M.W.M.; Kwakkel, G.; Witkamp, T.D.; Kappelle, L.J.; Prevo, A.J.H. Ischemic Lesion Volume Correlates with Long-Term Functional Outcome and Quality of Life of Middle Cerebral Artery Stroke Survivors. Restor. Neurol. Neurosci. 2005, 23, 257–263. [Google Scholar]
- Sperber, C.; Gallucci, L.; Mirman, D.; Arnold, M.; Umarova, R.M. Stroke Lesion Size—Still a Useful Biomarker for Stroke Severity and Outcome in Times of High-Dimensional Models. Neuroimage Clin. 2023, 40, 103511. [Google Scholar] [CrossRef] [PubMed]
- Gaudinski, M.R.; Henning, E.C.; Miracle, A.; Luby, M.; Warach, S.; Latour, L.L. Establishing Final Infarct Volume: Stroke Lesion Evolution Past 30 Days Is Insignificant. Stroke 2008, 39, 2765–2768. [Google Scholar] [CrossRef]
- Thomalla, G.; Cheng, B.; Ebinger, M.; Hao, Q.; Tourdias, T.; Wu, O.; Kim, J.S.; Breuer, L.; Singer, O.C.; Warach, S.; et al. DWI-FLAIR Mismatch for the Identification of Patients with Acute Ischaemic Stroke within 4·5 h of Symptom Onset (PRE-FLAIR): A Multicentre Observational Study. Lancet Neurol. 2011, 10, 978–986. [Google Scholar] [CrossRef]
- Etherton, M.R.; Wu, O.; Cougo, P.; Giese, A.-K.; Cloonan, L.; Fitzpatrick, K.M.; Kanakis, A.S.; Boulouis, G.; Karadeli, H.H.; Lauer, A.; et al. Integrity of Normal-Appearing White Matter and Functional Outcomes after Acute Ischemic Stroke. Neurology 2017, 88, 1701–1708. [Google Scholar] [CrossRef]
- Zhu, L.L.; Lindenberg, R.; Alexander, M.P.; Schlaug, G. Lesion Load of the Corticospinal Tract Predicts Motor Impairment in Chronic Stroke. Stroke 2010, 41, 910–915. [Google Scholar] [CrossRef]
- Bonkhoff, A.K.; Cohen, A.L.; Drew, W.; Ferguson, M.A.; Hussain, A.; Lin, C.; Schaper, F.L.W.V.J.; Bourached, A.; Giese, A.-K.; Oliveira, L.C.; et al. Prediction of Stroke Severity: Systematic Evaluation of Lesion Representations. Ann. Clin. Transl. Neurol. 2024, 11, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Montellano, F.A.; Ungethüm, K.; Ramiro, L.; Nacu, A.; Hellwig, S.; Fluri, F.; Whiteley, W.N.; Bustamante, A.; Montaner, J.; Heuschmann, P.U. Role of Blood-Based Biomarkers in Ischemic Stroke Prognosis: A Systematic Review. Stroke 2021, 52, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Adeleye, O.; Koester, S.W.; Winkler, E.A.; Hartke, J.N.; Karahalios, K.; Mihaljevic, S.; Rani, A.; Raikwar, S.; Rulney, J.D.; et al. Cerebrospinal Fluid Biomarkers for Diagnosis and the Prognostication of Acute Ischemic Stroke: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 10902. [Google Scholar] [CrossRef]
- Biswal, B.B. Resting State fMRI: A Personal History. Neuroimage 2012, 62, 938–944. [Google Scholar] [CrossRef]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Carter, A.R.; Astafiev, S.V.; Lang, C.E.; Connor, L.T.; Rengachary, J.; Strube, M.J.; Pope, D.L.W.; Shulman, G.L.; Corbetta, M. Resting Interhemispheric Functional Magnetic Resonance Imaging Connectivity Predicts Performance after Stroke. Ann. Neurol. 2010, 67, 365–375. [Google Scholar] [CrossRef]
- Golestani, A.-M.; Tymchuk, S.; Demchuk, A.; Goodyear, B.G.; VISION-2 Study Group. Longitudinal Evaluation of Resting-State FMRI after Acute Stroke with Hemiparesis. Neurorehabilit. Neural Repair 2013, 27, 153–163. [Google Scholar] [CrossRef]
- Rehme, A.K.; Volz, L.J.; Feis, D.-L.; Bomilcar-Focke, I.; Liebig, T.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Identifying Neuroimaging Markers of Motor Disability in Acute Stroke by Machine Learning Techniques. Cereb. Cortex 2015, 25, 3046–3056. [Google Scholar] [CrossRef]
- Wang, L.; Yu, C.; Chen, H.; Qin, W.; He, Y.; Fan, F.; Zhang, Y.; Wang, M.; Li, K.; Zang, Y.; et al. Dynamic Functional Reorganization of the Motor Execution Network after Stroke. Brain 2010, 133, 1224–1238. [Google Scholar] [CrossRef]
- Ismail, U.N.; Yahya, N.; Manan, H.A. Investigating Functional Connectivity Related to Stroke Recovery: A Systematic Review. Brain Res. 2024, 1840, 149023. [Google Scholar] [CrossRef]
- Tilwani, D.; O’Reilly, C.; Riccardi, N.; Shalin, V.L.; den Ouden, D.-B.; Fridriksson, J.; Shinkareva, S.V.; Sheth, A.P.; Desai, R.H. Benchmarking Machine Learning Models in Lesion-Symptom Mapping for Predicting Language Outcomes in Stroke Survivors. Front. Neuroimaging 2025, 4, 1573816. [Google Scholar] [CrossRef]
- Dobromyslin, V.I.; Zhou, W.; Alzheimer’s Disease Neuroimaging Initiative. Enhancing Cerebral Infarct Classification by Automatically Extracting Relevant fMRI Features. Brain Inform. 2025, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Huo, M.; Liu, W.; Mansouri, N.; Tanglay, O.; Young, I.; Osipowicz, K.; Hu, X.; Zhang, X.; Doyen, S.; et al. Connectomics Underlying Motor Functional Outcomes in the Acute Period Following Stroke. Front. Aging Neurosci. 2023, 15, 1131415. [Google Scholar] [CrossRef]
- White, A.; Saranti, M.; d’Avila Garcez, A.; Hope, T.M.H.; Price, C.J.; Bowman, H. Predicting Recovery Following Stroke: Deep Learning, Multimodal Data and Feature Selection Using Explainable AI. NeuroImage Clin. 2024, 43, 103638. [Google Scholar] [CrossRef]
- Karakis, R.; Gurkahraman, K.; Mitsis, G.D.; Boudrias, M.-H. Deep Learning Prediction of Motor Performance in Stroke Individuals Using Neuroimaging Data. J. Biomed. Inform. 2023, 141, 104357. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, C.; Huang, H.; Li, C.; Li, M.; Li, X.; Gao, Z.; Peng, M.; Xu, H.; Zhu, W. Machine Learning Models Based on Location-Radiomics Enable the Accurate Prediction of Early Neurological Function Deterioration for Acute Stroke in Elderly Patients. Front. Aging Neurosci. 2025, 17, 1582687. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.-L.; Shao, J.-M.; Wang, C.-H.; Li, S.-B.; Jiang, Y. Optimizing Early Neurological Deterioration Prediction in Acute Ischemic Stroke Patients Following Intravenous Thrombolysis: A LASSO Regression Model Approach. Front. Neurosci. 2024, 18, 1390117. [Google Scholar] [CrossRef]
- Campagnini, S.; Liuzzi, P.; Mannini, A.; Basagni, B.; Macchi, C.; Carrozza, M.C.; Cecchi, F. Cross-Validation of Predictive Models for Functional Recovery after Post-Stroke Rehabilitation. J. Neuroeng. Rehabil. 2022, 19, 96. [Google Scholar] [CrossRef]
- Lee, M.; Hong, Y.; An, S.; Park, U.; Shin, J.; Lee, J.; Oh, M.S.; Lee, B.-C.; Yu, K.-H.; Lim, J.-S.; et al. Machine Learning-Based Prediction of Post-Stroke Cognitive Status Using Electroencephalography-Derived Brain Network Attributes. Front. Aging Neurosci. 2023, 15, 1238274. [Google Scholar] [CrossRef]
- Tozlu, C.; Edwards, D.; Boes, A.; Labar, D.; Tsagaris, K.Z.; Silverstein, J.; Pepper Lane, H.; Sabuncu, M.R.; Liu, C.; Kuceyeski, A. Machine Learning Methods Predict Individual Upper-Limb Motor Impairment Following Therapy in Chronic Stroke. Neurorehabilit. Neural Repair 2020, 34, 428–439. [Google Scholar] [CrossRef]
- Lehmann, A.L.C.F.; Alfieri, D.F.; de Araújo, M.C.M.; Trevisani, E.R.; Nagao, M.R.; Pesente, F.S.; Gelinski, J.R.; de Freitas, L.B.; Flauzino, T.; Lehmann, M.F.; et al. Carotid Intima Media Thickness Measurements Coupled with Stroke Severity Strongly Predict Short-Term Outcome in Patients with Acute Ischemic Stroke: A Machine Learning Study. Metab. Brain Dis. 2021, 36, 1747–1761. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.-F.; Ku, H.-L.; Chen, D.Y.-T.; Tseng, Y.-C.; Chen, C.-J.; Lin, Y.-C.; Hsieh, Y.-C.; Chan, L.; Chiou, H.-Y.; Hsu, C.Y.; et al. Cerebral Motor Functional Connectivity at the Acute Stage: An Outcome Predictor of Ischemic Stroke. Sci. Rep. 2018, 8, 16803. [Google Scholar] [CrossRef]
- Puig, J.; Blasco, G.; Alberich-Bayarri, A.; Schlaug, G.; Deco, G.; Biarnes, C.; Navas-Martí, M.; Rivero, M.; Gich, J.; Figueras, J.; et al. Resting-State Functional Connectivity Magnetic Resonance Imaging and Outcome After Acute Stroke. Stroke 2018, 49, 2353–2360. [Google Scholar] [CrossRef]
- Jauch, E.C.; Saver, J.L.; Adams, H.P.; Bruno, A.; Connors, J.J.B.; Demaerschalk, B.M.; Khatri, P.; McMullan, P.W.; Qureshi, A.I.; Rosenfield, K.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 870–947. [Google Scholar] [CrossRef]
- Adams, H.P.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of Acute Cerebral Infarction: A Clinical Examination Scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Rankin, J. Cerebral Vascular Accidents in Patients over the Age of 60. II. Prognosis. Scott. Med. J. 1957, 2, 200–215. [Google Scholar] [CrossRef]
- De Coene, B.; Hajnal, J.V.; Gatehouse, P.; Longmore, D.B.; White, S.J.; Oatridge, A.; Pennock, J.M.; Young, I.R.; Bydder, G.M. MR of the Brain Using Fluid-Attenuated Inversion Recovery (FLAIR) Pulse Sequences. AJNR Am. J. Neuroradiol. 1992, 13, 1555–1564. [Google Scholar]
- Mugler, J.P.; Brookeman, J.R. Rapid Three-Dimensional T1-Weighted MR Imaging with the MP-RAGE Sequence. J. Magn. Reson. Imaging 1991, 1, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E.; Penny, W.D. Statistical Parametric Mapping. The Analysis of Functional Brain Images, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-12-372560-8. [Google Scholar]
- Gaser, C.; Dahnke, R.; Thompson, P.M.; Kurth, F.; Luders, E.; The Alzheimer’s Disease Neuroimaging Initiative. CAT: A Computational Anatomy Toolbox for the Analysis of Structural MRI Data. Gigascience 2024, 13, giae049. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, P.; Wink, L. LST: A Lesion Segmentation Tool for SPM. Manual/Documentation for Version 3.0.0; 2019. Available online: https://www.applied-statistics.de/LST_documentation.pdf (accessed on 15 May 2025).
- Boren, S.B.; Savitz, S.I.; Ellmore, T.M.; Arevalo, O.D.; Aronowski, J.; Silos, C.; George, S.; Haque, M.E. Longitudinal Resting-State Functional Magnetic Resonance Imaging Study: A Seed-Based Connectivity Biomarker in Patients with Ischemic and Intracerebral Hemorrhage Stroke. Brain Connect. 2023, 13, 498–507. [Google Scholar] [CrossRef]
- Rolls, E.T.; Huang, C.-C.; Lin, C.-P.; Feng, J.; Joliot, M. Automated Anatomical Labelling Atlas 3. Neuroimage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Babyak, M.A. What You See May Not Be What You Get: A Brief, Nontechnical Introduction to Overfitting in Regression-Type Models. Psychosom. Med. 2004, 66, 411–421. [Google Scholar] [CrossRef]
- Saver, J.L.; Johnston, K.C.; Homer, D.; Wityk, R.; Koroshetz, W.; Truskowski, L.L.; Haley, E.C. Infarct Volume as a Surrogate or Auxiliary Outcome Measure in Ischemic Stroke Clinical Trials. The RANTTAS Investigators. Stroke 1999, 30, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Thijs, V.N.; Lansberg, M.G.; Beaulieu, C.; Marks, M.P.; Moseley, M.E.; Albers, G.W. Is Early Ischemic Lesion Volume on Diffusion-Weighted Imaging an Independent Predictor of Stroke Outcome? A Multivariable Analysis. Stroke 2000, 31, 2597–2602. [Google Scholar] [CrossRef]
- Schiemanck, S.K.; Kwakkel, G.; Post, M.W.M.; Prevo, A.J.H. Predictive Value of Ischemic Lesion Volume Assessed with Magnetic Resonance Imaging for Neurological Deficits and Functional Outcome Poststroke: A Critical Review of the Literature. Neurorehabilit. Neural Repair 2006, 20, 492–502. [Google Scholar] [CrossRef]
- Johnston, K.C.; Wagner, D.P.; Wang, X.-Q.; Newman, G.C.; Thijs, V.; Sen, S.; Warach, S.; GAIN, Citicoline, and ASAP Investigators. Validation of an Acute Ischemic Stroke Model: Does Diffusion-Weighted Imaging Lesion Volume Offer a Clinically Significant Improvement in Prediction of Outcome? Stroke 2007, 38, 1820–1825. [Google Scholar] [CrossRef]
- Kim, S.M.; Kwon, S.U.; Kim, J.S.; Kang, D.-W. Early Infarct Growth Predicts Long-Term Clinical Outcome in Ischemic Stroke. J. Neurol. Sci. 2014, 347, 205–209. [Google Scholar] [CrossRef]
- Grefkes, C.; Fink, G.R. Connectivity-Based Approaches in Stroke and Recovery of Function. Lancet Neurol. 2014, 13, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Huang, G.; Chen, L.; Li, W.; Liang, Z. Structural and Functional Reorganization Following Unilateral Internal Capsule Infarction Contribute to Neurological Function Recovery. Neuroradiology 2019, 61, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, Z.; Wu, P.; Chen, J. Ability of an Altered Functional Coupling between Resting-State Networks to Predict Behavioral Outcomes in Subcortical Ischemic Stroke: A Longitudinal Study. Front. Aging Neurosci. 2022, 14, 933567. [Google Scholar] [CrossRef]
- Park, C.; Chang, W.H.; Ohn, S.H.; Kim, S.T.; Bang, O.Y.; Pascual-Leone, A.; Kim, Y.-H. Longitudinal Changes of Resting-State Functional Connectivity during Motor Recovery after Stroke. Stroke 2011, 42, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, D.; Zhang, H.; Wang, Y.; Wu, P.; Zhang, H.; Yang, Y.; Huang, W. Changes of Brain Connectivity in the Primary Motor Cortex After Subcortical Stroke: A Multimodal Magnetic Resonance Imaging Study. Medicine 2016, 95, e2579. [Google Scholar]
- Min, Y.-S.; Park, J.W.; Park, E.; Kim, A.-R.; Cha, H.; Gwak, D.-W.; Jung, S.-H.; Chang, Y.; Jung, T.-D. Interhemispheric Functional Connectivity in the Primary Motor Cortex Assessed by Resting-State Functional Magnetic Resonance Imaging Aids Long-Term Recovery Prediction among Subacute Stroke Patients with Severe Hand Weakness. J. Clin. Med. 2020, 9, 975. [Google Scholar] [CrossRef]
- Almeida, S.R.M.; Vicentini, J.; Bonilha, L.; De Campos, B.M.; Casseb, R.F.; Min, L.L. Brain Connectivity and Functional Recovery in Patients With Ischemic Stroke. J. Neuroimaging 2017, 27, 65–70. [Google Scholar] [CrossRef]
- Corbetta, M.; Siegel, J.S.; Shulman, G.L. On the Low Dimensionality of Behavioral Deficits and Alterations of Brain Network Connectivity after Focal Injury. Cortex 2018, 107, 229–237. [Google Scholar] [CrossRef]
- Thiebaut de Schotten, M.; Foulon, C.; Nachev, P. Brain Disconnections Link Structural Connectivity with Function and Behaviour. Nat. Commun. 2020, 11, 5094. [Google Scholar] [CrossRef]
- Pini, L.; Salvalaggio, A.; De Filippo De Grazia, M.; Zorzi, M.; Thiebaut de Schotten, M.; Corbetta, M. A Novel Stroke Lesion Network Mapping Approach: Improved Accuracy yet Still Low Deficit Prediction. Brain Commun. 2021, 3, fcab259. [Google Scholar] [CrossRef]
- Salvalaggio, A.; De Filippo De Grazia, M.; Zorzi, M.; Thiebaut de Schotten, M.; Corbetta, M. Post-Stroke Deficit Prediction from Lesion and Indirect Structural and Functional Disconnection. Brain 2020, 143, 2173–2188. [Google Scholar] [CrossRef]
- Bowren, M.; Bruss, J.; Manzel, K.; Edwards, D.; Liu, C.; Corbetta, M.; Tranel, D.; Boes, A.D. Post-Stroke Outcomes Predicted from Multivariate Lesion-Behaviour and Lesion Network Mapping. Brain 2022, 145, 1338–1353. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, L.; Oliveira, Ĺ.A.F.; Heij, J.; Priovoulos, N.; Kundu, P.; Leoni, R.F.; van der Zwaag, W. Advances in Resting State fMRI Acquisitions for Functional Connectomics. Neuroimage 2021, 243, 118503. [Google Scholar] [CrossRef]
- DiBella, E.V.R.; Sharma, A.; Richards, L.; Prabhakaran, V.; Majersik, J.J.; HashemizadehKolowri, S.K. Beyond Diffusion Tensor MRI Methods for Improved Characterization of the Brain after Ischemic Stroke: A Review. AJNR Am. J. Neuroradiol. 2022, 43, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.-L.; Yeh, C.-H.; Liang, X.; Vidyasagar, R.; Seitz, R.J.; Nilsson, M.; Connelly, A.; Carey, L.M. Structural Connectivity Remote From Lesions Correlates With Somatosensory Outcome Poststroke. Stroke 2021, 52, 2910–2920. [Google Scholar] [CrossRef] [PubMed]
- Gotts, S.J.; Jo, H.J.; Wallace, G.L.; Saad, Z.S.; Cox, R.W.; Martin, A. Two Distinct Forms of Functional Lateralization in the Human Brain. Proc. Natl. Acad. Sci. USA 2013, 110, E3435–E3444. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, C.; Jin, X.; Jiang, Y.; Yang, L.; Chen, Y.; Gong, G. Sex-Related Human Brain Asymmetry in Hemispheric Functional Gradients. Neuroimage 2021, 229, 117761. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, D.; Zeng, Y.; Liu, M. Repetitive Transcranial Magnetic Stimulation for Improving Function after Stroke. Cochrane Database Syst. Rev. 2013, 2013, CD008862. [Google Scholar] [CrossRef]
- Zeng, Y.; Ye, Z.; Zheng, W.; Wang, J. Efficacy of Cerebellar Transcranial Magnetic Stimulation for Post-Stroke Balance and Limb Motor Function Impairments: Meta-Analyses of Random Controlled Trials and Resting-State fMRI Studies. Cerebellum 2024, 23, 1678–1696. [Google Scholar] [CrossRef]
- Vatinno, A.A.; Simpson, A.; Ramakrishnan, V.; Bonilha, H.S.; Bonilha, L.; Seo, N.J. The Prognostic Utility of Electroencephalography in Stroke Recovery: A Systematic Review and Meta-Analysis. Neurorehabilit. Neural Repair 2022, 36, 255–268. [Google Scholar] [CrossRef]
- Jo, J.Y.; Lee, A.; Kim, M.S.; Park, E.; Chang, W.H.; Shin, Y.-I.; Kim, Y.-H. Prediction of Motor Recovery Using Quantitative Parameters of Motor Evoked Potential in Patients With Stroke. Ann. Rehabil. Med. 2016, 40, 806–815. [Google Scholar] [CrossRef]
- Zhang, J.J.; Sánchez Vidaña, D.I.; Chan, J.N.-M.; Hui, E.S.K.; Lau, K.K.; Wang, X.; Lau, B.W.M.; Fong, K.N.K. Biomarkers for Prognostic Functional Recovery Poststroke: A Narrative Review. Front. Cell Dev. Biol. 2022, 10, 1062807. [Google Scholar] [CrossRef]
- Andersen, S.M.; Rapcsak, S.Z.; Beeson, P.M. Cost Function Masking during Normalization of Brains with Focal Lesions: Still a Necessity? Neuroimage 2010, 53, 78–84. [Google Scholar] [CrossRef]
- Nachev, P.; Coulthard, E.; Jäger, H.R.; Kennard, C.; Husain, M. Enantiomorphic Normalization of Focally Lesioned Brains. Neuroimage 2008, 39, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.M.; Emsell, L.; Blommaert, J.; Zhylka, A.; Kovacs, S.; Theys, T.; Sollmann, N.; Dupont, P.; Sunaert, S. Virtual Brain Grafting: Enabling Whole Brain Parcellation in the Presence of Large Lesions. Neuroimage 2021, 229, 117731. [Google Scholar] [CrossRef]
- Solodkin, A.; Hasson, U.; Siugzdaite, R.; Schiel, M.; Chen, E.E.; Kotter, R.; Small, S.L. Virtual Brain Transplantation (VBT): A Method for Accurate Image Registration and Parcellation in Large Cortical Stroke. Arch. Ital. Biol. 2010, 148, 219–241. [Google Scholar] [PubMed]
- Yourganov, G.; Fridriksson, J.; Stark, B.; Rorden, C. Removal of Artifacts from Resting-State fMRI Data in Stroke. Neuroimage Clin. 2018, 17, 297–305. [Google Scholar] [CrossRef]
- Brett, M.; Leff, A.P.; Rorden, C.; Ashburner, J. Spatial Normalization of Brain Images with Focal Lesions Using Cost Function Masking. Neuroimage 2001, 14, 486–500. [Google Scholar] [CrossRef]
- Crinion, J.; Ashburner, J.; Leff, A.; Brett, M.; Price, C.; Friston, K. Spatial Normalization of Lesioned Brains: Performance Evaluation and Impact on fMRI Analyses. Neuroimage 2007, 37, 866–875. [Google Scholar] [CrossRef]


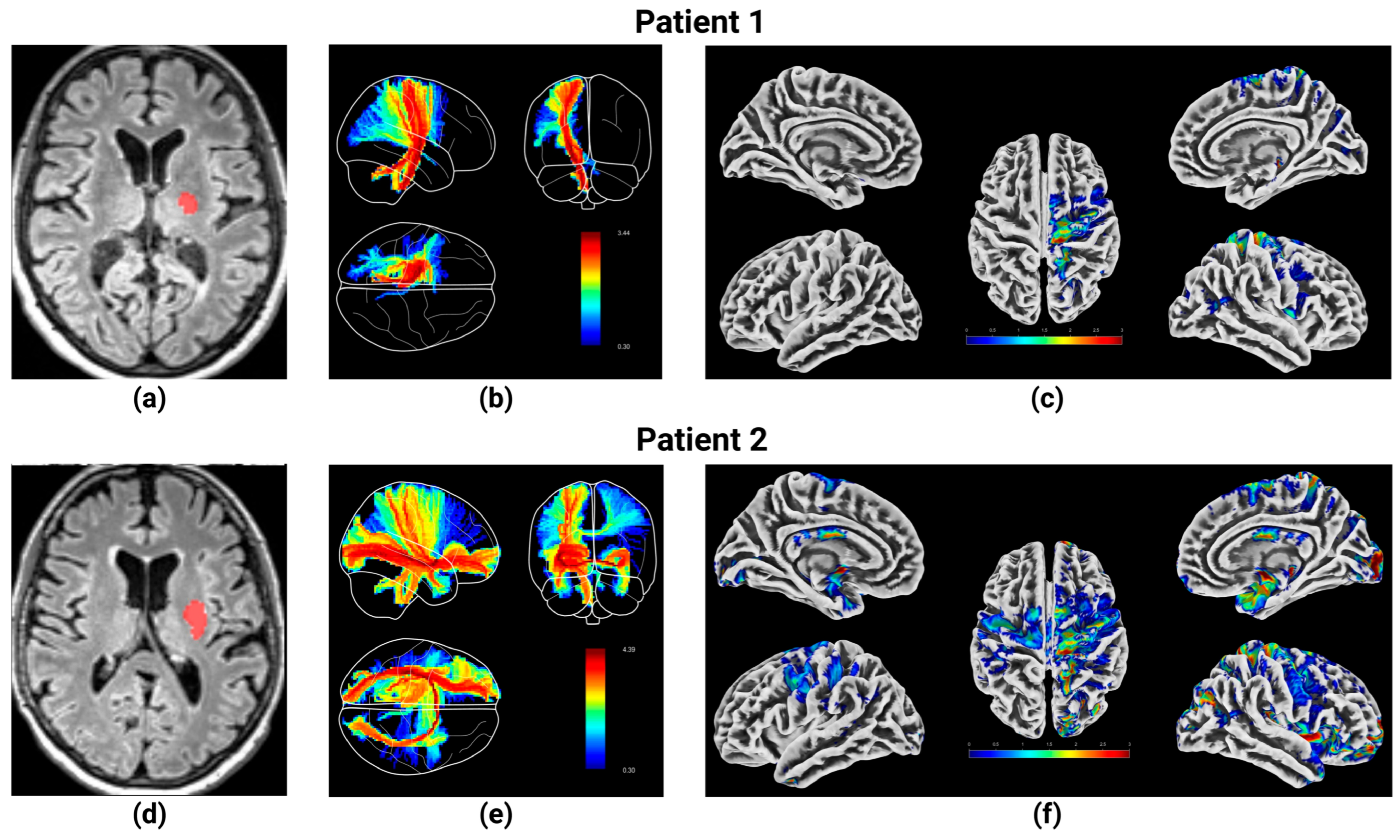
| N | 44 |
|---|---|
| Age (in years) | 68.11 ± 10.2 (68.50, 47–86) |
| Sex female/male (in %) | 21/23 (47.73/52.27%) |
| NIHSS score at admission | 4.30 ± 3.35 (3.00, 0–14) |
| NIHSS score at 24 h (early follow-up) | 2.86 ± 2.60 (2.00, 0–14) |
| NIHSS score at discharge | 1.84 ± 2.17 (1.00, 0–10, n = 43) |
| mRS pre-stroke level | 0.44 ± 0.84 (0.00, 0–3, n = 41) |
| mRS at discharge | 1.73 ± 1.18 (1.50, 0–4, n = 40) |
| Affected hemisphere right/left (in %) | 19/25 (43.18%/56.82%) |
| Premedication | |
| Platelet aggregation inhibition (single or dual; in %) | 11 (25.00%) |
| Oral anticoagulation (in %) | 2 (4.55%) |
| Acute treatment intervention | |
| Systemic thrombolysis (external or in-house, in %) | 10 (22.73%) |
| Mechanical thrombectomy (in %) | 4 (9.10%) |
| Both (thrombolysis and thrombectomy) | 3 (6.82%) |
| Acute carotid artery thrombendarteriectomy (in %) | 0 (0.00%) |
| Duration of hospitalization in days | 6.64 ± 2.84 (6.00, 2–14) |
| N | 44 |
|---|---|
| Anatomical region | |
| Basal ganglia | 28 (63.63%) |
| Thalamus | 7 (15.90%) |
| Centrum semiovale and periventricular regions | 5 (11.37%) |
| Other (e.g., hand knob, hippocampus) | 4 (9.10%) |
| Etiological subtype (TOAST classification) | |
| Cardioembolic | 14 (31.82%) |
| Cryptogenic | 12 (27.27%) |
| Microangiopathic | 11 (25.00%) |
| Macroangiopathic | 7 (15.91%) |
| Model Description | R-Squared | Adjusted R-Squared | AIC | BIC | Cross-Validated R-Squared | Cross-Validated MSE |
|---|---|---|---|---|---|---|
| Lesion Size Only | 0.48 | 0.47 | 205.39 | 208.96 | 0.49 | 6.62 |
| Best Connectivity Predictor Only (bilateral primary motor vs. left basal ganglia motor) | 0.21 | 0.19 | 224.17 | 227.73 | 0.21 | 9.45 |
| Best Predictors Without Lesion Size | 0.56 | 0.50 | 206.33 | 217.03 | 0.59 | 8.24 |
| Best Predictors Including Lesion Size | 0.71 | 0.67 | 188.00 | 198.70 | 0.73 | 5.37 |
| Best Predictors for Left Hemisphere Only | 0.54 | 0.48 | 207.74 | 218.45 | 0.57 | 8.28 |
| Best Predictors for Right Hemisphere Only | 0.42 | 0.35 | 218.14 | 228.85 | 0.45 | 9.87 |
| Best Predictors for Both Hemispheres (D.) | 0.48 | 0.41 | 213.69 | 224.40 | 0.52 | 10.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigel, K.; Gaser, C.; Brodoehl, S.; Wagner, F.; Jochmann, E.; Güllmar, D.; Mayer, T.E.; Klingner, C.M. Acute Stroke Severity Assessment: The Impact of Lesion Size and Functional Connectivity. Brain Sci. 2025, 15, 735. https://doi.org/10.3390/brainsci15070735
Weigel K, Gaser C, Brodoehl S, Wagner F, Jochmann E, Güllmar D, Mayer TE, Klingner CM. Acute Stroke Severity Assessment: The Impact of Lesion Size and Functional Connectivity. Brain Sciences. 2025; 15(7):735. https://doi.org/10.3390/brainsci15070735
Chicago/Turabian StyleWeigel, Karolin, Christian Gaser, Stefan Brodoehl, Franziska Wagner, Elisabeth Jochmann, Daniel Güllmar, Thomas E. Mayer, and Carsten M. Klingner. 2025. "Acute Stroke Severity Assessment: The Impact of Lesion Size and Functional Connectivity" Brain Sciences 15, no. 7: 735. https://doi.org/10.3390/brainsci15070735
APA StyleWeigel, K., Gaser, C., Brodoehl, S., Wagner, F., Jochmann, E., Güllmar, D., Mayer, T. E., & Klingner, C. M. (2025). Acute Stroke Severity Assessment: The Impact of Lesion Size and Functional Connectivity. Brain Sciences, 15(7), 735. https://doi.org/10.3390/brainsci15070735







