Biofeedback for Motor and Cognitive Rehabilitation in Parkinson’s Disease: A Comprehensive Review of Non-Invasive Interventions
Abstract
1. Introduction
- -
- Electromyographic (EMG) biofeedback, which facilitates voluntary control over muscle activity by providing real-time visual or auditory feedback on electromyographic signals. This method has been investigated for its potential to reduce rigidity, improve postural stability, and enhance motor coordination in PD patients [27,28,29,30,31].
- -
- Heart rate variability (HRV) biofeedback, which targets autonomic nervous system (ANS) dysregulation by training patients to modulate their breathing patterns to influence heart rate variability and vagal tone. This approach has shown promise in reducing PD-related anxiety, depression, and orthostatic hypotension [32,33,34].
- -
- Electroencephalographic (EEG) neurofeedback, which focuses on training individuals to modulate dysfunctional brain oscillations associated with motor impairment, executive dysfunction, and cognitive decline. EEG–NF interventions have demonstrated potential for improving motor coordination, cognitive flexibility, and emotional self-regulation in PD [35,36,37].
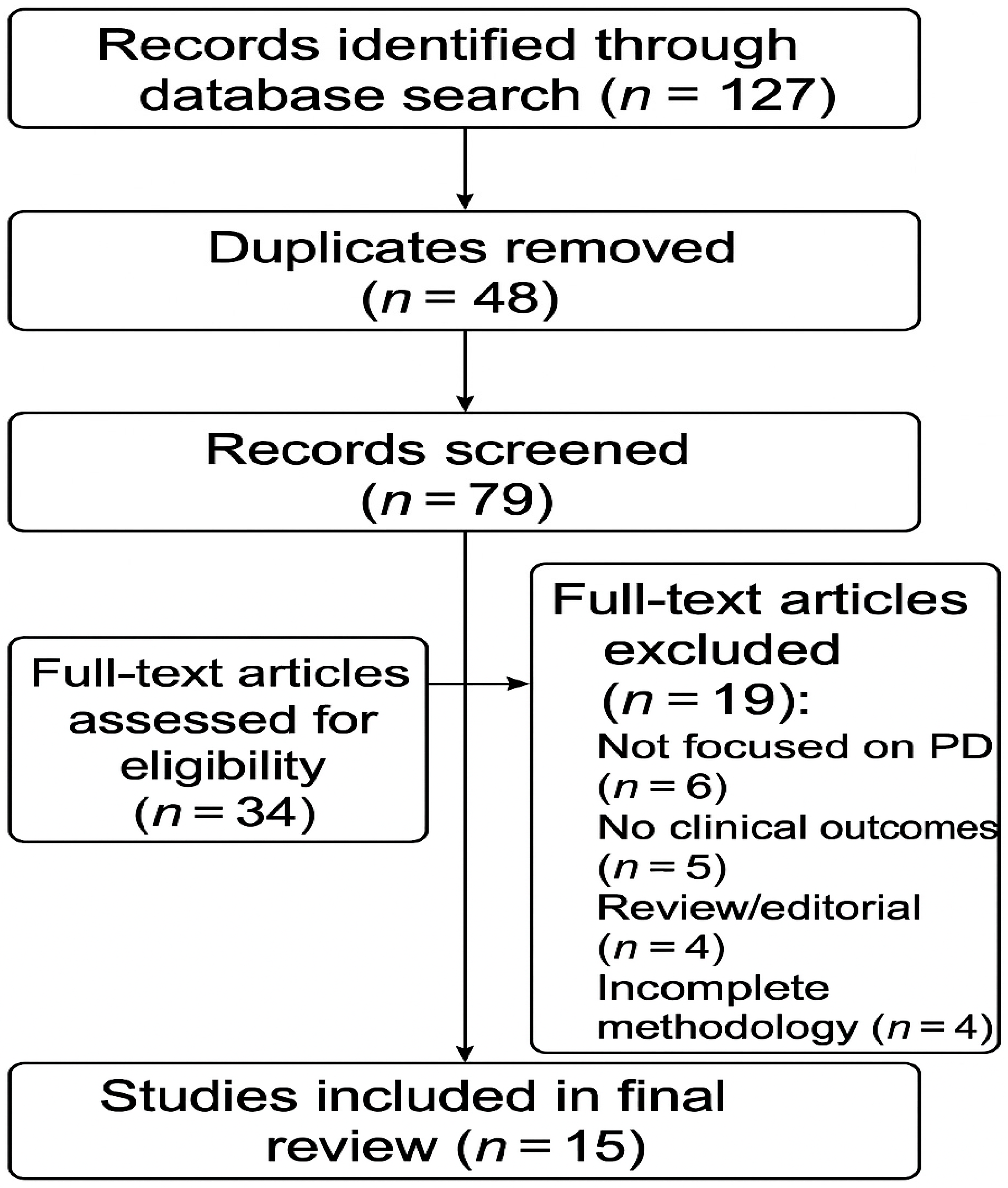
2. Methods
3. Results
3.1. Effects of EMG Biofeedback on Motor Outcomes in Parkinson’s Disease
3.2. Effects of EMG Biofeedback on Non-Motor Outcomes
3.3. Effects of HRV Biofeedback on Autonomic Regulation and Emotional Function
3.4. Clinical Application Models for Biofeedback Integration in Parkinson’s Disease Rehabilitation
3.5. Trends and Clinical Implications
3.6. Methodological Constraints in Reviewed Studies
- -
- Small sample sizes, often below the threshold for statistical power, which limit the generalizability of findings.
- -
- Short intervention durations and lack of long-term follow-up, which restrict the ability to assess sustained effects of biofeedback interventions.
- -
- Heterogeneity in populations, including studies with mixed cohorts (e.g., PD and stroke), or the use of healthy controls rather than PD patients.
- -
- Limited use of control groups and randomization, reducing internal validity.
- -
- Absence of blinding, particularly in pilot or feasibility studies, increasing risk of bias.
- -
- Variability in outcome measures, which hinders cross-study comparisons.
- -
- Focus on feasibility or acceptability over efficacy, especially in studies involving novel technologies (e.g., VR- or AI-driven biofeedback).
4. Discussion
5. Study Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Zhang, B.; Ren, Q.; Zhong, Q.; Li, Y.; Liu, G.; Zhao, C. Eye movement especially vertical oculomotor impairment as an aid to assess Parkinson’s disease. Neurol. Sci. 2021, 42, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Kouli, A.; Torsney, K.M.; Kuan, W.L. Parkinson’s disease: Etiology, neuropathology, and pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, A., Greenland, C., Eds.; Codon Publications: Singapore, 2018. [Google Scholar]
- Abusrair, A.; Elsekaily, W.; Bohlega, S. Tremor in Parkinson’s disease: From pathophysiology to advanced therapies. Tremor Other Hyperkinetic Mov. 2022, 12, 29. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- McKay, J.L.; Hackney, M.E.; Factor, S.A.; Ting, L.H. Lower limb rigidity is associated with frequent falls in Parkinson’s disease. Mov. Disord. Clin. Pract. 2019, 6, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Silva, A.B.R.L.; de Oliveira, R.W.G.; Diógenes, G.P.; de Castro Aguiar, M.F.; Sallem, C.C.; Lima, M.P.P.; de Albuquerque Filho, L.B.; de Medeiros, S.D.P.; de Mendonça, L.L.P.; de Santiago Filho, P.C.; et al. Premotor, nonmotor and motor symptoms of Parkinson’s disease: A new clinical state of the art. Ageing Res. Rev. 2023, 84, 101834. [Google Scholar] [CrossRef]
- Diaconu, S.; Monescu, V.; Filip, R.; Marian, L.; Kakucs, C.; Murasan, I.; Chaudhuri, K.R.; Jianu, D.C.; Falup-Pecurariu, C.; Opritoiu, B. The impact of fatigue on sleep and other non-motor symptoms in Parkinson’s disease. Brain Sci. 2024, 14, 397. [Google Scholar] [CrossRef]
- Li, X.; Chen, C.; Pan, T.; Zhou, X.; Sun, X.; Zhang, Z.; Wu, D.; Chen, X. Trends and hotspots in non-motor symptoms of Parkinson’s disease: A 10-year bibliometric analysis. Front. Aging Neurosci. 2024, 16, 1335550. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 509–524. [Google Scholar] [CrossRef]
- Golińska, P.; Bieleninik, Ł.; Harciarek, M.; Bidzan, M. The impact of cognitive impairment of individuals with Parkinson’s disease on their caregivers’ mental health: A systematic review protocol. PLoS ONE 2022, 17, e0271480. [Google Scholar] [CrossRef]
- Khan, A.; Ezeugwa, J.; Ezeugwu, V.E. A systematic review of the associations between sedentary behavior, physical inactivity, and non-motor symptoms of Parkinson’s disease. PLoS ONE 2024, 19, e0293382. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Schapira, A.H. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Furdu-Lunguț, E.; Antal, C.; Turcu, S.; Costea, D.O.; Mitran, M.; Mitran, L.; Diaconescu, A.S.; Novac, M.B.; Gorecki, G.P. Study on pharmacological treatment of impulse control disorders in Parkinson’s disease. J. Clin. Med. 2024, 13, 6708. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2021, 17, 496–510. [Google Scholar] [CrossRef]
- Policastro, G.; Brunelli, M.; Tinazzi, M.; Chiamulera, C.; Emerich, D.F.; Paolone, G. Cytokine-, neurotrophin-, and motor rehabilitation-induced plasticity in Parkinson’s disease. Neural Plast. 2020, 2020, 8814028. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Lang, A.E.; Olanow, C.W. Levodopa-induced dyskinesias in Parkinson disease: Current and evolving concepts. Ann. Neurol. 2018, 84, 797–811. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Liu, Z.; Rao, J.; Wang, J.; Wang, P.; Gong, X.; Wen, Y. Transcranial direct current stimulation for Parkinson’s disease: A systematic review and meta-analysis. Front. Aging Neurosci. 2021, 13, 746797. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, P.; Li, Z.; Holmes, C.F.; Zhong, Y.; Liu, H.; Bao, X.; Xie, J. Efficacy of repetitive transcranial magnetic stimulation over the supplementary motor area on motor function in Parkinson’s disease. Am. J. Phys. Med. Rehabil. 2024, 104, 318–324. [Google Scholar] [CrossRef]
- Weaver, F.M.; Follett, K.A.; Stern, M.; Hur, K.; Harris, C.L.; Marks, W.J.; Reda, D.J. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: A randomized controlled trial. JAMA 2012, 301, 63–73. [Google Scholar] [CrossRef]
- Greenland, J.C.; Williams-Gray, C.H.; Barker, R.A. The clinical heterogeneity of Parkinson’s disease and its therapeutic implications. Eur. J. Neurosci. 2018, 49, 328–338. [Google Scholar] [CrossRef]
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schäfer, H.; Bötzel, K.; German Parkinson Study Group. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Radder, D.L.M.; Sturkenboom, I.H.; Nimwegen, M.v.; Keus, S.; Bloem, B.R.; Vries, N.M.d. Physical therapy and occupational therapy in Parkinson’s disease. Int. J. Neurosci. 2017, 127, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Volpe, D.; Ellis, T.D.; Hirsch, M.A.; Johnson, J.; Wood, J.T.; Aragon, A.; Biundo, R.; Di Rocco, A.; Kasman, G.S.; et al. Delivering multidisciplinary rehabilitation care in Parkinson’s disease: An international consensus statement. J. Park. Dis. 2024, 14, 135–166. [Google Scholar] [CrossRef]
- Budzynski, T.H.; Budzynski, H.K.; Evans, J.R.; Abarbanel, A. Introduction to Quantitative EEG and Neurofeedback: Advanced Theory and Applications; Academic Press: Cambridge, UK, 2009. [Google Scholar]
- Bowman, T.; Gervasoni, E.; Arienti, C.; Lazzarini, S.G.; Negrini, S.; Crea, S.; Cattaneo, D.; Carrozza, M.C. Wearable devices for biofeedback rehabilitation: A systematic review and meta-analysis to design application rules and estimate the effectiveness on balance and gait outcomes in neurological diseases. Sensors 2021, 21, 3444. [Google Scholar] [CrossRef] [PubMed]
- Freitas, G.S.D.; Mituuti, C.T.; Furkim, A.M.; Busanello-Stella, A.R.; Stefani, F.M.; Arone, M.M.A.D.S.; Berretin-Felix, G. Electromyography biofeedback in the treatment of neurogenic orofacial disorders: Systematic review of the literature. Audiol.-Commun. Res. 2016, 21, e1671. [Google Scholar] [CrossRef]
- Albuquerque, L.C.A.; Pernambuco, L.; da Silva, C.M.; Chateaubriand, M.M.; da Silva, H.J. Effects of electromyographic biofeedback as an adjunctive therapy in the treatment of swallowing disorders: A systematic review of the literature. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 927–938. [Google Scholar] [CrossRef]
- Thibault, R.T.; Lifshitz, M.; Birbaumer, N.; Raz, A. Neurofeedback, self-regulation, and brain imaging: Clinical science and fad in the service of mental disorders. Psychol. Sci. Public Interest 2018, 19, 103–186. [Google Scholar] [CrossRef]
- Battel, I.; Walshe, M. An intensive neurorehabilitation programme with sEMG biofeedback to improve swallowing in idiopathic Parkinson’s disease (IPD): A feasibility study. Int. J. Lang. Commun. Disord. 2023, 58, 813–825. [Google Scholar] [CrossRef]
- Lanzani, V.; Brambilla, C.; Scano, A. A methodological scoping review on EMG processing and synergy-based results in muscle synergy studies in Parkinson’s disease. Front. Bioeng. Biotechnol. 2025, 12, 1445447. [Google Scholar] [CrossRef]
- Arone, M.M.A.D.S.; Brasolotto, A.G.; Luccas, G.R.D.; Gatti, M.; Mituuti, C.T.; Berretin-Felix, G. Biofeedback eletromiográfico como coadjuvante pode ajudar a manter os resultados da terapia profilática de deglutição em longo prazo na doença de Parkinson? Um estudo piloto. Audiol. -Commun. Res. 2021, 26, e2542. [Google Scholar] [CrossRef]
- Heimrich, K.G.; Lehmann, T.; Schlattmann, P.; Prell, T. Heart rate variability analyses in Parkinson’s disease: A systematic review and meta-analysis. Brain Sci. 2021, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Morrow, C.B.; Seemiller, J.; Mills, K.A.; Pontone, G.M. The effect of dysautonomia on motor, behavioral, and cognitive fluctuations in Parkinson’s disease. Mov. Disord. 2025, 40, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, X.; Jing, W.; Omorodion, I.; Liu, L. Association between heart rate variability and Parkinson’s disease: A meta-analysis. Curr. Pharm. Des. 2021, 27, 2056–2067. [Google Scholar] [CrossRef]
- Matzilevich, E.U.; Daniel, P.L.; Little, S. Towards therapeutic electrophysiological neurofeedback in Parkinson’s disease. Park. Relat. Disord. 2024, 121, 106010. [Google Scholar] [CrossRef] [PubMed]
- Cooke, A.; Hindle, J.; Lawrence, C.; Bellomo, E.; Pritchard, A.W.; MacLeod, C.A.; Martin-Forbes, P.; Jones, S.; Bracewell, M.; Linden, D.E.J.; et al. Effects of home-based EEG neurofeedback training as a non-pharmacological intervention for Parkinson’s disease. Neurophysiol. Clin. 2024, 54, 102997. [Google Scholar] [CrossRef]
- Pei, G.; Wu, J.; Chen, D.; Guo, G.; Liu, S.; Hong, M.; Yan, T. Effects of an integrated neurofeedback system with dry electrodes: EEG acquisition and cognition assessment. Sensors 2018, 18, 3396. [Google Scholar] [CrossRef]
- Blaznik, L.; Marusic, U. Exploring the Impact of Electroencephalography-Based Neurofeedback (EEG NFB) on Motor Deficits in Parkinson’s Disease: A Targeted Literature Review. Appl. Sci. 2025, 15, 2496. [Google Scholar] [CrossRef]
- Mirelman, A.; Herman, T.; Nicolai, S.; Zijlstra, A.; Zijlstra, W.; Becker, C.; Hausdorff, J.M. Audio-biofeedback training for posture and balance in patients with Parkinson’s disease. J. Neuroeng. Rehabil. 2011, 8, 1–7. [Google Scholar] [CrossRef]
- Nanhoe-Mahabier, W.; Allum, J.H.J.; Overeem, S.; Borm, G.F.; Bloem, B.R. The effect of vibrotactile biofeedback on trunk sway during stance tasks in Parkinson’s disease. Mov. Disord. 2012, 27, 532–537. [Google Scholar]
- Caudron, S.; Benvenuti, F.; Bentivoglio, A.R.; Hallemans, A. Visual feedback improves postural stability in Parkinson’s disease: A single-session study. Gait Posture 2014, 40, 611–615. [Google Scholar]
- Kober, S.E.; Witte, M.; Stangl, M.; Väljamäe, A.; Neuper, C.; Wood, G. Shutting down sensorimotor interference unblocks the networks for stimulus processing: An SMR neurofeedback training study. Clin. Neurophysiol. 2015, 126, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Byl, N.; Zhang, W.; Coo, S.; Tomizuka, M. Clinical impact of gait training enhanced with visual kinematic biofeedback: Patients with Parkinson’s disease and patients stable post stroke. Neuropsychologia 2015, 79, 332–343. [Google Scholar] [CrossRef]
- Carpinella, I.; Cattaneo, D.; Bonora, G.; Bowman, T.; Martina, L.; Montesano, A.; Ferrarin, M. Wearable sensor-based biofeedback training for balance and gait in Parkinson disease: A pilot randomized controlled trial. Arch. Phys. Med. Rehabil. 2017, 98, 622–630. [Google Scholar] [CrossRef]
- Roskopf, C.; Hepp-Reymond, M.-C.; Dietz, V. Vibrotactile feedback improves postural control in patients with Parkinson’s disease. J. NeuroEng. Rehabil. 2019, 16, 78. [Google Scholar]
- Marcos-Martínez, D.; Martínez-Cagigal, V.; Santamaría-Vázquez, E.; Pérez-Velasco, S.; Hornero, R. Neurofeedback training based on motor imagery strategies increases EEG complexity in elderly population. Entropy 2021, 23, 1574. [Google Scholar] [CrossRef] [PubMed]
- McMaster, T.; Hillier, S.; McGinley, J. Real-time trunk lean visual biofeedback improves gait in individuals with Parkinson’s disease: A pilot study. Gait Posture 2022, 92, 258–263. [Google Scholar]
- Shi, Z.; Ding, L.; Han, X.; Jiang, B.; Zhang, J.; Suo, D.; Wu, J.; Pei, G.; Fang, B.; Yan, T. Multimodal biofeedback for Parkinson’s disease motor and nonmotor symptoms. Brain Sci. Adv. 2023, 9, 136–154. [Google Scholar] [CrossRef]
- Romero, J.P.; Moreno-Verdú, M.; Arroyo-Ferrer, A.; Serrano, J.I.; Herreros-Rodríguez, J.; García-Caldentey, J.; Rocon de Lima, E.; Del Castillo, M.D. Clinical and neurophysiological effects of bilateral repetitive transcranial magnetic stimulation and EEG-guided neurofeedback in Parkinson’s disease: A randomized, four-arm controlled trial. J. Neuroeng. Rehabil. 2024, 21, 135. [Google Scholar] [CrossRef]
- Xu, S.; Kantarcigil, C.; Rangwala, R.; Nellis, A.; San Chun, K.; Richards, D.; Martin-Harris, B. Digital health technology for Parkinson’s disease with comprehensive monitoring and artificial intelligence-enabled haptic biofeedback for bulbar dysfunction. J. Parkinson’s Dis. 2024, 15, 630–645. [Google Scholar] [CrossRef]
- da Cruz, A.; Martins, M.P.; Pereira, F. Virtual reality combined with EMG biofeedback and rhythmic auditory stimulation for voice rehabilitation in Parkinson’s disease: A controlled trial. J. Neurorehabil. Neural Repair. 2025, 39, 158–167. [Google Scholar]
- Bonora, G. Biofeedback Based Physical Rehabilitation in Parkinson’s Disease Aimed at Self-Enhancement. Ph.D. Thesis, Alma Mater Studiorum Università di Bologna, Bologna, Italy, 2016. [Google Scholar] [CrossRef]
- Hasegawa, N.; Asaka, T. Motor learning on postural control using auditory biofeedback training. Impact 2021, 2021, 55–57. [Google Scholar] [CrossRef]
- Iłżecka, J. Biofeedback as a form of neurorehabilitation in Parkinson’s disease. J. Educ. Health Sport 2021, 11, 33–39. [Google Scholar] [CrossRef]
- Lucas, I.; Solé-Morata, N.; Baenas, I.; Rosinska, M.; Fernández-Aranda, F.; Jiménez-Murcia, S. Biofeedback interventions for impulsivity-related processes in addictive disorders. Curr. Addict. Rep. 2023, 10, 543–552. [Google Scholar] [CrossRef]
- Knežević, S. Brain-Computer Interfaces In Neurorehabilitation For Central Nervous System Diseases: Applications in Stroke, Multiple Sclerosis And Parkinson’s Disease. Sanamed 2025, 20, 49–59. [Google Scholar] [CrossRef]
- Kouzak, V.; Clotilde Tavares, M.; Isabel Silvestre da Silva, W. The Effects of Neurofeedback Training in Patients with Parkinson’s Disease; IntechOpen: London, UK, 2024. [Google Scholar]
- Little, S.; Brown, P. The functional role of beta oscillations in Parkinson’s disease. Park. Relat. Disord. 2014, 20, 44–48. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Anil, K.; Hall, S.D.; Demain, S.; Freeman, J.A.; Ganis, G.; Marsden, J. A systematic review of neurofeedback for the management of motor symptoms in Parkinson’s disease. Brain Sci. 2021, 11, 1292. [Google Scholar] [CrossRef]
- Esmail, S.; Linden, D.E. Neural networks and neurofeedback in Parkinson’s disease. Neuroregulation 2014, 1, 240. [Google Scholar] [CrossRef]
- Caliskan, A.; Badem, H.; Basturk, A.; Yuksel, M. Diagnosis of the Parkinson disease by using deep neural network classifier. IU-J. Electr. Electron. Eng. 2017, 17, 3311–3318. [Google Scholar]
- Ananías, J.; Vidal, C.; Ortiz-Muñoz, L.; Irarrázaval, S.; Besa, P. Use of electromyographic biofeedback in rehabilitation following anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Physiotherapy 2024, 123, 19–29. [Google Scholar] [CrossRef]
- Arone, M.M.A.D.S.; Brasolotto, A.G.; Luccas, G.R.D.; Gatti, M.; Mituuti, C.T.; Berretin-Felix, G. Can adjunctive electromyographic biofeedback help maintain long-term prophylactic swallowing therapy results in Parkinson’s disease? A pilot study. Audiol. Commun. Res. 2021, 26, e2542. [Google Scholar] [CrossRef]
- Imama, S.; Zameer, Z. Emerging Paradigms in Exercise-Based Neuro-Physiotherapy for Holistic Motor and Cognitive Rehabilitation in Parkinson’s Disease: Neuro-Physiotherapy in Parkinson’s Disease. Therapist (J. Therapies Rehabil. Sci.) 2024, 5, 2–10. [Google Scholar] [CrossRef]
- Murthy, Y.S.; Bansal, R.; Chodisetty, R.C.M.; Chakravorty, C.; Sai, K.P. Transforming Minds AI and Machine Learning Applications in Cognitive Neuroscience. In Transforming Neuropsychology and Cognitive Psychology with AI and Machine Learning; IGI Global Scientific Publishing: Hershey, PA, USA, 2025; pp. 107–128. [Google Scholar]
- Shah, R.; Dave, B.; Parekh, N.; Srivastava, K. Parkinson’s disease detection-an interpretable approach to temporal audio classification. In Proceedings of the 2022 IEEE 3rd Global Conference for Advancement in Technology (GCAT), Bangalore, India, 7–9 October 2022; pp. 1–6. [Google Scholar]
- Lim, S.B.; Horslen, B.C.; Davis, J.R.; Allum, J.H.; Carpenter, M.G. Benefits of multi-session balance and gait training with multi-modal biofeedback in healthy older adults. Gait Posture 2016, 47, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.; Lockhart, T.E.; Lieberman, A. Motor learning deficits in Parkinson’s disease (PD) and their effect on training response in gait and balance: A narrative review. Front. Neurol. 2019, 10, 62. [Google Scholar] [CrossRef]
| Authors (Year) | Sample | Type of Biofeedback | Intervention Duration | Main Outcomes | Key Limitations | Study Design | |
|---|---|---|---|---|---|---|---|
| 1 | Mirelman et al. (2011) [40] | PD patients | Audio-based | 6 weeks | ↑ Posture, balance | Small sample; limited to short-term follow-up | Controlled trial |
| 2 | Nanhoe-Mahabier et al. (2012) [41] | 20 PD patients | Vibrotactile | Single session | ↓ Trunk sway; ↑ Balance | Single session; no control group | Pre–post experimental |
| 3 | Caudron et al. (2014) [42] | 17 PD patients | Visual | Single session | ↓ Postural bias; ↑ Orientation | Single session; lacks generalizability | Pre–post experimental |
| 4 | Kober et al. (2014) [43] | 20 (10 exp/10 ctrl) | EEG (SMR) | 10 sessions | ↑ Attention, memory, ERP | Small sample size; healthy subjects only | RCT |
| 5 | Byl et al. (2015) [44] | 20 PD + stroke patients | Visual (Gait) | 8 weeks | ↑ Gait parameters, motor control | Mixed population (PD and stroke); no long-term follow-up | Interventional study |
| 6 | Carpinella et al. (2017) [45] | 42 PD patients | Sensor-based (Wearable) | 20 sessions | ↑ Balance, gait | Pilot RCT; short duration | Pilot RCT |
| 7 | Roskopf et al. (2019) [46] | PD patients | Vibrotactile | 4 weeks | ↓ Postural sway; ↑ Balance | Small sample; lack of blinded assessment | Controlled trial |
| 8 | Arone et al. (2021) [32] | 6 PD patients | EMG (Swallowing) | 18 sessions | ↑ Swallowing retention | Very small sample; no randomization | Pilot study |
| 9 | Bowman et al. (2021) [26] | PD patients | Visual + Auditory | 6 weeks | ↑ Gait speed, step length | No blinding; limited sample | Randomized controlled trial |
| 10 | Marcos-Martínez et al. (2021) [47] | 11 elderly subjects | Motor Imagery EEG | 5 sessions | ↑ EEG complexity, cognition | Elderly subjects only; no PD patients | Pilot study |
| 11 | McMaster et al. (2022) [48] | PD patients | Visual (Trunk lean) | 1 week + follow-up | ↓ Trunk lean; ↑ Gait | Short follow-up; no control group | Pilot study |
| 12 | Shi et al. (2023) [49] | 21 PD patients | Multimodal (EEG, HRV, PPG) | 5 sessions | ↓ Depression; ↑ Balance, gait | Small sample; limited intervention sessions | Pilot study |
| 13 | Romero et al. (2024) [50] | 40 PD patients | EEG + rTMS | 8 sessions | ↑ Motor symptoms, QoL | Limited follow-up; moderate sample size | Randomized controlled trial |
| 14 | Xu et al. (2024) [51] | 20 PD patients | AI-driven Haptic | Pilot phase | ↑ Swallowing freq; high acceptance | Pilot design; acceptability prioritized over efficacy | Pilot study |
| 15 | da Cruz et al. (2025) [52] | 30 PD patients | VR + EMG + RAS | 2 sessions (7 days apart) | ↓ Vocal jitter/shimmer; ↑ Engagement | Short duration; limited to voice parameters | Controlled trial |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diotaiuti, P.; Marotta, G.; Vitiello, S.; Di Siena, F.; Palombo, M.; Langiano, E.; Ferrara, M.; Mancone, S. Biofeedback for Motor and Cognitive Rehabilitation in Parkinson’s Disease: A Comprehensive Review of Non-Invasive Interventions. Brain Sci. 2025, 15, 720. https://doi.org/10.3390/brainsci15070720
Diotaiuti P, Marotta G, Vitiello S, Di Siena F, Palombo M, Langiano E, Ferrara M, Mancone S. Biofeedback for Motor and Cognitive Rehabilitation in Parkinson’s Disease: A Comprehensive Review of Non-Invasive Interventions. Brain Sciences. 2025; 15(7):720. https://doi.org/10.3390/brainsci15070720
Chicago/Turabian StyleDiotaiuti, Pierluigi, Giulio Marotta, Salvatore Vitiello, Francesco Di Siena, Marco Palombo, Elisa Langiano, Maria Ferrara, and Stefania Mancone. 2025. "Biofeedback for Motor and Cognitive Rehabilitation in Parkinson’s Disease: A Comprehensive Review of Non-Invasive Interventions" Brain Sciences 15, no. 7: 720. https://doi.org/10.3390/brainsci15070720
APA StyleDiotaiuti, P., Marotta, G., Vitiello, S., Di Siena, F., Palombo, M., Langiano, E., Ferrara, M., & Mancone, S. (2025). Biofeedback for Motor and Cognitive Rehabilitation in Parkinson’s Disease: A Comprehensive Review of Non-Invasive Interventions. Brain Sciences, 15(7), 720. https://doi.org/10.3390/brainsci15070720








