Are Changes in Corticomotor Excitability Associated with Improved Arm Functional Performance Following a Tailored Strength Training Intervention in Chronic Stroke Survivors?
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Assessment
2.2. TMS Assessment
2.3. Strength Training and tDCS Intervention
2.4. TMS Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. Variations in TMS Measures with the Intervention
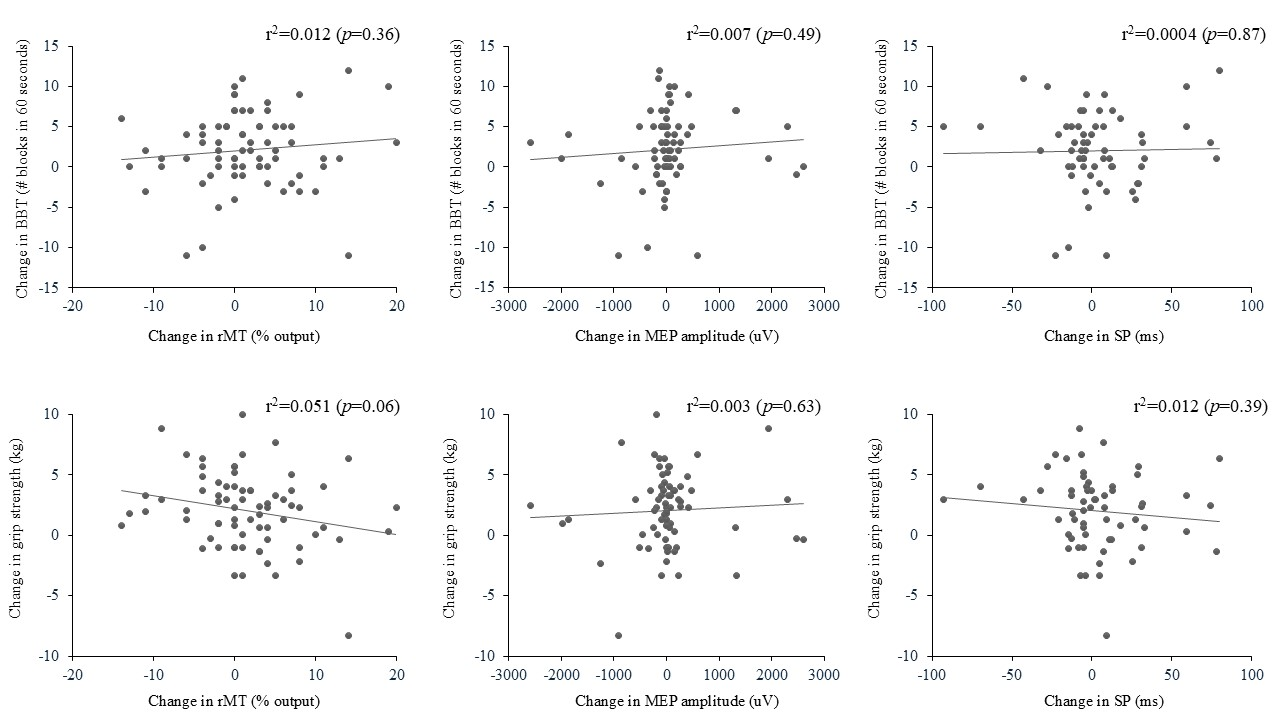
3.3. Association Between Changes in TMS Outcome Measures and Changes in Arm Function
4. Discussion
4.1. Effects of the Intervention on TMS Outcome Measures
4.2. Association Between Changes in TMS Outcome Measures and Arm Functional Performance
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Xu, W. Enhancing Brain Plasticity to Promote Stroke Recovery. Front. Neurol. 2020, 11, 554089. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xu, H.; Yu, C. Brain connectivity plasticity in the motor network after ischemic stroke. Neural Plast. 2013, 2013, 924192. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Saini, M.; Kumar, N.; Srivastava, M.V.P.; Mehndiratta, A. Evidence of neuroplasticity with robotic hand exoskeleton for post-stroke rehabilitation: A randomized controlled trial. J. Neuroeng. Rehabil. 2021, 18, 76. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Milot, M.H. Changes in transcranial magnetic stimulation outcome measures in response to upper-limb physical training in stroke: A systematic review of randomized controlled trials. Ann. Phys. Rehabil. Med. 2018, 61, 224–234. [Google Scholar] [CrossRef]
- Demir, Y.P.; Balci, N.C.; Unluer, N.O.; Ulug, N.; Dogru, E.; Kilinc, M.; Yildirim, S.A.; Yilmaz, O. Three different points of view in stroke rehabilitation: Patient, caregiver, and physiotherapist. Top. Stroke Rehabil. 2015, 22, 377–385. [Google Scholar] [CrossRef]
- Cherni, Y.; Tremblay, A.; Simon, M.; Bretheau, F.; Blanchette, A.K.; Mercier, C. Corticospinal Responses Following Gait-Specific Training in Stroke Survivors: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 15585. [Google Scholar] [CrossRef]
- Turco, C.V.; Nelson, A.J. Transcranial Magnetic Stimulation to Assess Exercise-Induced Neuroplasticity. Front. Neuroergon. 2021, 2, 679033. [Google Scholar] [CrossRef]
- Patten, C.; Lexell, J.; Brown, H.E. Weakness and strength training in persons with poststroke hemiplegia: Rationale, method, and efficacy. J. Rehabil. Res. Dev. 2004, 41, 293–312. [Google Scholar] [CrossRef]
- Harris, J.E.; Eng, J.J. Strength training improves upper-limb function in individuals with stroke: A meta-analysis. Stroke 2010, 41, 136–140. [Google Scholar] [CrossRef]
- Milot, M.H.; Palimeris, S.; Corriveau, H.; Tremblay, F.; Boudrias, M.H. Effects of a tailored strength training program of the upper limb combined with transcranial direct current stimulation (tDCS) in chronic stroke patients: Study protocol for a randomised, double-blind, controlled trial. BMC Sports Sci. Med. Rehabil. 2019, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Palimeris, S.; Ansari, Y.; Remaud, A.; Tremblay, F.; Corriveau, H.; Boudrias, M.H.; Milot, M.H. Effect of a tailored upper extremity strength training intervention combined with direct current stimulation in chronic stroke survivors: A Randomized Controlled Trial. Front. Rehabil. Sci. 2022, 3, 978257. [Google Scholar] [CrossRef] [PubMed]
- Franck, J.A.; Smeets, R.; Seelen, H.A.M. Changes in actual arm-hand use in stroke patients during and after clinical rehabilitation involving a well-defined arm-hand rehabilitation program: A prospective cohort study. PLoS ONE 2019, 14, e0214651. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.; Condliffe, E.G.; Dairaghi, C.A.; Lum, P.S. Concurrent neuromechanical and functional gains following upper-extremity power training post-stroke. J. Neuroeng. Rehabil. 2013, 10, 1. [Google Scholar] [CrossRef]
- Wallace, A.C.; Talelli, P.; Dileone, M.; Oliver, R.; Ward, N.; Cloud, G.; Greenwood, R.; Di Lazzaro, V.; Rothwell, J.C.; Marsden, J.F. Standardizing the intensity of upper limb treatment in rehabilitation medicine. Clin. Rehabil. 2010, 24, 471–478. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Kim, B.; Winstein, C. Can Neurological Biomarkers of Brain Impairment Be Used to Predict Poststroke Motor Recovery? A Systematic Review. Neurorehabilit. Neural Repair 2017, 31, 3–24. [Google Scholar] [CrossRef]
- Stinear, C.M.; Byblow, W.D.; Ackerley, S.J.; Barber, P.A.; Smith, M.C. Predicting Recovery Potential for Individual Stroke Patients Increases Rehabilitation Efficiency. Stroke 2017, 48, 1011–1019. [Google Scholar] [CrossRef]
- Talelli, P.; Greenwood, R.J.; Rothwell, J.C. Arm function after stroke: Neurophysiological correlates and recovery mechanisms assessed by transcranial magnetic stimulation. Clin. Neurophysiol. 2006, 117, 1641–1659. [Google Scholar] [CrossRef]
- Hupfeld, K.E.; Swanson, C.W.; Fling, B.W.; Seidler, R.D. TMS-induced silent periods: A review of methods and call for consistency. J. Neurosci. Methods 2020, 346, 108950. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Trompetto, C. Clinical and research methods for evaluating cortical excitability. J. Clin. Neurophysiol. 2002, 19, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Brum, M.; Cabib, C.; Valls-Sole, J. Clinical Value of the Assessment of Changes in MEP Duration with Voluntary Contraction. Front. Neurosci. 2015, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Bembenek, J.P.; Kurczych, K.; Karli Nski, M.; Czlonkowska, A. The prognostic value of motor-evoked potentials in motor recovery and functional outcome after stroke—A systematic review of the literature. Funct. Neurol. 2012, 27, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J. Motor cortex excitability in stroke before and after constraint-induced movement therapy. Cogn. Behav. Neurol. 2006, 19, 41–47. [Google Scholar] [CrossRef]
- Koski, L.; Mernar, T.J.; Dobkin, B.H. Immediate and long-term changes in corticomotor output in response to rehabilitation: Correlation with functional improvements in chronic stroke. Neurorehabilit. Neural Repair 2004, 18, 230–249. [Google Scholar] [CrossRef]
- Sehle, A.; Stuerner, J.; Hassa, T.; Spiteri, S.; Schoenfeld, M.A.; Liepert, J. Behavioral and neurophysiological effects of an intensified robot-assisted therapy in subacute stroke: A case control study. J. Neuroeng. Rehabil. 2021, 18, 6. [Google Scholar] [CrossRef]
- Teasell, R.; Salbach, N.M.; Foley, N.; Mountain, A.; Cameron, J.I.; de Jong, A.; Acerra, N.E.; Bastasi, D.; Carter, S.L.; Fung, J.; et al. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. Int. J. Stroke 2020, 15, 763–788. [Google Scholar] [CrossRef]
- Simonetti, D.; Zollo, L.; Milighetti, S.; Miccinilli , S.; Bravi, M.; Ranieri, F.; Magrone, G.; Guglielmelli, E.; Di Lazzaro, V.; Sterzi, S. Literature Review on the Effects of tDCS Coupled with Robotic Therapy in Post Stroke Upper Limb Rehabilitation. Front. Hum. Neurosci. 2017, 11, 268. [Google Scholar] [CrossRef]
- Cha, T.H.; Hwang, H.S. Rehabilitation Interventions Combined with Noninvasive Brain Stimulation on Upper Limb Motor Function in Stroke Patients. Brain Sci. 2022, 12, 994. [Google Scholar] [CrossRef]
- Lee, J.H.; Jeun, Y.J.; Park, H.Y.; Jung, Y.J. Effect of Transcranial Direct Current Stimulation Combined with Rehabilitation on Arm and Hand Function in Stroke Patients: A Systematic Review and Meta-Analysis. Healthcare 2021, 9, 1705. [Google Scholar] [CrossRef]
- Bastani, A.; Cofre Lizama, L.E.; Zoghi, M.; Blashki, G.; Davis, S.; Kaye, A.H.; Khan, F.; Galea, M.P. The combined effect of cranial-nerve non-invasive neuromodulation with high-intensity physiotherapy on gait and balance in a patient with cerebellar degeneration: A case report. Cerebellum Ataxias 2018, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.; Teo, W.P.; Tang, N.; Ang, K.K.; Ng, Y.S.; Zhou, J.H.; Teh, I.; Phua, K.S.; Zhao, L.; Guan, C. Using Transcranial Direct Current Stimulation to Augment the Effect of Motor Imagery-Assisted Brain-Computer Interface Training in Chronic Stroke Patients-Cortical Reorganization Considerations. Front. Neurol. 2020, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Cortes, M.; Rykman-Peltz, A.; Chang, J.; Elder, J.; Thickbroom, G.; Mariman, J.J.; Gerber, L.M.; Oromendia, C.; Krebs, H.I.; et al. Clinical improvement with intensive robot-assisted arm training in chronic stroke is unchanged by supplementary tDCS. Restor. Neurol. Neurosci. 2019, 37, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Krebs, H.I.; Rykman, A.; Zipse, J.; Thickbroom, G.W.; Mastaglia, F.L.; Pascual-Leone, A.; Volpe, B.T. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restor. Neurol. Neurosci. 2009, 27, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hummel, F.; Celnik, P.; Giraux, P.; Floel, A.; Wu, W.H.; Gerloff, C.; Cohen, L.G. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005, 128 Pt 3, 490–499. [Google Scholar] [CrossRef]
- Li, L.L.; Wu, J.J.; Li, K.P.; Jin, J.; Xiang, Y.T.; Hua, X.Y.; Zheng, M.X.; Xu, J.G. Comparative efficacy of different noninvasive brain stimulation protocols on upper-extremity motor function and activities of daily living after stroke: A systematic review and network meta-analysis. Neurol. Sci. 2024, 45, 3641–3681. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Blanchette, A.K.; Mercier, C.; Bernard-Larocque, V.; Milot, M.H. Efficacy, safety, and tolerability of bilateral transcranial direct current stimulation combined to a resistance training program in chronic stroke survivors: A double-blind, randomized, placebo-controlled pilot study. Restor. Neurol. Neurosci. 2019, 37, 333–346. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef]
- Mishory, A.; Molnar, C.; Koola, J.; Li, X.; Kozel, F.A.; Myrick, H.; Stroud, Z.; Nahas, Z.; George, M.S. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. J. ECT 2004, 20, 160–165. [Google Scholar] [CrossRef]
- Milot, M.H.; Spencer, S.J.; Chan, V.; Allington, J.P.; Klein, J.; Chou, C.; Pearson-Fuhrhop, K.; Bobrow, J.E.; Reinkensmeyer, D.J.; Cramer, S.C. Corticospinal excitability as a predictor of functional gains at the affected upper limb following robotic training in chronic stroke survivors. Neurorehabilit. Neural Repair 2014, 28, 819–827. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.W.; Tremblay, F. Evidence of alterations in transcallosal motor inhibition as a possible long-term consequence of concussions in sports: A transcranial magnetic stimulation study. Clin. Neurophysiol. 2016, 127. [Google Scholar] [CrossRef]
- Iannone, A.; Santiago, I.; Ajao, S.T.; Brasil-Neto, J.; Rothwell, J.C.; Spampinato, D.A. Comparing the effects of focal and conventional tDCS on motor skill learning: A proof of principle study. Neurosci. Res. 2022, 178, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Jonker, Z.D.; Gaiser, C.; Tulen, J.H.M.; Ribbers, G.M.; Frens, M.A.; Selles, R.W. No effect of anodal tDCS on motor cortical excitability and no evidence for responders in a large double-blind placebo-controlled trial. Brain Stimul. 2021, 14, 100–109. [Google Scholar] [CrossRef]
- Bastani, A.; Jaberzadeh, S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: A systematic review and meta-analysis. Clin. Neurophysiol. 2012, 123, 644–657. [Google Scholar] [CrossRef]
- Hordacre, B.; McCambridge, A.B.; Ridding, M.C.; Bradnam, L.V. Can Transcranial Direct Current Stimulation Enhance Poststroke Motor Recovery? Development of a Theoretical Patient-Tailored Model. Neurology 2021, 97, 170–180. [Google Scholar] [CrossRef]
- Labruna, L.; Stark-Inbar, A.; Breska, A.; Dabit, M.; Vanderschelden, B.; Nitsche, M.A.; Ivry, R.B. Individual differences in TMS sensitivity influence the efficacy of tDCS in facilitating sensorimotor adaptation. Brain Stimul. 2019, 12, 992–1000. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Trudgen, A.; Cirillo, J.; Byblow, W.D. Somatosensory and transcranial direct current stimulation effects on manual dexterity and motor cortex function: A metaplasticity study. Brain Stimul. 2019, 12, 938–947. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Seeber, A.; Frommann, K.; Klein, C.C.; Rochford, C.; Nitsche, M.S.; Fricke, K.; Liebetanz, D.; Lang, N.; Antal, A.; et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J. Physiol. 2005, 568 Pt 1, 291–303. [Google Scholar] [CrossRef]
- Lavin, K.M.; Roberts, B.M.; Fry, C.S.; Moro, T.; Rasmussen, B.B.; Bamman, M.M. The Importance of Resistance Exercise Training to Combat Neuromuscular Aging. Physiology 2019, 34, 112–122. [Google Scholar] [CrossRef]
- Adkins, D.L.; Boychuk, J.; Remple, M.S.; Kleim, J.A. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J. Appl. Physiol. 2006, 101, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, T.; Granacher, U.F.; Fernandez-del-Olmo, M.; Howatson, G.; Manca, A.; Deriu, F.; Taube, W.; Gruber, M.; Marquez, G.; Lundbye-Jensen, J.; et al. Functional relevance of resistance training-induced neuroplasticity in health and disease. Neurosci. Biobehav. Rev. 2021, 122, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Corp, D.T.; Bereznicki, H.G.K.; Clark, G.M.; Youssef, G.J.; Fried, P.J.; Jannati, A.; Davies, C.B.; Gomes-Osman, J.; Kirkovski, M.; Albein-Urios, N.; et al. Large-scale analysis of interindividual variability in single and paired-pulse TMS data. Clin. Neurophysiol. 2021, 132, 2639–2653. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Feria, J.; Martin-Rodriguez, J.F.; Mir, P. Corticospinal adaptations following resistance training and its relationship with strength: A systematic review and multivariate meta-analysis. Neurosci. Biobehav. Rev. 2023, 152, 105289. [Google Scholar] [CrossRef]
- Ward, N.S.; Newton, J.M.; Swayne, O.B.; Lee, L.; Frackowiak, R.S.; Thompson, A.J.; Greenwood, R.J.; Rothwell, J.C. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur. J. Neurosci. 2007, 25, 1865–1873. [Google Scholar] [CrossRef]
- Kim, A.R.; Kim, D.H.; Park, S.Y.; Kyeong, S.; Kim, Y.W.; Lee, S.K.; Kim, D.Y. Can the integrity of the corticospinal tract predict the long-term motor outcome in poststroke hemiplegic patients? Neuroreport 2018, 29, 453–458. [Google Scholar] [CrossRef]
- Thickbroom, G.W.; Byrnes, M.L.; Archer, S.A.; Mastaglia, F.L. Motor outcome after subcortical stroke: MEPs correlate with hand strength but not dexterity. Clin. Neurophysiol. 2002, 113, 2025–2029. [Google Scholar] [CrossRef]
- Facciorusso, S.; Guanziroli, E.; Brambilla, C.; Spina, S.; Giraud, M.; Molinari Tosatti, L.; Santamato, A.; Molteni, F.; Scano, A. Muscle synergies in upper limb stroke rehabilitation: A scoping review. Eur. J. Phys. Rehabil. Med. 2024, 60, 767–792. [Google Scholar] [CrossRef]
- Oquita, R.; Cuello, V.; Uppati, S.; Mannuru, S.; Salinas, D.; Dobbs, M.; Potter-Baker, K.A. Moving toward elucidating alternative motor pathway structures post-stroke: The value of spinal cord neuroimaging. Front. Neurol. 2024, 15, 1282685. [Google Scholar] [CrossRef]
- Wechsler, L.R.; Bates, D.; Stroemer, P.; Andrews-Zwilling, Y.S.; Aizman, I. Cell Therapy for Chronic Stroke. Stroke 2018, 49, 1066–1074. [Google Scholar] [CrossRef]
- Ziemann, U.; Paulus, W.; Nitsche, M.A.; Pascual-Leone, A.; Byblow, W.D.; Berardelli, A.; Siebner, H.R.; Classen, J.; Cohen, L.G.; Rothwell, J.C. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008, 1, 164–182. [Google Scholar] [CrossRef]
| tDCS Real (N = 36) | tDCS Sham (N = 36) | All (N = 72) | p Value | |
|---|---|---|---|---|
| Age (years, Mean ± SD) | 64(12) | 67(10) | 65(11) | 0.39 |
| Handedness (N, right/left) | 31/5 | 36/0 | 67/5 | 0.02 |
| Gender (N, male/female) | 24/12 | 23/13 | 47/25 | 0.80 |
| Time since stroke (years) | 5(3) | 6(4) | 6(4) | 0.12 |
| Type of stroke (N, I/H/O) | 31/3/2 | 29/7/0 | 60/10/2 | 0.16 |
| Side of stroke (N, right/left) | 16/20 | 23/13 | 39/33 | 0.09 |
| Real tDCS Group | Sham tDCS Group | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| rMT (% stimulator output) | 51 (16) [24–85] | 49 (15) [28–94] | 49 (14) [25–90] | 47 (12) [28–71] |
| MEP amplitude (uV) | 494 (626) [29–2979] | 523 (753) [53–3486] | 628 (863) [28–4372] | 666 (1011) [21–4331] |
| SP (ms) | 164 (66) [58–389] | 159 (61) [64–358] | 155 (72) [60–351] | 154 (89) [44–436] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palimeris, S.; Ansari, Y.; Remaud, A.; Tremblay, F.; Corriveau, H.; Boudrias, M.-H.; Milot, M.-H. Are Changes in Corticomotor Excitability Associated with Improved Arm Functional Performance Following a Tailored Strength Training Intervention in Chronic Stroke Survivors? Brain Sci. 2025, 15, 700. https://doi.org/10.3390/brainsci15070700
Palimeris S, Ansari Y, Remaud A, Tremblay F, Corriveau H, Boudrias M-H, Milot M-H. Are Changes in Corticomotor Excitability Associated with Improved Arm Functional Performance Following a Tailored Strength Training Intervention in Chronic Stroke Survivors? Brain Sciences. 2025; 15(7):700. https://doi.org/10.3390/brainsci15070700
Chicago/Turabian StylePalimeris, Stephania, Yekta Ansari, Anthony Remaud, François Tremblay, Hélène Corriveau, Marie-Hélène Boudrias, and Marie-Hélène Milot. 2025. "Are Changes in Corticomotor Excitability Associated with Improved Arm Functional Performance Following a Tailored Strength Training Intervention in Chronic Stroke Survivors?" Brain Sciences 15, no. 7: 700. https://doi.org/10.3390/brainsci15070700
APA StylePalimeris, S., Ansari, Y., Remaud, A., Tremblay, F., Corriveau, H., Boudrias, M.-H., & Milot, M.-H. (2025). Are Changes in Corticomotor Excitability Associated with Improved Arm Functional Performance Following a Tailored Strength Training Intervention in Chronic Stroke Survivors? Brain Sciences, 15(7), 700. https://doi.org/10.3390/brainsci15070700







