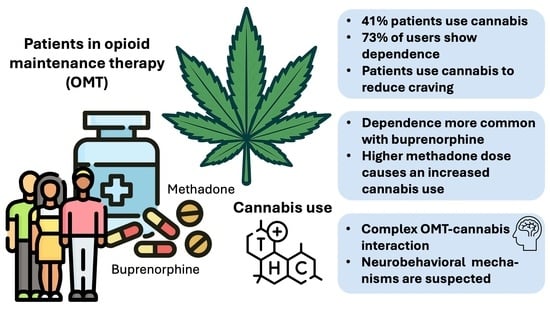
Cannabis Use in Opioid Maintenance Therapy: Prevalence, Clinical Correlates and Reasons for Use
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| CB1 | Cannabinoid Receptor Type 1 |
| CI | Confidence Intervals |
| CPQ | Cannabis Problems Questionnaire |
| ICD-10 | International Classification of Diseases, 10th Revision |
| MMM | Marijuana Motives Measure |
| MOR | μ-opioid receptor (Mu-Opioid Receptor) |
| MSHQ | Marijuana Smoking History Questionnaire |
| ML | Marijuana Ladder |
| OMT | Opioid Maintenance Therapy |
| OUD | Opioid Use Disorder |
| SDS | Severity of Dependence Scale |
| SPSS | Statistical Package for the Social Sciences |
| TCH | Delta-9-Tetrahydrocannabinol |
References
- Degenhardt, L.; Grebely, J.; Stone, J.; Hickman, M.; Vickerman, P.; Marshall, B.D.; Bruneau, J.; Altice, F.L.; Henderson, G.; Rahimi-Movaghar, A. Global patterns of opioid use and dependence: Harms to populations, interventions, and future action. Lancet 2019, 394, 1560–1579. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Blanco, C. Substance use disorders: A comprehensive update of classification, epidemiology, neurobiology, clinical aspects, treatment and prevention. World Psychiatry 2023, 22, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Blanco, C. The changing opioid crisis: Development, challenges and opportunities. Mol. Psychiatry 2021, 26, 218–233. [Google Scholar] [CrossRef]
- Wilson, J.; Mills, K.; Freeman, T.P.; Sunderland, M.; Visontay, R.; Marel, C. Weeding out the truth: A systematic review and meta-analysis on the transition from cannabis use to opioid use and opioid use disorders, abuse or dependence. Addiction 2022, 117, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Strang, J. Medication treatment of opioid use disorder. Biol. Psychiatry 2020, 87, 82–88. [Google Scholar] [CrossRef]
- Sordo, L.; Barrio, G.; Bravo, M.J.; Indave, B.I.; Degenhardt, L.; Wiessing, L.; Ferri, M.; Pastor-Barriuso, R. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ 2017, 357, j1550. [Google Scholar] [CrossRef]
- Hser, Y.I.; Evans, E.; Huang, D.; Weiss, R.; Saxon, A.; Carroll, K.M.; Woody, G.; Liu, D.; Wakim, P.; Matthews, A.G. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction 2016, 111, 695–705. [Google Scholar] [CrossRef]
- Roncero, C.; Barral, C.; Rodríguez-Cintas, L.; Pérez-Pazos, J.; Martinez-Luna, N.; Casas, M.; Torrens, M.; Grau-López, L. Psychiatric comorbidities in opioid-dependent patients undergoing a replacement therapy programme in Spain: The PROTEUS study. Psychiatry Res. 2016, 243, 174–181. [Google Scholar] [CrossRef]
- Jones, C.M.; McCance-Katz, E.F. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alcohol Depend. 2019, 197, 78–82. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Booklet 3: Drug Market Trends—Cannabis and Opioids. In World Drug Report 2021; United Nations Office on Drugs and Crime: New York, NY, USA, 2021; pp. 11–41. [Google Scholar]
- European Union Drugs Agency. European Drug Report 2020: Trends and Developments. 2020. Available online: https://www.euda.europa.eu/publications/edr/trends-developments/2020_en (accessed on 23 March 2025).
- Green, B.; Kavanagh, D.; Young, R.M. Reasons for cannabis use in men with and without psychosis. Drug Alcohol Rev. 2004, 23, 445–453. [Google Scholar] [CrossRef]
- Wedekind, D.; Jacobs, S.; Karg, I.; Luedecke, C.; Schneider, U.; Cimander, K.; Baumann, P.; Ruether, E.; Poser, W.; Havemann-Reinecke, U. Psychiatric comorbidity and additional abuse of drugs in maintenance treatment with L-and D, L-methadone. World J. Biol. Psychiatry 2010, 11, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Bryson, W.C.; Morasco, B.J.; Cotton, B.P.; Thielke, S.M. Cannabis use and nonfatal opioid overdose among patients enrolled in methadone maintenance treatment. Subst. Use Misuse 2021, 56, 697–703. [Google Scholar] [CrossRef]
- Leung, J.; Chan, G.C.; Hides, L.; Hall, W.D. What is the prevalence and risk of cannabis use disorders among people who use cannabis? A systematic review and meta-analysis. Addict. Behav. 2020, 109, 106479. [Google Scholar] [CrossRef]
- Budney, A.J.; Bickel, W.K.; Amass, L. Marijuana use and treatment outcome among opioid-dependent patients. Addiction 1998, 93, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Balhara, Y.P.S.; Jain, R. Cannabis use among opioid-dependent individuals on opioid substitution therapy. J. Pharmacol. Pharmacother. 2014, 5, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Rosic, T.; Kapoor, R.; Panesar, B.; Naji, L.; Chai, D.B.; Sanger, N.; Marsh, D.C.; Worster, A.; Thabane, L.; Samaan, Z. The association between cannabis use and outcome in pharmacological treatment for opioid use disorder. Harm Reduct. J. 2021, 18, 24. [Google Scholar] [CrossRef]
- Hsu, G.; Kovács, B. Association between county level cannabis dispensary counts and opioid related mortality rates in the United States: Panel data study. BMJ 2021, 372, m4957. [Google Scholar] [CrossRef]
- Vierke, C.; Marxen, B.; Boettcher, M.; Hiemke, C.; Havemann-Reinecke, U. Buprenorphine–cannabis interaction in patients undergoing opioid maintenance therapy. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 847–856. [Google Scholar] [CrossRef]
- Socías, M.E.; Wood, E.; Lake, S.; Nolan, S.; Fairbairn, N.; Hayashi, K.; Shulha, H.P.; Liu, S.; Kerr, T.; Milloy, M.J. High-intensity cannabis use is associated with retention in opioid agonist treatment: A longitudinal analysis. Addiction 2018, 113, 2250–2258. [Google Scholar] [CrossRef]
- Socías, M.E.; Choi, J.; Lake, S.; Wood, E.; Valleriani, J.; Hayashi, K.; Kerr, T.; Milloy, M.-J. Cannabis use is associated with reduced risk of exposure to fentanyl among people on opioid agonist therapy during a community-wide overdose crisis. Drug Alcohol Depend. 2021, 219, 108420. [Google Scholar] [CrossRef]
- DuPont, R.L.; Saylor, K.E. Marijuana and benzodiazepines in patients receiving methadone treatment. JAMA 1989, 261, 3409. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, D.A.; Weinstein, M.G.; Havassy, B.E.; Hall, S.M. Factors associated with lapses to heroin use during methadone maintenance. Drug Alcohol Depend. 1998, 52, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Wiese, B.; Wilson-Poe, A.R. Emerging evidence for cannabis’ role in opioid use disorder. Cannabis Cannabinoid Res. 2018, 3, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moreno, J.; Lopez-Jimenez, A.; Gorriti, M.; de Fonseca, F.R. Functional interactions between endogenous cannabinoid and opioid systems: Focus on alcohol, genetics and drug-addicted behaviors. Curr. Drug Targets 2010, 11, 406–428. [Google Scholar] [CrossRef]
- Singh, M.E.; Verty, A.N.; McGregor, I.S.; Mallet, P.E. A cannabinoid receptor antagonist attenuates conditioned place preference but not behavioural sensitization to morphine. Brain Res. 2004, 1026, 244–253. [Google Scholar] [CrossRef]
- Wills, K.L.; Parker, L.A. Effect of pharmacological modulation of the endocannabinoid system on opiate withdrawal: A review of the preclinical animal literature. Front. Pharmacol. 2016, 7, 187. [Google Scholar] [CrossRef]
- Fattore, L.; Spano, M.; Melis, V.; Fadda, P.; Fratta, W. Differential effect of opioid and cannabinoid receptor blockade on heroin-seeking reinstatement and cannabinoid substitution in heroin-abstinent rats. Br. J. Pharmacol. 2011, 163, 1550–1562. [Google Scholar] [CrossRef]
- Navarro, M.; Carrera, M.; Fratta, W.; Valverde, O.; Cossu, G.; Fattore, L.; Chowen, J.; Gomez, R.; Del Arco, I.; Villanua, M. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J. Neurosci. 2001, 21, 5344–5350. [Google Scholar] [CrossRef]
- Lazary, J.; Juhasz, G.; Hunyady, L.; Bagdy, G. Personalized medicine can pave the way for the safe use of CB1 receptor antagonists. Trends Pharmacol. Sci. 2011, 32, 270–280. [Google Scholar] [CrossRef]
- Boehnke, K.F.; Litinas, E.; Clauw, D.J. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J. Pain 2016, 17, 739–744. [Google Scholar] [CrossRef]
- Kral, A.H.; Wenger, L.; Novak, S.P.; Chu, D.; Corsi, K.F.; Coffa, D.; Shapiro, B.; Bluthenthal, R.N. Is cannabis use associated with less opioid use among people who inject drugs? Drug Alcohol Depend. 2015, 153, 236–241. [Google Scholar] [CrossRef]
- Degenhardt, L.; Lintzeris, N.; Campbell, G.; Bruno, R.; Cohen, M.; Farrell, M.; Hall, W.D. Experience of adjunctive cannabis use for chronic non-cancer pain: Findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend. 2015, 147, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Olfson, M.; Wall, M.M.; Liu, S.-M.; Blanco, C. Cannabis use and risk of prescription opioid use disorder in the United States. Am. J. Psychiatry 2018, 175, 47–53. [Google Scholar] [CrossRef]
- Campbell, G.; Hall, W.D.; Peacock, A.; Lintzeris, N.; Bruno, R.; Larance, B.; Nielsen, S.; Cohen, M.; Chan, G.; Mattick, R.P. Effect of cannabis use in people with chronic non-cancer pain prescribed opioids: Findings from a 4-year prospective cohort study. Lancet Public Health 2018, 3, e341–e350. [Google Scholar] [CrossRef]
- Lucas, P.; Boyd, S.; Milloy, M.-J.; Walsh, Z. Cannabis significantly reduces the use of prescription opioids and improves quality of life in authorized patients: Results of a large prospective study. Pain Med. 2021, 22, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, C.; Scherbaum, N.; Bonnet, U.; Roser, P. Cannabinoids in the treatment of cannabis use disorder: Systematic review of randomized controlled trials. Front. Psychiatry 2022, 13, 867878. [Google Scholar] [CrossRef]
- Freeman, T.P.; Hindocha, C.; Baio, G.; Shaban, N.D.; Thomas, E.M.; Astbury, D.; Freeman, A.M.; Lees, R.; Craft, S.; Morrison, P.D. Cannabidiol for the treatment of cannabis use disorder: A phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry 2020, 7, 865–874. [Google Scholar] [CrossRef]
- Katsidoni, V.; Anagnostou, I.; Panagis, G. Cannabidiol inhibits the reward-facilitating effect of morphine: Involvement of 5-HT1A receptors in the dorsal raphe nucleus. Addict. Biol. 2013, 18, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Markos, J.R.; Harris, H.M.; Gul, W.; ElSohly, M.A.; Sufka, K.J. Effects of cannabidiol on morphine conditioned place preference in mice. Planta Medica 2018, 84, 221–224. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Yoon, M.; Manini, A.F.; Hernandez, S.; Olmedo, R.; Ostman, M.; Jutras-Aswad, D. Early phase in the development of cannabidiol as a treatment for addiction: Opioid relapse takes initial center stage. Neurotherapeutics 2015, 12, 807–815. [Google Scholar] [CrossRef]
- Ren, Y.; Whittard, J.; Higuera-Matas, A.; Morris, C.V.; Hurd, Y.L. Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J. Neurosci. 2009, 29, 14764–14769. [Google Scholar] [CrossRef] [PubMed]
- Bisaga, A.; Sullivan, M.A.; Glass, A.; Mishlen, K.; Pavlicova, M.; Haney, M.; Raby, W.N.; Levin, F.R.; Carpenter, K.M.; Mariani, J.J. The effects of dronabinol during detoxification and the initiation of treatment with extended release naltrexone. Drug Alcohol Depend. 2015, 154, 38–45. [Google Scholar] [CrossRef]
- Jicha, C.J.; Lofwall, M.R.; Nuzzo, P.A.; Babalonis, S.; Elayi, S.C.; Walsh, S.L. Safety of oral dronabinol during opioid withdrawal in humans. Drug Alcohol Depend. 2015, 157, 179–183. [Google Scholar] [CrossRef]
- Scavone, J.L.; Sterling, R.C.; Weinstein, S.P.; Van Bockstaele, E.J. Impact of Cannabis Use during Stabilization on Methadone Maintenance Treatment. Am. J. Addict. 2013, 22, 344–351. [Google Scholar] [CrossRef]
- Hermann, D.; Klages, E.; Welzel, H.; Mann, K.; Croissant, B. Low efficacy of non-opioid drugs in opioid withdrawal symptoms. Addict. Biol. 2005, 10, 165–169. [Google Scholar] [CrossRef]
- Nava, F.; Manzato, E.; Lucchini, A. Chronic cannabis use does not affect the normalization of hypothalamic–pituitary–adrenal (HPA) axis induced by methadone in heroin addicts. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Lafaye, G.; Laurent, K.; Lisa, B.; Benyamina, A. Cannabis, cannabinoids, and health. Dialogues Clin. Neurosci. 2017, 19, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, C.; Spradlin, A. Measuring cannabis consumption: Psychometric properties of the daily sessions, frequency, age of onset, and quantity of cannabis use inventory (DFAQ-CU). PLoS ONE 2017, 12, e0178194. [Google Scholar] [CrossRef]
- Simons, J.; Correia, C.J.; Carey, K.B.; Borsari, B.E. Validating a five-factor marijuana motives measure: Relations with use, problems, and alcohol motives. J. Couns. Psychol. 1998, 45, 265. [Google Scholar] [CrossRef]
- Narcotics Division, Security Bureau. Beat Drugs Fund Evaluation Questions Set No. 13: Contemplation Ladder. 2013. Available online: https://www.nd.gov.hk/en/beat_questions_2010R2.html (accessed on 15 February 2025).
- Biener, L.; Abrams, D.B. The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991, 10, 360. [Google Scholar] [CrossRef]
- Slavet, J.D.; Stein, L.; Colby, S.M.; Barnett, N.P.; Monti, P.M.; Golembeske Jr, C.; Lebeau-Craven, R. The Marijuana Ladder: Measuring motivation to change marijuana use in incarcerated adolescents. Drug Alcohol Depend. 2006, 83, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Baumeister, S.E.; Kraus, L. Severity of Dependence Scale: Establishing a cut-off point for cannabis dependence in the German adult population. Sucht 2008, 54, 57–63. [Google Scholar] [CrossRef]
- Gossop, M.; Darke, S.; Griffiths, P.; Hando, J.; Powis, B.; Hall, W.; Strang, J. The Severity of Dependence Scale (SDS): Psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 1995, 90, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Mills, K.L.; Sunderland, M.; Freeman, T.P.; Teesson, M.; Haber, P.S.; Marel, C. The long-term Relationship Between Cannabis and Heroin Use: An 18- to 20-year Follow-up of the Australian Treatment outcome Study (ATOS). Am. J. Psychiatry 2024, 181, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.P.A.; Nunes, J.C.; Heringer, D.L.; Anand, A.; de Aquino, J.P. The impact of cannabis on non-medical opioid use among individuals receiving pharmacotherapies for opioid use disorder: A systematic review and meta-analysis of longitudinal studies. Am. J. Drug Alcohol Abus. 2024, 50, 12–16. [Google Scholar] [CrossRef]
- Khantzian, E.J. The Self-Medication Hypothesis of Addictive Disorders: Focus on Heroin and Cocaine Dependence. Am. J. Psychiatry 1985, 142, 1259–1264. [Google Scholar] [CrossRef]
- Lake, S.; Buxton, J.; Walsh, Z.; Cooper, Z.D.; Socias, M.E.; Fairbairn, N.; Hayashi, K.; Milroy, M.-J. Methadone Dose, Cannabis use, and Treatment Retention: Findings from a Community-based sample of People Who use Unregulated Drugs. J. Addict. Med. 2023, 17, e18–e26. [Google Scholar] [CrossRef]
- De Aquino, J.P.; Bahji, A.; Gómez, O.; Sofuoglu, M. Alleviation of opioid withdrawal by cannabis and delta-9-tetrahydrocannabinol: A systematic review of observational and experimental studies. Drug Alcohol Depend. 2022, 241, 109702. [Google Scholar] [CrossRef]
- Lake, S.; Kerr, T.; Buxton, J.; Walsh, Z.; Cooper, Z.D.; Socias, M.E.; Fairbairn, N.; Hayashi, K.; Milroy, M.-R. The Cannabis-Dependent Relationship between Methadone treatment dose and Illicit Opioid use in a Community-Based Cohort of People Who use Drugs. Cannabis Cannabinoid Res. 2023, 8, 155–165. [Google Scholar] [CrossRef]
- Bagra, I.; Krishnan, V.; Rao, R.; Agarwal, A. Does cannabis use influence opioid outcomes and quality of life among buprenorphine maintained patients? A cross-sectional Comparative Study. J. Addict. Med. 2018, 12, 315–320. [Google Scholar] [CrossRef]
- Flückiger, L.; Meyer, M.; Steinauer, R.; Walter, M. Cannabis regulation—An international challenge. Suchtmedizin Forsch. Und Prax. 2021, 23, 319–326. [Google Scholar]
- Renard, D.; Gaillard, N. Brain haemorrhage and cerebral vasospasm associated with chronic use of cannabis and buprenorphine. Cerebrovasc. Dis. 2008, 25, 282–283. [Google Scholar] [CrossRef]
- Backmund, M.; Meyer, K.; Eichenlaub, D.; Schütz, C.G. Predictors for completing an inpatient detoxification program among intravenous heroin users, methadone substituted and codeine substituted patients. Alcohol Drug Depend. 2001, 64, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bradford, A.C.; Bradford, W.D.; Abraham, A.; Bagwell Adams, G. Association between US state medical cannabis laws and opioid prescribing in the medicare part D population. JAMA Intern. Med. 2018, 178, 667–672. [Google Scholar] [CrossRef]
- Castillo-Carniglia, A.; Rivera-Aguirre, A.; Santaella-Tenorio, J.; Fink, D.S.; Crystal, S.; Ponicki, W.; Gruenewald, P.; Martins, S.S.; Keyes, K.M.; Credá, M. Changes in opioid and benzodiazepine poisoning deaths after cannabis legalization in the US: A county-level analysis, 2002–2020. Epidemiology 2023, 34, 467–475. [Google Scholar] [CrossRef]
- Sover, C.L.; Davis, C.S.; Gordon, S.C.; Humphreys, K. Association between medical cannabis laws and opioid overdose mortality has reversed over time. Proc. Natl. Acad. Sci. USA 2019, 116, 12624–12626. [Google Scholar] [CrossRef]
- De Aquino, J.P.; Sofuoglu, M.; Stefanovics, E.; Rosenheck, R. Adverse consequences of co-occuring opioid use disorder and cannabis use disorder compared to opioid use disorder only. Am. J. Drug Alcohol Abus. 2019, 45, 527. [Google Scholar] [CrossRef] [PubMed]
- Darke, S.; Williamson, A.; Ross, J.; Teesson, M. Reductions in heroin use are not associated with increases in other drug use: 2-year findings from the Australian Treatment Outcome Study. Drug Alcohol Depend. 2006, 84, 201–205. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Spriggs, S.; Alishayev, J.; Winkel, G.; Gurgov, K.; Kudrich, C.; Oprescu, A.M.; Salsitz, E. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: A double-blind randomized placebo-controlled trial. Am. J. Psychiatry 2019, 176, 911–922. [Google Scholar] [CrossRef]
- Reddon, H.; Lake, S.; Socias, M.E.; Hayashi, K.; DeBeck, K.; Walsh, Z.; Milloy, M.-J. Cannabis use to manage opioid cravings among people who use unregulated opioids during a toxicity crisis. Int. J. Drug Policy 2023, 119, 104113. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Backmund, M.; Zámbó, G.G.; Schöfl, S.; Soyka, M. Cannabis Use in Opioid Maintenance Therapy: Prevalence, Clinical Correlates and Reasons for Use. Brain Sci. 2025, 15, 699. https://doi.org/10.3390/brainsci15070699
Backmund M, Zámbó GG, Schöfl S, Soyka M. Cannabis Use in Opioid Maintenance Therapy: Prevalence, Clinical Correlates and Reasons for Use. Brain Sciences. 2025; 15(7):699. https://doi.org/10.3390/brainsci15070699
Chicago/Turabian StyleBackmund, Markus, Greta G. Zámbó, Susanne Schöfl, and Michael Soyka. 2025. "Cannabis Use in Opioid Maintenance Therapy: Prevalence, Clinical Correlates and Reasons for Use" Brain Sciences 15, no. 7: 699. https://doi.org/10.3390/brainsci15070699
APA StyleBackmund, M., Zámbó, G. G., Schöfl, S., & Soyka, M. (2025). Cannabis Use in Opioid Maintenance Therapy: Prevalence, Clinical Correlates and Reasons for Use. Brain Sciences, 15(7), 699. https://doi.org/10.3390/brainsci15070699