Mapping the Olfactory Brain: A Systematic Review of Structural and Functional Magnetic Resonance Imaging Changes Following COVID-19 Smell Loss
Abstract
1. Introduction
2. Materials
2.1. Search Strategy and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Bias and Quality Assessment
3. Results
3.1. Demographic Information
3.2. Studies Using MRI Reported Olfactory Bulb Volume (OBV) and Olfactory Sulcus Depth (OSD)
3.3. Olfactory Bulb Volume (OBV)
3.4. Olfactory Sulcus Depth (OSD)
3.5. Studies Reported MRI on Grey Matter and White Matter Changes
3.6. Studies Reported Resting State-fMRI (rs-fMRI)
3.7. Studies Reported Task-Based fMRI (tb-fMRI)
3.8. Studies Reported Diffusion Tensor Imaging (DTI)
3.9. Studies Reported T2-Weighted Imaging (T2WI) and Postcontrast 3D T2 FLAIR, Diffusion-Weighted Imaging (DWI) and 3D High-Resolution T1-Weighted Imaging (T1WI)
4. Discussion
4.1. Olfactory Bulb Volume (OBV) and Olfactory Sulcus Depth (OSD)
4.1.1. OBV Reduction in COVID-19 Patients
4.1.2. OSD Reduction and Its Association with OBV and OD Type
4.1.3. Duration of Olfactory Loss as a Mediator in OBV and OSD Variability
4.2. The Effect of SARS-CoV-2 on Grey Matter and White Matter Changes
4.2.1. Grey Matter Changes and Their Association with Olfactory Dysfunction
4.2.2. White Matter Changes and COVID-19-Related Olfactory Dysfunction
4.2.3. Impact of Demographics and Symptom Profile on GM and WM Changes
4.3. The Effect of SARS-CoV-2 on Brain Activation and Connectivity
4.3.1. Resting-State fMRI Observations in COVID-19-Related OD
4.3.2. Task-Based fMRI Observations and Brain Activation in COVID-19-Related OD
4.3.3. Correlation Between rs-fMRI and tb-fMRI Findings on OD Duration and Type
4.4. DTI, T2WI, DWI, and 3D High-Resolution T1WI-Based Findings in Olfactory Dysfunction
4.4.1. White Matter Tracts and Olfactory Dysfunction
4.4.2. Structural Changes in White and Gray Matter Through DWI and T1WI
4.4.3. Correlating Imaging Findings with OD Duration and Type
4.5. Brain Plasticity and Responses to Environmental Stress and External Stimuli: Implications for Post-COVID-19 Recovery
4.6. Potential Confounding by Undiagnosed Neurodegenerative Conditions
4.7. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boesveldt, S.; Parma, V. The importance of the olfactory system in human well-being, through nutrition and social behavior. Cell Tissue Res. 2021, 383, 559–567. [Google Scholar] [CrossRef]
- Bratman, G.N.; Bembibre, C.; Daily, G.C.; Doty, R.L.; Hummel, T.; Jacobs, L.F.; Kahn, P.H., Jr.; Lashus, C.; Majid, A.; Miller, J.D.; et al. Nature and human well-being: The olfactory pathway. Sci. Adv. 2024, 10, eadn3028. [Google Scholar] [CrossRef]
- Melis, M.; Tomassini Barbarossa, I.; Sollai, G. The Implications of Taste and Olfaction in Nutrition and Health. Nutrients 2023, 15, 3412. [Google Scholar] [CrossRef]
- Schäfer, L.; Schriever, V.A.; Croy, I. Human olfactory dysfunction: Causes and consequences. Cell Tissue Res. 2021, 383, 569–579. [Google Scholar] [CrossRef]
- Georgiopoulos, C.; Buechner, M.A.; Falkenburger, B.; Engström, M.; Hummel, T.; Haehner, A. Differential connectivity of the posterior piriform cortex in Parkinson’s disease and postviral olfactory dysfunction: An fMRI study. Sci. Rep. 2024, 14, 6256. [Google Scholar] [CrossRef]
- Gottfried, J.A. Central mechanisms of odour object perception. Nat. Rev. Neurosci. 2010, 11, 628–641. [Google Scholar] [CrossRef]
- Kadohisa, M. Effects of odor on emotion, with implications. Front. Syst. Neurosci. 2013, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.T.; Peace, S.T.; Cleland, T.A. Properties and mechanisms of olfactory learning and memory. Front. Behav. Neurosci. 2014, 8, 238. [Google Scholar] [CrossRef]
- Gellrich, J.; Han, P.; Manesse, C.; Betz, A.; Junghanns, A.; Raue, C.; Schriever, V.A.; Hummel, T. Brain volume changes in hyposmic patients before and after olfactory training. Laryngoscope 2018, 128, 1531–1536. [Google Scholar] [CrossRef]
- Rombaux, P.; Duprez, T.; Hummel, T. Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology 2009, 47, 3–9. [Google Scholar] [PubMed]
- Whitcroft, K.L.; Mancini, L.; Yousry, T.; Hummel, T.; Andrews, P.J. Functional septorhinoplasty alters brain structure and function: Neuroanatomical correlates of olfactory dysfunction. Front. Allergy 2023, 4, 1079945. [Google Scholar] [CrossRef]
- Zahnert, F.; Kleinholdermann, U.; Belke, M.; Keil, B.; Menzler, K.; Pedrosa, D.J.; Timmermann, L.; Kircher, T.; Nenadić, I.; Knake, S. The connectivity-based architecture of the human piriform cortex. Neuroimage 2024, 297, 120747. [Google Scholar] [CrossRef]
- Iravani, B.; Peter, M.G.; Arshamian, A.; Olsson, M.J.; Hummel, T.; Kitzler, H.H.; Lundström, J.N. Acquired olfactory loss alters functional connectivity and morphology. Sci. Rep. 2021, 11, 16422. [Google Scholar] [CrossRef]
- Ruser, P.; Koeppel, C.J.; Kitzler, H.H.; Hummel, T.; Croy, I. Individual odor hedonic perception is coded in temporal joint network activity. Neuroimage 2021, 229, 117782. [Google Scholar] [CrossRef]
- Thaploo, D.; Joshi, A.; Yilmaz, E.; Yildirim, D.; Altundag, A.; Hummel, T. Functional connectivity patterns in parosmia. Behav. Brain Funct. 2023, 19, 24. [Google Scholar] [CrossRef]
- Waymel, A.; Friedrich, P.; Bastian, P.A.; Forkel, S.J.; Thiebaut de Schotten, M. Anchoring the human olfactory system within a functional gradient. Neuroimage 2020, 216, 116863. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, J.; Wu, Y.; Xiong, J.; Lv, H.; Li, J.; Kuang, H.; Jiang, X.; Chen, Y. Abnormal functional connectivity of the core olfactory network in patients with chronic rhinosinusitis accompanied by olfactory dysfunction. Front. Neurol. 2023, 14, 1295556. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, L.; Sighinolfi, G.; Mitolo, M.; Ferri, L.; Jane Rochat, M.; Pensato, U.; Taruffi, L.; Testa, C.; Masullo, M.; Cortelli, P.; et al. Cognitive and functional connectivity impairment in post-COVID-19 olfactory dysfunction. Neuroimage Clin. 2023, 38, 103410. [Google Scholar] [CrossRef]
- Tan, H.Q.; Choi, J.S.; Choi, Y.J.; Lee, J.C. A systematic review of olfactory dysfunction and its neuroimaging findings n COVID-19 patients. Laryngoscope 2022, 132, 1002–1012. [Google Scholar] [CrossRef]
- Mohammadi, A.; Dehghani, A.; Parsa, A.B.; Sepehry, A.A. Structural and functional alterations in the brain associated with olfactory dysfunction in COVID-19 patients: A systematic review. Neuroradiology 2023, 65, 23–40. [Google Scholar]
- Manan, A.A.; Yahya, N.; Idris, Z.; Manan, H.A. The Utilization of Diffusion Tensor Imaging as an Image-Guided Tool in Brain Tumor Resection Surgery: A Systematic Review. Cancers 2022, 14, 2466. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.B.; Lantos, J.E.; Heier, L.A.; Shatzkes, D.R.; Phillips, C.D. Olfactory Bulb Signal Abnormality in Patients with COVID-19 Who Present with Neurologic Symptoms. AJNR Am. J. Neuroradiol. 2020, 41, 1882–1887. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, D.; Kandemirli, S.G.; Tekcan Sanli, D.E.; Akinci, O.; Altundag, A. A Comparative Olfactory MRI, DTI and fMRI Study of COVID-19 Related Anosmia and Post Viral Olfactory Dysfunction. Acad. Radiol. 2022, 29, 31–41. [Google Scholar] [CrossRef]
- Güney, B.; Bacaksızlar Sarı, F.; Özdemir, M.Y.; Çullu, N.; Doğan, E.; Togan, T. Changes in olfactory bulbus volume and olfactory sulcus depth in the chronic period after COVID-19 infection. Acta Otolaryngol. 2021, 141, 786–790. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Geng, D.; Mei, N.; Wu, P.Y.; Huang, C.C.; Jia, T.; Zhao, Y.; Wang, D.; Xiao, A.; et al. Cerebral Micro-Structural Changes in COVID-19 Patients—An MRI-based 3-month Follow-up Study. EClinicalMedicine 2020, 25, 100484. [Google Scholar] [CrossRef] [PubMed]
- Altunisik, E.; Baykan, A.H.; Sahin, S.; Aydin, E.; Erturk, S.M. Quantitative Analysis of the Olfactory System in COVID-19: An MR Imaging Study. AJNR Am. J. Neuroradiol. 2021, 42, 2207–2214. [Google Scholar] [CrossRef]
- Sherif, F.; Elmokadem, A.H.; Abdel Razek, A.; Kamal, E.; Abdou, E.H.E.; Salem, M.A.; Ghoneim, M.M. DTI of the Olfactory Bulb in COVID-19-Related Anosmia: A Pilot Study. AJNR Am. J. Neuroradiol. 2022, 43, 1180–1183. [Google Scholar] [CrossRef] [PubMed]
- Bispo, D.D.C.; Brandão, P.R.P.; Pereira, D.A.; Maluf, F.B.; Dias, B.A.; Paranhos, H.R.; von Glehn, F.; de Oliveira, A.C.P.; Soares, A.A.S.M.; Descoteaux, M.; et al. Altered structural connectivity in olfactory disfunction after mild COVID-19 using probabilistic tractography. Sci. Rep. 2023, 13, 12886. [Google Scholar] [CrossRef]
- Akkaya, H.; Kizilog Lu, A.; Dilek, O.; Belibag Li, C.; Kaya, Ö.; Yılmaz, C.; Gülek, B. Evaluation of the olfactory bulb volume and morphology in patients with coronavirus disease 2019: Can differences create predisposition to anosmia? Rev. Assoc. Med. Bras. (1992) 2021, 67, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Parlak, A.E.; Selçuk, Ö.T.; Yilmaz, G.Ö.; Aydenizoz, D.; Selçuk, N.T.; Öcal, R.; Seyman, D.; Yilmaz, M.; Eyigör, H. Olfactory Bulb Volume and Morphology Changes in COVID-19 Patients With Olfactory Disorders Using Magnetic Resonance Imaging. J. Comput. Assist. Tomogr. 2024, 48, 317–322. [Google Scholar] [CrossRef]
- Iravani, K.; Malekpour, B.; Rasekhi, A.; Faramarzi, A.; Soltaniesmaeili, A.; Golkhar, B.; Jahanandish, F.; Babaei, A. Functional magnetic resonance imaging in coronavirus disease 2019 induced olfactory dysfunction. J. Laryngol. Otol. 2024, 138, 178–183. [Google Scholar] [CrossRef]
- Perlaki, G.; Darnai, G.; Arató, Á.; Alhour, H.A.; Szente, A.; Áfra, E.; Nagy, S.A.; Horváth, R.; Kovács, N.; Dóczi, T.; et al. Gray Matter Changes Following Mild COVID-19: An MR Morphometric Study in Healthy Young People. J. Magn. Reson. Imaging 2024, 59, 2152–2161. [Google Scholar] [CrossRef]
- Arrigoni, A.; Previtali, M.; Bosticardo, S.; Pezzetti, G.; Poloni, S.; Capelli, S.; Napolitano, A.; Remuzzi, A.; Zangari, R.; Lorini, F.L.; et al. Brain microstructure and connectivity in COVID-19 patients with olfactory or cognitive impairment. Neuroimage Clin. 2024, 43, 103631. [Google Scholar] [CrossRef]
- Abdou, E.H.E.; Ebada, H.A.; Salem, M.A.; Ghoneim, M.M.R.; Sherif, F.; Kamal, E. Clinical and Imaging Evaluation of COVID-19-Related Olfactory Dysfunction. Am. J. Rhinol. Allergy 2023, 37, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Wingrove, J.; Makaronidis, J.; Prados, F.; Kanber, B.; Yiannakas, M.C.; Magee, C.; Castellazzi, G.; Grandjean, L.; Golay, X.; Tur, C.; et al. Aberrant olfactory network functional connectivity in people with olfactory dysfunction following COVID-19 infection: An exploratory, observational study. EClinicalMedicine 2023, 58, 101883. [Google Scholar] [CrossRef]
- Capelli, S.; Caroli, A.; Barletta, A.; Arrigoni, A.; Napolitano, A.; Pezzetti, G.; Longhi, L.G.; Zangari, R.; Lorini, F.L.; Sessa, M.; et al. MRI evidence of olfactory system alterations in patients with COVID-19 and neurological symptoms. J. Neurol. 2023, 270, 1195–1206. [Google Scholar] [CrossRef]
- Esposito, F.; Cirillo, M.; De Micco, R.; Caiazzo, G.; Siciliano, M.; Russo, A.G.; Monari, C.; Coppola, N.; Tedeschi, G.; Tessitore, A. Olfactory Loss and Brain Connectivity after COVID-19: Structural Follow-Up at One Year. Neural Plast. 2023, 2023, 6496539. [Google Scholar] [CrossRef] [PubMed]
- Burulday, V.; Bayar Muluk, N.; Akgül, M.H.; Sayar, M.S. Diffusion-weighted imaging measurements of central smell regions in COVID-19 patients: Insular gyrus, corpus amygdala, and thalamus. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3201–3207. [Google Scholar]
- Caroli, A.; Capelli, S.; Napolitano, A.; Cabrini, G.; Arrigoni, A.; Pezzetti, G.; Previtali, M.; Longhi, L.G.; Zangari, R.; Lorini, F.L.; et al. Brain diffusion alterations in patients with COVID-19 pathology and neurological manifestations. Neuroimage Clin. 2023, 37, 103338. [Google Scholar] [CrossRef]
- Besteher, B.; Machnik, M.; Troll, M.; Toepffer, A.; Zerekidze, A.; Rocktäschel, T.; Heller, C.; Kikinis, Z.; Brodoehl, S.; Finke, K.; et al. Larger gray matter volumes in neuropsychiatric long-COVID syndrome. Psychiatry Res. 2022, 317, 114836. [Google Scholar] [CrossRef]
- Zhang, H.; Chung, T.W.; Wong, F.K.; Hung, I.F.; Mak, H.K. Changes in the Intranetwork and Internetwork Connectivity of the Default Mode Network and Olfactory Network in Patients with COVID-19 and Olfactory Dysfunction. Brain Sci. 2022, 12, 511. [Google Scholar] [CrossRef]
- Burulday, V.; Yıldız, S.; Tuncer, A.; Atagün, İ.; Yiğit, D.; Gürkan, H.; Genç, E.; Alkan, A. Structural brain changes in patients with long COVID: A voxel-based morphometry study. Brain Commun. 2022, 12, 1486. [Google Scholar]
- Esposito, F.; Cirillo, M.; De Micco, R.; Caiazzo, G.; Siciliano, M.; Russo, A.G.; Monari, C.; Coppola, N.; Tedeschi, G.; Tessitore, A. Olfactory loss and brain connectivity after COVID-19. Hum. Brain Mapp. 2022, 43, 1548–1560. [Google Scholar]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef]
- Butowt, R.; Meunier, N.; Bryche, B.; von Bartheld, C.S. The olfactory nerve is not a likely route to brain infection in COVID-19: A critical review of data from humans and animal models. Acta Neuropathol. 2021, 141, 809–822. [Google Scholar] [CrossRef]
- Tsukahara, T.; Ueha, R.; Kondo, K.; Yamasoba, T. Pathological findings of the olfactory mucosa in persistent COVID-19–related olfactory dysfunction. Laryngoscope 2023, 133, 1246–1253. [Google Scholar]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Butowt, R.; Bilinska, K.; von Bartheld, C.S. Olfactory dysfunction in COVID-19: New insights into the underlying mechanisms. Trends Neurosci. 2023, 46, 75–90. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Radley, J.J.; Sapolsky, R.M. Stress-induced neuronal remodeling in the hippocampus: Implications for the pathophysiology of mood disorders. Neurobiol. Dis. 2006, 22, 199–210. [Google Scholar]
- Chou, S.H.Y.; Beghi, E.; Helms, S. COVID-19 neurological complications and brain plasticity. Lancet Neurol. 2021, 20, 349–357. [Google Scholar]
- Li, Z.; Gebler, J.; Joshi, A.; Xu, X.; Thaploo, D.; Hähner, A.; Avaro, V.; Calegari, F.; Hummel, T. Functional but Not Structural Brain Changes After Olfactory Training in Women with COVID-19-Associated Olfactory Dysfunction. Laryngoscope 2025, 135, 2089–2096. [Google Scholar] [CrossRef]

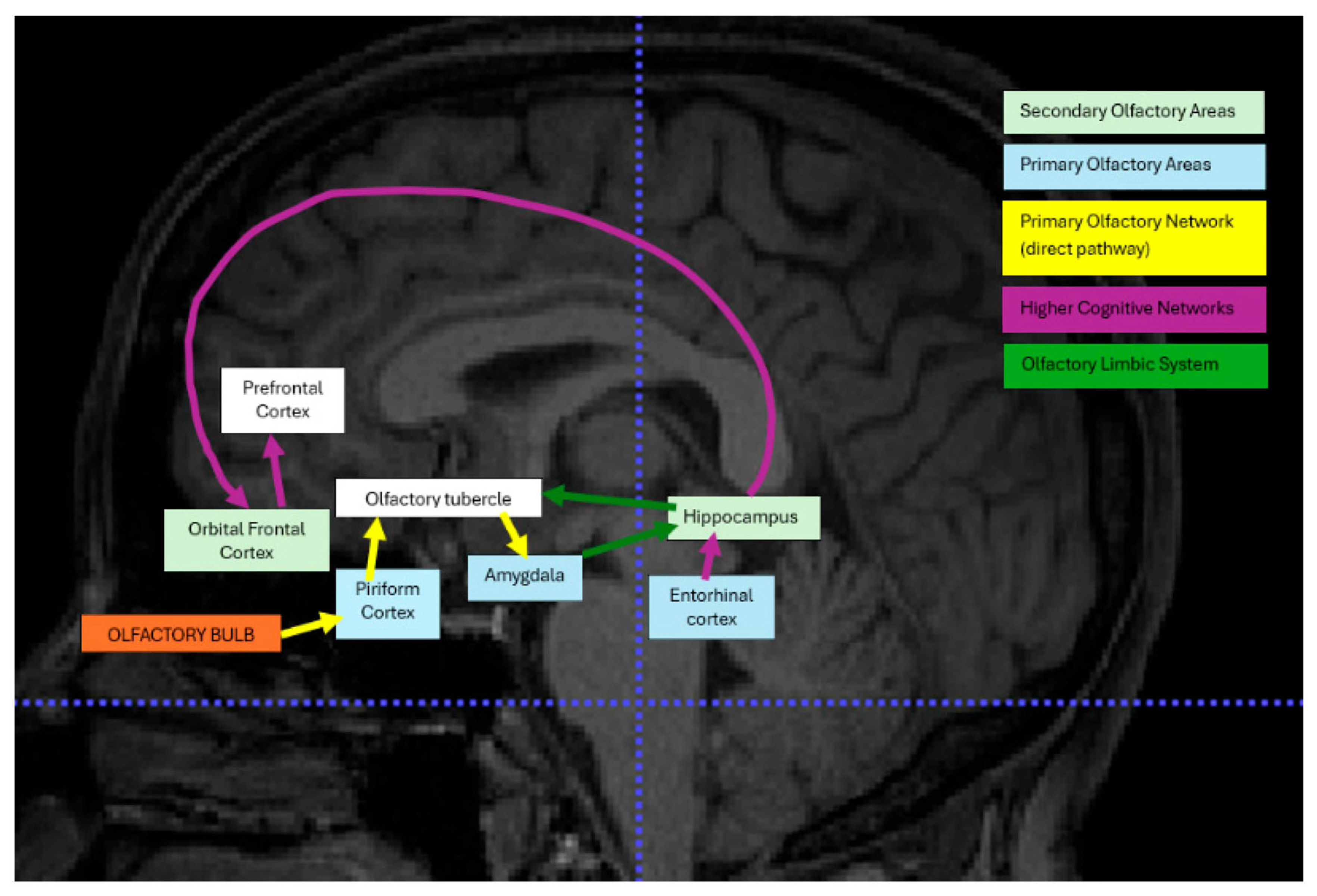
| Population | Adults Above 18 to 81 Years Old with Post-COVID-19-Related OD |
|---|---|
| Interventions | All types of MRI techniques (including rs-fMRI, tb-fMRI, DTI) |
| Comparison | COVID-19 patients (diagnosis confirmed; olfactory status based on clinical assessment or self-reporting, including OD: anosmia, hyposmia, or normosmia) |
| Outcome | OBV, OSD, brain activation, brain connectivity, white matter integrity changes |
| Study design | except for case reports, case series, all types of reviews, letters to the editor |
| Study (Author, Year) | Number of Patients | Number of Controls | Mean Age (Age Range; In Years) | Male (%) | Imaging Techniques |
|---|---|---|---|---|---|
| Yildirim D et al., 2022 [25] | 31 (100% anosmic) | 97 (19% hyposmic, 81% anosmic) | 42.6 ± 14.1(19–80 years) (both groups) | 38% | MRI, tb-fMRI, and DTI |
| Güney B et al., 2021 –no access [26] | 41 | 42 | 40.27 ± 14.5(–missing age range) | 49% (patients) | MRI |
| Lu Y et al., 2020, [27] | 60 (2 anosmic) | 39 | Patients: 44.10 ± 16.00 Controls: 45.88 ± 13.90 | 57% | MRI and DTI |
| Douaud G et al., 2022 [24] | 401 | 384 | Patients: 58.9 ± 7.0 Controls: 60.2 ± 7.4 | 43% | MRI |
| Altunisik E et al., 2021 [28] | 36 | 80 | Patients: 37.33 ± 7.38 Controls: 35.74 ± 8.38 | 46% | MRI |
| Strauss SB et al., 2020 [23] | 12 (1 anosmic) | 12 (7 anosmic, 2 phanthosmic, 3 hyposmic) | Patients: 58.0 ± 13.8 Controls: 58.25 ± 14.9 | 38% | MRI |
| Sherif F et al., 2022 [29] | 62 (100% anosmic) | 23 | Patients: 16–83 Controls: 17–61 | 24% | MRI, rs-fMRI, and DTI |
| Bispo DDC et al., 2023 [30] | 33 (50% hyposmic) | 20 | Patients: 36.4 ± 9.5 Controls: 39.3 ± 12.9 | 30% | MRI, rs-fMRI, and DTI |
| Akkaya H, 2021 [31] | 59 (100% anosmic) | 64 | Patients: 54.5 (21–71) Controls: 55 (19–80) | 54% | MRI |
| Parlak AE et al., 2024 [32] | 31 (68% anosmic, 32% hyposmic) | 35 | Patients: 54 ± 13.8 Controls: 59.9 ± 17.4 | Not significant | MRI |
| Iravani K et al., 2024 [33] | 15 | 5 | 33.33 ± 1.53 (both groups) | 60% | MRI, tb-fMRI |
| Perlaki G et al., 2024 [34] | 38 (100% anosmic) | 37 | Patients: 26 (23–29.3) Controls: 25 (24–27.5) | 37% | MRI |
| Arrigoni A et al., 2024 [35] | 51 (35 OD, 16 CM) (42 anosmic) | 14 (CRTL) | COVID-OD: 40 (31–53) COVID-CM: 56 CTRL: 62 (45–70) | 32% | MRI, rs-fMRI and DTI |
| Abdou EHE et al., 2023 [36] | 110 | 50 | Patients: 31.82 ± 10.15 Controls: 35.14 ± 12.37 | 28% | MRI |
| Wingrove J et al., 2023 [37] | 28 (100% anosmic) | 29 (11 + 18) | CoV: 37.02 ± 9.08 Long: 52.25 ± 12.17 Recov: 50.79 ± 8.91 Young: 27.73 ± 1.87 Controls: 38.89 ± 11.39 | 32% | MRI, rs-fMRI |
| Capelli S et al., 2023 [38] | 196 | 39 | Patients: 53 (42–60) Controls: 55 (46–66) | 46% | MRI |
| Esposito F et al., 2023 [39] | 18 | 10 | Patients: 38.7 Controls: 33.1 | 53% | MRI, rs-fMRI |
| Muccioli L et al., 2023 [18] | 23 (1 anosmic, most hyposmic) | 26 | Patients: 37 ± 14 Controls: 38.5 ± 13.7 | 49% | MRI, rs-fMRI |
| Burulday V et al., 2023 [40] | 27 | 27 | Patients: 35.25 ± 13.99 Controls: 35.62 ± 13.47 | Not significant | MRI |
| Caroli A et al., 2023 [41] | 215 (84 hyposmic) | 36 | Patients: 48 (36–55) Controls: 52 [42–65] | 57% | MRI |
| Besteher B et al., 2022 [42] | 30 | 20 | Patients: 47.5 ± 11.5 Controls: 42.95 ± 13.41 | 46% | MRI |
| Zhang H et al., 2022 [43] | 24 | 13 | Patients: 43.6 ± 14.0 Controls: 45.0 ± 13.2 | 38% | MRI, rs-fMRI |
| Esposito F et al., 2022 [39] | 27 | 18 | Patients: 40.0 ± 7.6 Controls: 36.0 ± 7.1 | 36% | MRI, rs-fMRI |
| Burulday V et al., 2022 [44] | 23 | 23 | Patients: 37.08 Controls: 36.82 | 59% | MRI |
| Study | COVID-19 Group OBV (mm3) | Control Group OBV (mm3) | Type of OD | Duration of OD |
|---|---|---|---|---|
| Güney et al. (2021) [26] | 68.0 ± 14.3 | 94.2 ± 7.6 | Anosmia (3), Hyposmia (38) | Not reported |
| Parlak et al. (2024) [32] | Right: 38.0 ± 8.5, Left: 37.1 ± 8.4 | Right: 56.3 ± 17.1, Left: 49.1 ± 13.5 | Anosmia (67.7%), Hy-posmia (rest) | 3 weeks to 2 months |
| Capelli et al. (2023) [38] | Left: 40.2 ± 9.7 | Left: 50.8 ± 11.3 | Not reported | 1 to 582 days |
| Altunisik et al. (2021) [28] | Reduced OBV | Not provided | Not reported | Not reported |
| Perlaki et al. (2024) [34] | 52.3 ± 13.6 | 61.0 ± 15.8 | Anosmia, Hypogeusia | ~60 days |
| Burulday et al. (2022) [44] | 45.6 ± 10.4 | 60.2 ± 12.7 | Not reported | Not reported |
| Akkaya et al. (2021) [31] | No significant difference | Not provided | Anosmia | Timing of MRI not reported |
| Muccioli et al. (2023) [18] | No significant difference | Not provided | Hyposmia (majority), Anosmia (1) | 2 to 19 months |
| Abdou et al. (2023) [36] | 72.1 ± 15.2 | 65.4 ± 14.8 | Not reported | Not reported |
| Study | COVID-19 Group OSD (mm) | Control Group OSD (mm) | Type of OD |
|---|---|---|---|
| Güney et al. (2021) [26] | 7.98 ± 0.37 | 8.82 ± 0.74 | Not reported |
| Parlak et al. (2024) [32] | Right: 7.4 ± 0.1, Left: 7.4 ± 1.0 | Right: 9.6 ± 0.8, Left: 9.4 ± 0.8 | Not reported |
| Altunisik et al. (2021) [28] | 7.5 ± 0.6 | 8.9 ± 0.5 | Not reported |
| Perlaki et al. (2024) [34] | 7.6 ± 0.9 | 8.8 ± 0.7 | Anosmia |
| Burulday et al. (2022) [44] | 7.3 ± 0.4 | 8.7 ± 0.6 | Not reported |
| Capelli et al. (2023) [38] | 7.7 ± 0.5 | 8.9 ± 0.8 | Not reported |
| Muccioli et al. (2023) [18] | No significant difference | Not provided | Hyposmia, Anosmia (1) |
| Akkaya et al. (2021) [31] | No significant difference | Not provided | Anosmia |
| Abdou et al. (2023) [36] | 8.1 ± 0.3 | 7.9 ± 0.4 | Not reported |
| Study | Sample Size | Duration of Olfactory Loss | Brain Regions Involved | Findings | Types of OD |
|---|---|---|---|---|---|
| Douaud et al. (2022) [24] | 401 COVID-19 patients, 384 controls The older population (UK Biobank, 51–81 years | 141 days | The orbitofrontal cortex, parahippocampal gyrus | Significant grey matter reduction in primary and secondary olfactory areas | Not specified |
| Perlaki et al. (2024) [34] | 50 patients Younger cohort (average age: 27 years) | 60 days | Primary olfactory cortex | Tissue damage/reduction in primary olfactory areas | Anosmia, hypogeusia |
| Besteher et al. (2022) [42] | 45 patients A varied age group | 2–16 months | Secondary olfactory areas, hippocampus, amygdala | Increased GMV in limbic regions (e.g., hippocampus, amygdala) in long-COVID patients with neuropsychiatric symptoms | Long-COVID, neuropsychiatric symptoms |
| Capelli et al. (2023) [38] | 75 COVID-19 patients, 60 controls Higher proportion of females in COVID-19 group | Not reported | Global brain regions | Brain volume differences attributed to a higher proportion of females; smaller brain volumes observed | Not specified |
| Study | Sample Size | Duration of OD | Regions/Tracts Investigated | Findings | Networks Involved |
|---|---|---|---|---|---|
| Esposito et al. (2023) [39] | 21 patients | 29–93 days | Anterior piriform cortex, olfactory cortex | Increased FC in the olfactory cortex as a compensatory mechanism for anosmia and hyposmia | ON, DMN |
| Esposito et al. (2022) [45] | 18 patients | 0–21 days | Olfactory cortex | Increased FC in olfactory regions, patients were hyposmic and hypogeusia | ON |
| Zhang et al. (2022) [43] | 40 patients | 91–268 days | Olfactory cortex, DMN, OFC | Altered FC in olfactory regions, associated with clinical measures of olfactory impairment | DMN, OFC |
| Wingrove et al. (2023) [37] | 50 patients | 168 ± 43 days | Hippocampus, insula, cingulate cortex | Altered FC in limbic regions correlated with olfactory impairment | Limbic System, ON |
| Arrigoni et al. (2024) [35] | 100+ participants | Not specified | Orbitofrontal cortex, piriform cortex | Reduced connectivity between the orbitofrontal and piriform cortex | ON, OFC |
| Muccioli et al. (2023) [18] | 13 patients | Not specified | Amygdala, hippocampus | Disruptions in limbic system FC are linked to emotional and memory-related olfactory perception. | Limbic System |
| Sherif et al. (2022) [29] | 25 patients | Not specified | Olfactory bulb | Altered structural connectivity; reduced FA and elevated MD values in the olfactory bulb | ON |
| Bispo et al. (2023) [30] | 30 patients | 92 ± 26 days | Left lateral orbital gyrus, parietal regions | Reduced structural connectivity and local network efficiency in regions associated with smell | DMN, ON |
| Study | Population | Olfactory Dysfunction | Brain Regions Involved | Findings | Task |
|---|---|---|---|---|---|
| Yildirim et al. (2022) [25] | COVID-19 patients (14% anosmic, 86% hyposmic) | COVID-19-related OD vs. post-infectious OD | Olfactory cortex (OFC), Entorhinal cortex | Enhanced activity in OFC and entorhinal cortices in COVID-19-related OD cases. Trigemino-sensory activity is more robust in COVID-19 patients. | fMRI, olfactory stimuli task |
| Iravani et al. (2024) [33] | COVID-19 patients (anosmic and microsmic) | Persistent olfactory dysfunction post-COVID | Upper frontal lobe, Basal ganglia | Significantly reduced activation in the upper frontal lobe and basal ganglia compared to healthy controls. Reduced ability to process olfactory stimuli. | Tb-fMRI, olfactory stimuli task |
| Study | DTI Metrics | Involved Regions/White Matter Tracts | Findings | Patient Group |
|---|---|---|---|---|
| Sherif et al. (2022) [29] | ↓ FA, ↑ MD | Olfactory bulb | Reduced FA, elevated MD in olfactory bulb | Anosmic COVID-19 patients |
| Yildirim et al. (2022) [25] | ↑ QA | Orbitofrontal cortex, entorhinal cortex | Microstructural damage/reorganization | Anosmic and hyposmic patients |
| Bispo et al. (2023) [30] | ↓ Structural connectivity | Left lateral orbital gyrus, parietal brain regions | Reduced local network efficiency | Hyposmic patients (mean duration of olfactory loss: 92 ± 26 days) |
| Burulday et al. (2023) [40] | ↓ ADC | Thalamus | Lower ADC bilaterally in the thalamus | COVID-19 patients |
| Lu et al. (2020) [27] | ↑ FA, ↓ MD | Superior frontal-occipital fasciculus (SFF), corona radiata | Subtle microstructural WM changes | COVID-19 patients (mean duration of olfactory loss: 98 ± 8 days) |
| Lu et al. (2020) [27] | FA < 0.22, MD > 1.5 | Olfactory bulb | Defined diagnostic thresholds for FA and MD | Anosmic patients |
| Yildirim et al. (2022) [25] | N/A | Olfactory regions | Structural changes were noted, but no diagnostic thresholds | Anosmic and hyposmic patients |
| Study | Population | Olfactory Dysfunction | Imaging Techniques | Brain Regions Involved | Findings | Duration of Olfactory Loss |
|---|---|---|---|---|---|---|
| Strauss et al. (2020) [23] | COVID-19 patients vs. anosmia control group | COVID-19-related anosmia (higher OB T2 FLAIR intensity) | T2-weighted imaging (T2WI), postcontrast 3D T2 FLAIR | OB | Higher T2 FLAIR signal intensity in OB in COVID-19 patients, indicating structural or inflammatory changes. | Mean 14 days, range 0–32 days. |
| Caroli et al. (2023) [41] | COVID-19 patients with hyposmia (hospitalized and non-hospitalized) | COVID-19-related hyposmia (with microstructural brain changes) | MRI, DWI, ADC | WM, GM | Elevated ADC values in WM and GM regions, especially in patients with neurological symptoms. Correlation between ADC changes and symptom severity. | Hospitalized: 77 days; Non-hospitalized: 252 days |
| Lu et al. (2020) [27] | COVID-19 patients | COVID-19-related olfactory dysfunction | 3D high-resolution T1-weighted imaging (T1WI) | Olfactory cortices, Hippocampus | Increased gray matter volume in key olfactory-related regions (olfactory cortices, hippocampi). | Mean 97.46 ± 8.01 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Manan, H.; de Jesus, R.; Thaploo, D.; Hummel, T. Mapping the Olfactory Brain: A Systematic Review of Structural and Functional Magnetic Resonance Imaging Changes Following COVID-19 Smell Loss. Brain Sci. 2025, 15, 690. https://doi.org/10.3390/brainsci15070690
Abdul Manan H, de Jesus R, Thaploo D, Hummel T. Mapping the Olfactory Brain: A Systematic Review of Structural and Functional Magnetic Resonance Imaging Changes Following COVID-19 Smell Loss. Brain Sciences. 2025; 15(7):690. https://doi.org/10.3390/brainsci15070690
Chicago/Turabian StyleAbdul Manan, Hanani, Rafaela de Jesus, Divesh Thaploo, and Thomas Hummel. 2025. "Mapping the Olfactory Brain: A Systematic Review of Structural and Functional Magnetic Resonance Imaging Changes Following COVID-19 Smell Loss" Brain Sciences 15, no. 7: 690. https://doi.org/10.3390/brainsci15070690
APA StyleAbdul Manan, H., de Jesus, R., Thaploo, D., & Hummel, T. (2025). Mapping the Olfactory Brain: A Systematic Review of Structural and Functional Magnetic Resonance Imaging Changes Following COVID-19 Smell Loss. Brain Sciences, 15(7), 690. https://doi.org/10.3390/brainsci15070690






