Sensory Attenuation and Agency in Cooperative and Individual Contexts: Exploring the Role of Empathy in Action Perception
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.2.1. Perceptual Judgment Task
- Individual context self-press: the participant pressed the spacebar with the right index finger, without being involved in any interaction with the experimenter;
- Individual context other-press: the participant saw the experimenter pressing the spacebar with the right index finger, without being involved in any interaction with the experimenter;
- Cooperative context self-press: the participant pressed the spacebar with the right index finger, whenever the experimenter requested her to do so by touching the participant’s right forearm with her right hand, which was occluded to prevent the visual anticipation of touch;
- Cooperative context other-press: the experimenter pressed the spacebar with the right index finger whenever the participant requested her to do so by touching the experimenter’s right forearm with her right hand, which was likewise occluded (as before, to avoid any visual anticipation of touch).
2.2.2. Empathy
2.3. Statistical Analysis
2.3.1. Perceptual Judgment Task
2.3.2. Empathy
3. Results
3.1. Perceptual Judgment Task
3.2. Empathy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallagher, S. Philosophical Conceptions of the Self: Implications for Cognitive Science. Trends Cogn. Sci. 2000, 4, 14–21. [Google Scholar] [CrossRef]
- Frith, C.D.; Blakemore, S.J.; Wolpert, D.M. Abnormalities in the Awareness and Control of Action. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1771–1788. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.W.; Middleton, D.; Haggard, P.; Fletcher, P.C. Exploring Implicit and Explicit Aspects of Sense of Agency. Conscious. Cogn. 2012, 21, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.J.; Wolpert, D.M.; Frith, C.D. Central Cancellation of Self-Produced Tickle Sensation. Nat. Neurosci. 1998, 1, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.J.; Wolpert, D.; Frith, C.D. Why Can’t You Tickle Yourself? NeuroReport 2000, 3, 11–16. [Google Scholar] [CrossRef]
- Gu, J.; Buidze, T.; Zhao, K.; Gläscher, J.; Fu, X. The Neural Network of Sensory Attenuation: A Neuroimaging Meta-Analysis. Psychon. Bull. Rev. 2024, 32, 31–35. [Google Scholar] [CrossRef]
- Synofzik, M.; Vosgerau, G.; Newen, A. Beyond the Comparator Model: A Multifactorial Two-Step Account of Agency. Conscious. Cogn. 2008, 17, 219–239. [Google Scholar] [CrossRef]
- Martikainen, M.H. Suppressed Responses to Self-Triggered Sounds in the Human Auditory Cortex. Cereb. Cortex 2004, 15, 299–302. [Google Scholar] [CrossRef]
- Weiss, C.; Herwig, A.; Schütz-Bosbach, S. The Self in Action Effects: Selective Attenuation of Self-Generated Sounds. Cognition 2011, 121, 207–218. [Google Scholar] [CrossRef]
- Blakemore, S.J.; Frith, C. The Role of Motor Contagion in the Prediction of Action. Neuropsychologia 2005, 43, 260–267. [Google Scholar] [CrossRef]
- de Vignemont, F.; Haggard, P. Action Observation and Execution: What Is Shared? Soc. Neurosci. 2008, 3, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Schütz-Bosbach, S.; Prinz, W. Perceptual Resonance: Action-Induced Modulation of Perception. Trends Cogn. Sci. 2007, 11, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Jeannerod, M. The Mechanism of Self-Recognition in Humans. Behav. Brain Res. 2003, 142, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Krol, M.A.; Schutter, D.J.L.G.; Jellema, T. Sensorimotor cortex activation during anticipation of upcoming predictable but not unpredictable actions. Soc. Neurosci. 2020, 15, 214–226. [Google Scholar] [CrossRef]
- Bonini, L.; Rotunno, C.; Arcuri, E.; Gallese, V. Mirror neurons 30 years later: Implications and applications. Trends Cogn. Sci. 2022, 26, 767–781. [Google Scholar] [CrossRef]
- Kilner, J.M.; Friston, K.J.; Frith, C.D. Predictive coding: An account of the mirror neuron system. Cogn. Process. 2007, 8, 159–166. [Google Scholar] [CrossRef]
- Maranesi, M.; Livi, A.; Fogassi, L.; Rizzolatti, G.; Bonini, L. Mirror Neuron Activation Prior to Action Observation in a Predictable Context. J. Neurosci. 2014, 34, 14827–14832. [Google Scholar] [CrossRef]
- Krol, M.A.; Jellema, T. Sensorimotor representation of observed dyadic actions with varying agent involvement: An EEG mu study. Cogn. Neurosci. 2022, 14, 25–35. [Google Scholar] [CrossRef]
- Di Plinio, S.; Perrucci, M.G.; Ebisch, S.J.H. The Prospective Sense of Agency is Rooted in Local and Global Properties of Intrinsic Functional Brain Networks. J. Cogn. Neurosci. 2020, 32, 1764–1779. [Google Scholar] [CrossRef]
- Sidarus, N.; Vuorre, M.; Haggard, P. Integrating prospective and retrospective cues to the sense of agency: A multi-study investigation. Neurosci. Conscious. 2017, 2017, nix012. [Google Scholar] [CrossRef]
- Harrison, A.W.; Mannion, D.J.; Jack, B.N.; Griffiths, O.; Hughes, G.; Whitford, T.J. Sensory attenuation is modulated by the contrasting effects of predictability and control. NeuroImage 2021, 237, 118103. [Google Scholar] [CrossRef] [PubMed]
- Weiss, C.; Herwig, A.; Schütz-Bosbach, S. The Self in Social Interactions: Sensory Attenuation of Auditory Action Effects Is Stronger in Interactions with Others. PLoS ONE 2011, 6, e22723. [Google Scholar] [CrossRef]
- Desantis, A.; Weiss, C.; Schütz-Bosbach, S.; Waszak, F. Believing and Perceiving: Authorship Belief Modulates Sensory Attenuation. PLoS ONE 2012, 7, e37959. [Google Scholar] [CrossRef] [PubMed]
- Sommerville, J.A.; Decety, J. Social cognition: Development across the life span. In Social Cognition: Development Across the Life Span; Routledge: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Eslinger, P.J. Neurological and Neuropsychological Bases of Empathy. Eur. Neurol. 1998, 39, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Decety, J.; Lamm, C. Human Empathy Through the Lens of Social Neuroscience. Sci. World J. 2006, 6, 1146–1163. [Google Scholar] [CrossRef]
- Lamm, C.; Batson, C.D.; Decety, J. The Neural Substrate of Human Empathy: Effects of Perspective-Taking and Cognitive Appraisal. J. Cogn. Neurosci. 2007, 19, 42–58. [Google Scholar] [CrossRef]
- Krizman, J.; Bonacina, S.; Kraus, N. Sex differences in subcortical auditory processing emerge across development. Hear. Res. 2019, 380, 166–174. [Google Scholar] [CrossRef]
- Rochat, M.J. Sex and Gender Differences in the Development of Empathy. J. Neurosci. Res. 2023, 101, 718–729. [Google Scholar] [CrossRef]
- Mathôt, S.; Schreij, D.; Theeuwes, J. OpenSesame: An open-source, graphical experiment builder for the social sciences. Behav. Res. 2012, 44, 314–324. [Google Scholar] [CrossRef]
- Albiero, P.; Ingoglia, S.; Lo Coco, A. Contributo All’adattamento Italiano Dell’Interpersonal Reactivity Index. Test. Psicometria Metodol. 2006, 13, 107–125. [Google Scholar]
- Davis, M.H. A Multidimensional Approach to Individual Differences in Empathy. J. Personal. Soc. Psychol. 1980, 10, 85. [Google Scholar]
- Zhang, F.; Mcguire, K.; Firestone, G.; Dalrymple, K.; Greinwald, J.; Fu, Q.J. Cortical Processing of Location and Frequency Changes of Sounds in Normal Hearing Listeners. Hear. Res. 2021, 400, 108110. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Maddaluno, O.; Aiello, E.N.; Roncoroni, C.; Prunas, A.; Bolognini, N. The Reading the Mind in the Eyes Test, Iowa Gambling Task and Interpersonal Reactivity Index: Normative Data in an Italian Population Sample. Arch. Clin. Neuropsychol. 2022, 37, 929–938. [Google Scholar] [CrossRef]
- Beyer, F.; Sidarus, N.; Bonicalzi, S.; Haggard, P. Beyond Self-Serving Bias: Diffusion of Responsibility Reduces Sense of Agency and Outcome Monitoring. Soc. Cogn. Affect. Neurosci. 2017, 12, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Beyer, F.; Sidarus, N.; Fleming, S.; Haggard, P. Losing Control in Social Situations: How the Presence of Others Affects Neural Processes Related to Sense of Agency. eNeuro 2018, 5, ENEURO.033617.2018. [Google Scholar] [CrossRef]
- Prior, N.H.; Bentz, E.J.; Ophir, A.G. Reciprocal Processes of Sensory Perception and Social Bonding: An Integrated Social-sensory Framework of Social Behavior. Genes. Brain Behav. 2022, 21, e12781. [Google Scholar] [CrossRef]
- Khalighinejad, N.; Bahrami, B.; Caspar, E.A.; Haggard, P. Social Transmission of Experience of Agency: An Experimental Study. Front. Psychol. 2016, 7, 1315. [Google Scholar] [CrossRef]
- Tomasello, M.; Carpenter, M.; Call, J.; Behne, T.; Moll, H. Understanding and sharing intentions: The origins of cultural cognition. Behav. Brain Sci. 2005, 28, 675–691. [Google Scholar] [CrossRef]
- Parthasharathy, M.; Mantini, D.; Orban de Xivry, J.J. Increased upper-limb sensory attenuation with age. J. Neurophysiol. 2022, 127, 474–492. [Google Scholar] [CrossRef]
- Wolpe, N.; Ingram, J.N.; Tsvetanov, K.A.; Geerligs, L.; Kievit, R.A.; Henson, R.N.; Wolpert, D.M.; Rowe, J.B. Ageing increases reliance on sensorimotor prediction through structural and functional differences in frontostriatal circuits. Nat. Commun. 2016, 7, 13034. [Google Scholar] [CrossRef] [PubMed]
- Peelle, J.E. Age-Related Sensory Deficits and Their Consequences. In The Cambridge Handbook of Cognitive Aging: A Life Course Perspective; Thomas, A.K., Gutchess, A., Eds.; Cambridge Handbooks in Psychology; Cambridge University Press: Cambridge, UK, 2020; pp. 179–199. [Google Scholar]
- Timar, L.; Job, X.; Orban de Xivry, J.J.; Kilteni, K. Aging exerts a limited influence on the perception of self-generated and externally generated touch. J. Neurophysiol. 2023, 130, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Luo, Z.; Zhang, W.; Li, W.; Li, X. Age-related differences in affective and cognitive empathy: Self-report and performance-based evidence. Aging Neuropsychol. Cogn. 2018, 25, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Seghezzi, S.; Zirone, E.; Paulesu, E.; Zapparoli, L. The Brain in (Willed) Action: A Meta-Analytical Comparison of Imaging Studies on Motor Intentionality and Sense of Agency. Front. Psychol. 2019, 10, 804. [Google Scholar] [CrossRef]
- Zito, G.A.; Wiest, R.; Aybek, S. Neural Correlates of Sense of Agency in Motor Control: A Neuroimaging Meta-Analysis. PLoS ONE 2020, 15, e0234321. [Google Scholar] [CrossRef]
- Fan, Y.; Duncan, N.W.; de Greck, M.; Northoff, G. Is There a Core Neural Network in Empathy? An FMRI Based Quantitative Meta-Analysis. Neurosci. Biobehav. Rev. 2011, 35, 903–911. [Google Scholar] [CrossRef]

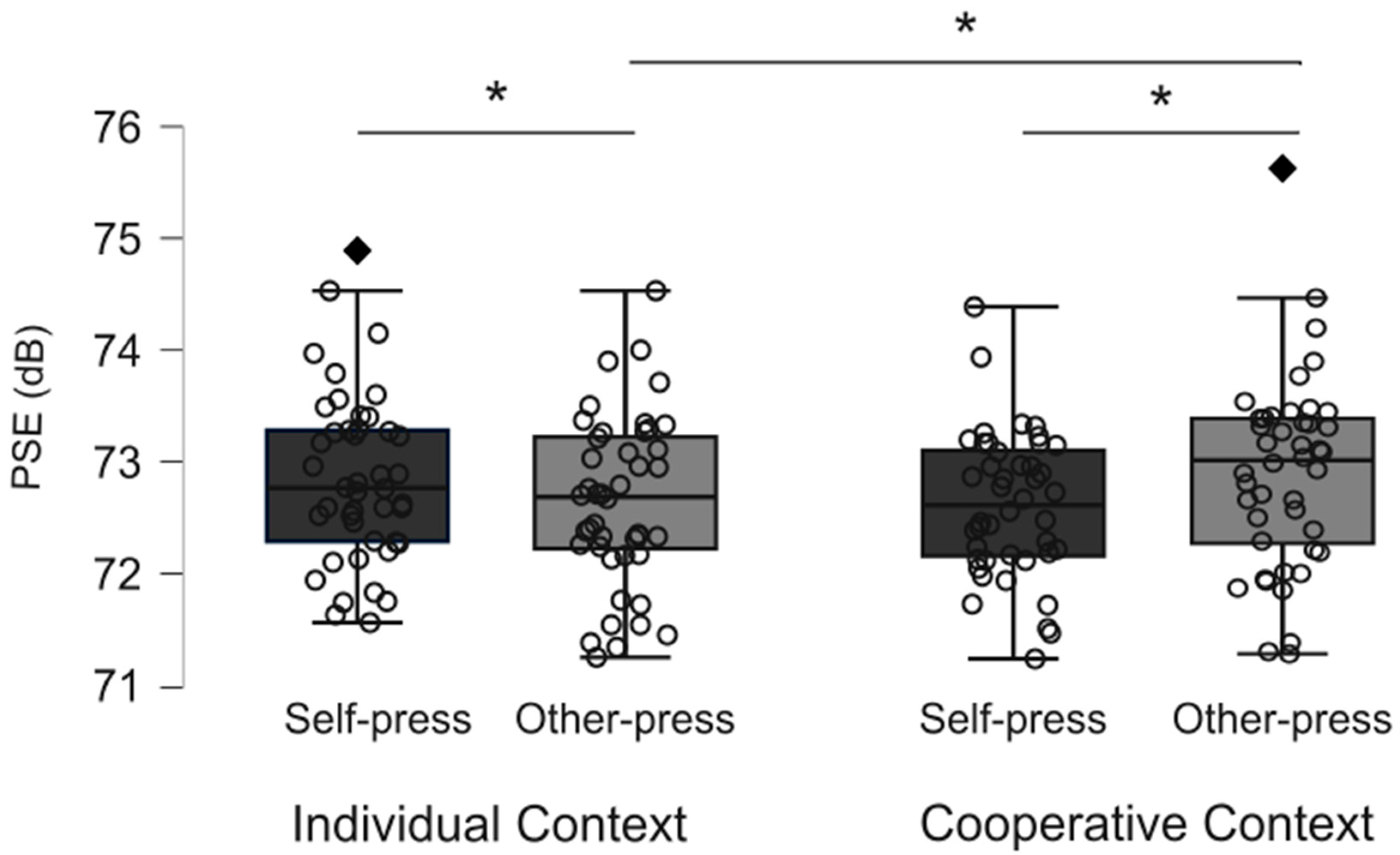
| Mean dB (SD) | Min | Max | Shapiro–Wilk’s | One-Sample t-Tests | |
| Individual context | |||||
| Self-press | 72.85 (0.76) | 71.56 | 74.89 | 0.97, p = 0.42 | t43 = 11.85, p < 0.001, d = 1.51 |
| Other-press | 72.63 (0.76) | 71.25 | 74.53 | 0.98, p = 0.58 | t43= 10.04, p < 0.001, d = 1.79 |
| Mean dB (SD) | Min | Max | Shapiro–Wilk’s | One-Sample t-Tests | |
| Cooperative context | |||||
| Self-press | 72.65 (0.65) | 71.28 | 74.43 | 0.98, p = 0.58 | t43= 13.83, p < 0.001, d = 2.09 |
| Other-press | 72.90 (0.86) | 71.28 | 75.63 | 0.96, p = 0.13 | t43= 8.49, p < 0.001, d = 1.28 |
| IRI | Mean | SD | Min | Max |
|---|---|---|---|---|
| Fantasy | 20 | 4.4 | 12 | 29 |
| Emphatic Concern | 25 | 4.35 | 11 | 32 |
| Perspective-Taking | 24.2 | 4.65 | 14 | 33 |
| Personal Distress | 16.9 | 4.91 | 6 | 27 |
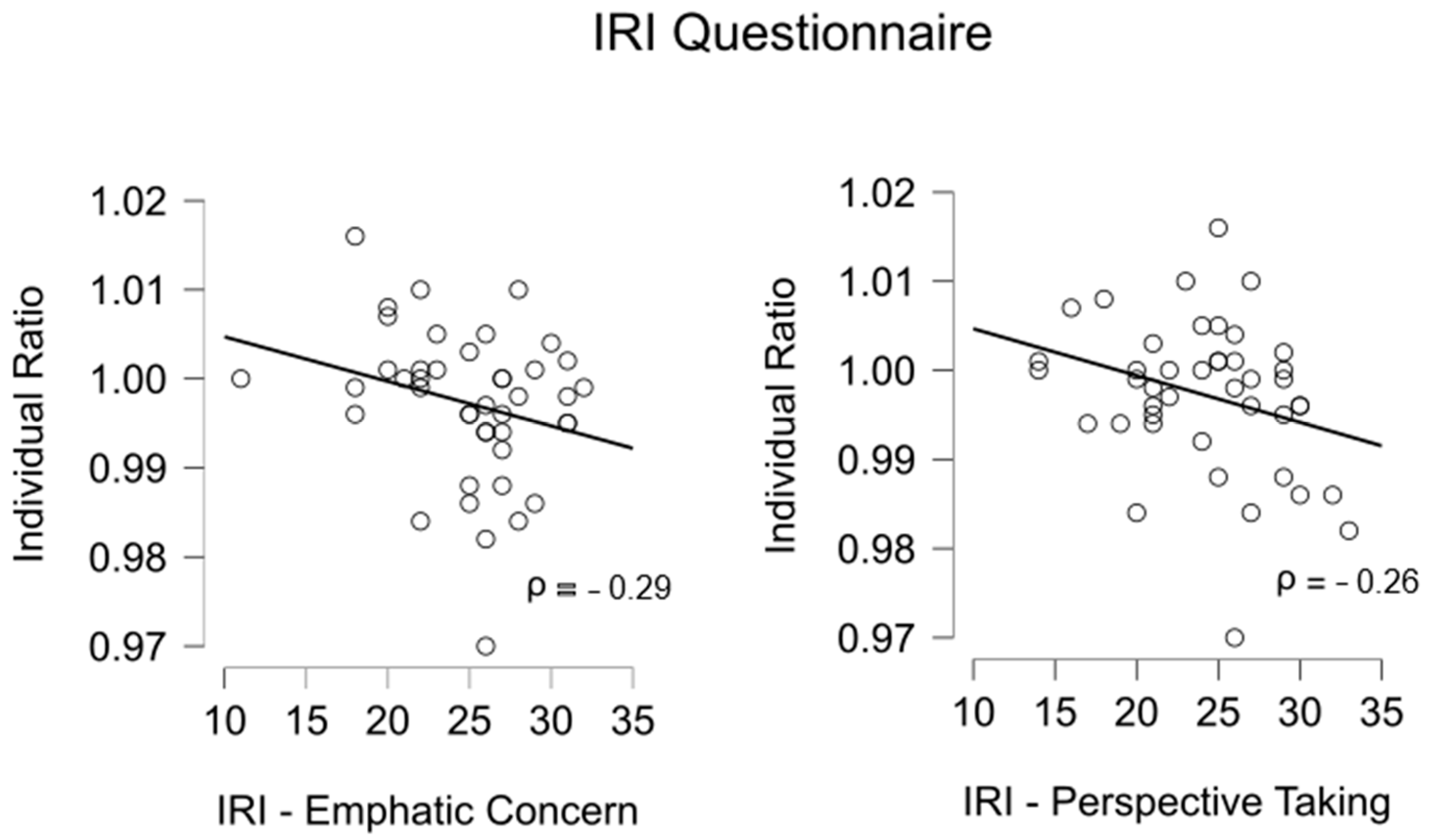
| Interpersonal Reactivity Index (IRI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| IRI-FS | IRI-EC | IRI-PT | IRI-PD | |||||
| ρ | p-Value | ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| Individual ratio | 0.04 | 0.60 | −0.29 | 0.030 | −0.26 | 0.045 | 0.23 | 0.93 |
| Cooperative ratio | −0.03 | 0.44 | −0.08 | 0.300 | 0.12 | 0.770 | −0.09 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagini, S.; Ghiggia, A.; Falco, S.; Castelli, L.; Mauro, A.; Scarpina, F. Sensory Attenuation and Agency in Cooperative and Individual Contexts: Exploring the Role of Empathy in Action Perception. Brain Sci. 2025, 15, 688. https://doi.org/10.3390/brainsci15070688
Tagini S, Ghiggia A, Falco S, Castelli L, Mauro A, Scarpina F. Sensory Attenuation and Agency in Cooperative and Individual Contexts: Exploring the Role of Empathy in Action Perception. Brain Sciences. 2025; 15(7):688. https://doi.org/10.3390/brainsci15070688
Chicago/Turabian StyleTagini, Sofia, Ada Ghiggia, Sara Falco, Lorys Castelli, Alessandro Mauro, and Federica Scarpina. 2025. "Sensory Attenuation and Agency in Cooperative and Individual Contexts: Exploring the Role of Empathy in Action Perception" Brain Sciences 15, no. 7: 688. https://doi.org/10.3390/brainsci15070688
APA StyleTagini, S., Ghiggia, A., Falco, S., Castelli, L., Mauro, A., & Scarpina, F. (2025). Sensory Attenuation and Agency in Cooperative and Individual Contexts: Exploring the Role of Empathy in Action Perception. Brain Sciences, 15(7), 688. https://doi.org/10.3390/brainsci15070688





