Neural Effects of Creative Movement, General Movement, and Sedentary Play Interventions on Interpersonal Synchrony in Children with Autism Spectrum Disorder: A Preliminary fNIRS Study †
Abstract
1. Introduction
1.1. IPS Difficulties in Children with ASD
1.2. Neural Mechanisms Underlying IPS
1.3. ASD-Related Differences in Cortical Activation During IPS
1.4. Behavioral Effects of Creative Movement Interventions in Children with ASD
1.5. Knowledge Gaps in Neural Effects of Movement-Based Interventions
2. Materials and Methods
2.1. Participants
2.2. Study Procedures
2.3. Training Protocol
2.4. fNIRS Testing Protocol
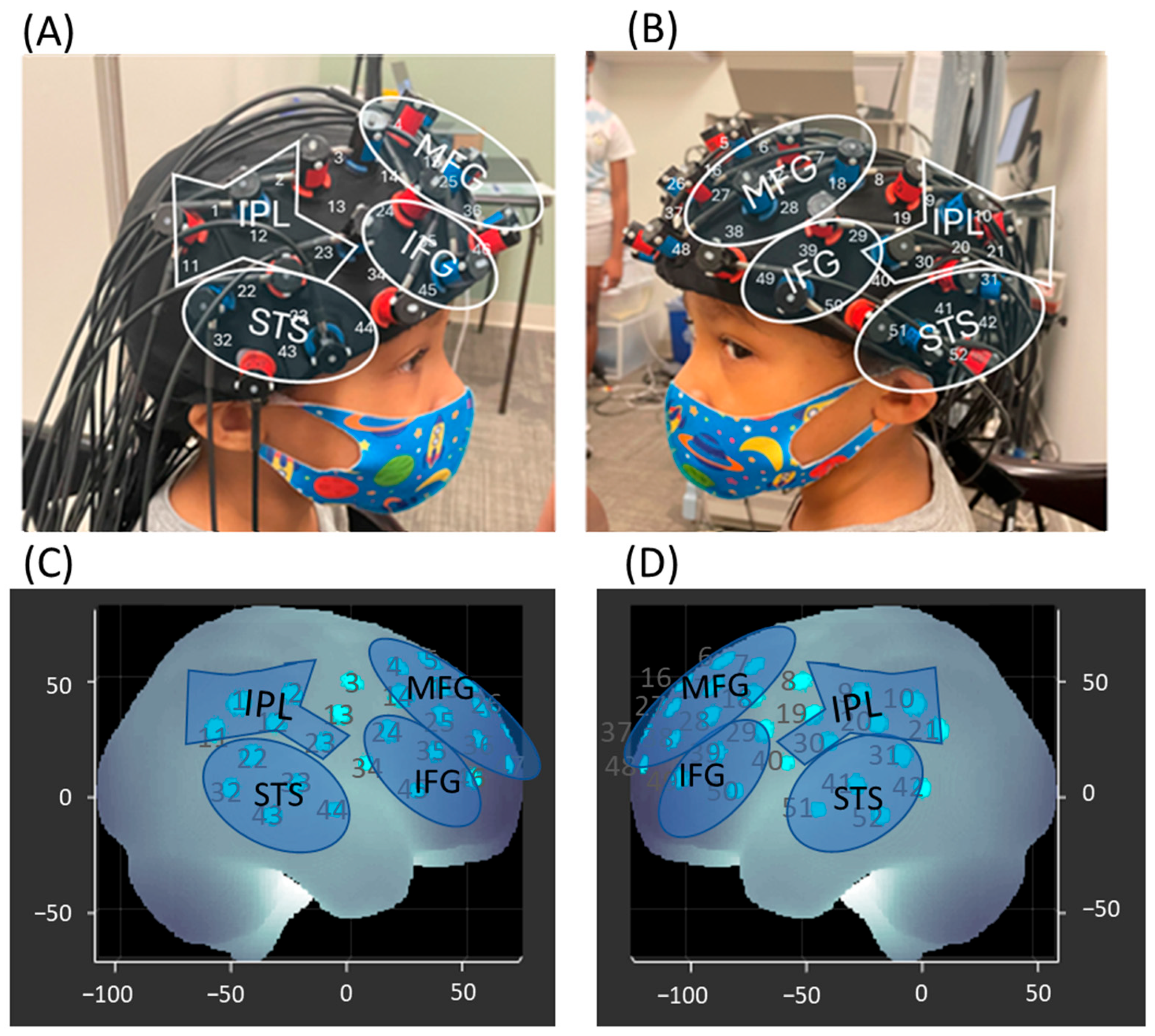
2.5. fNIRS Data Collection
2.6. Spatial Registration
2.7. fNIRS Data Processing
2.8. Video Coding and Exclusion
2.9. Statistical Analysis
3. Results
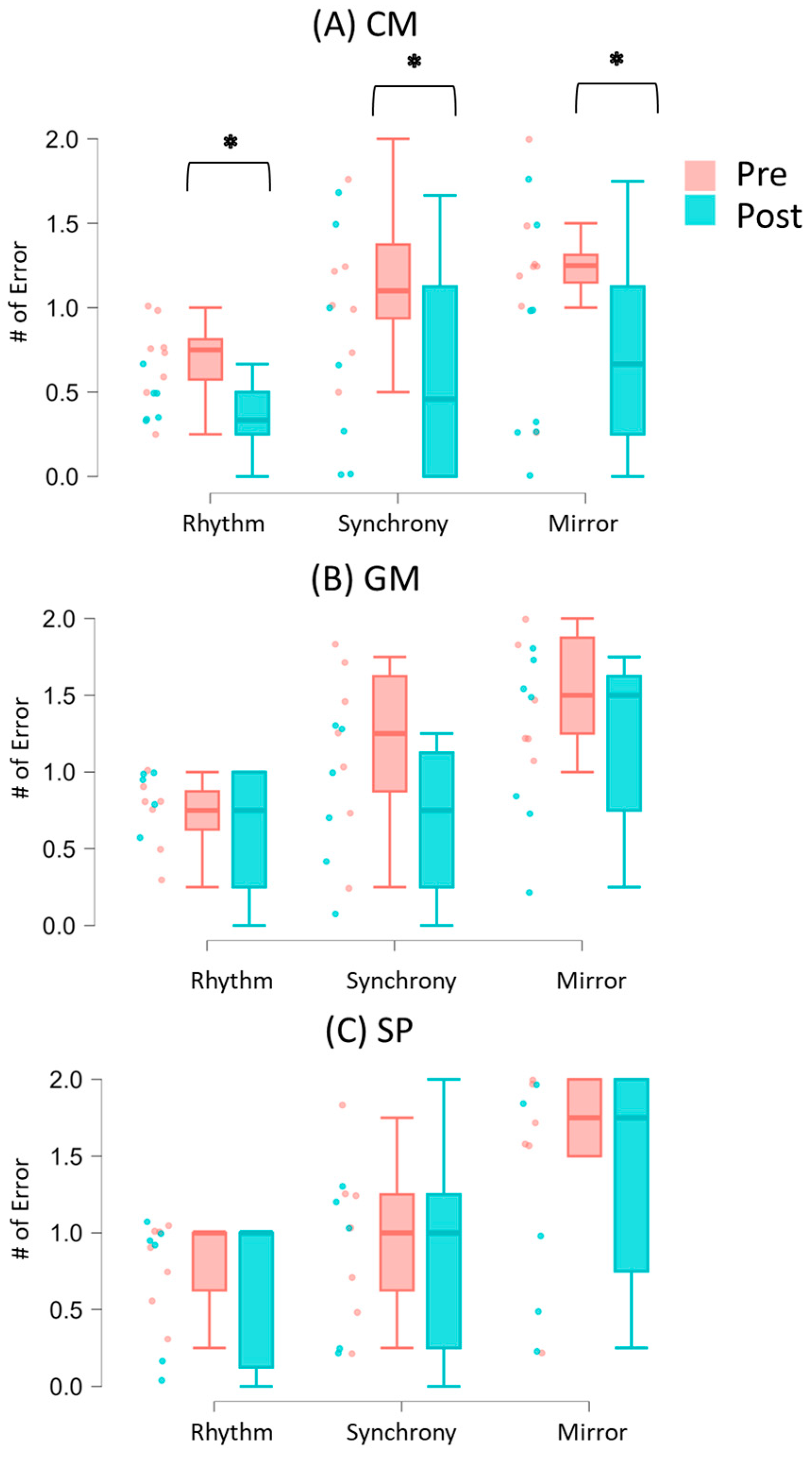
3.1. Synchrony Performance During Solo and Social Conditions
3.2. Cortical Activation During IPS
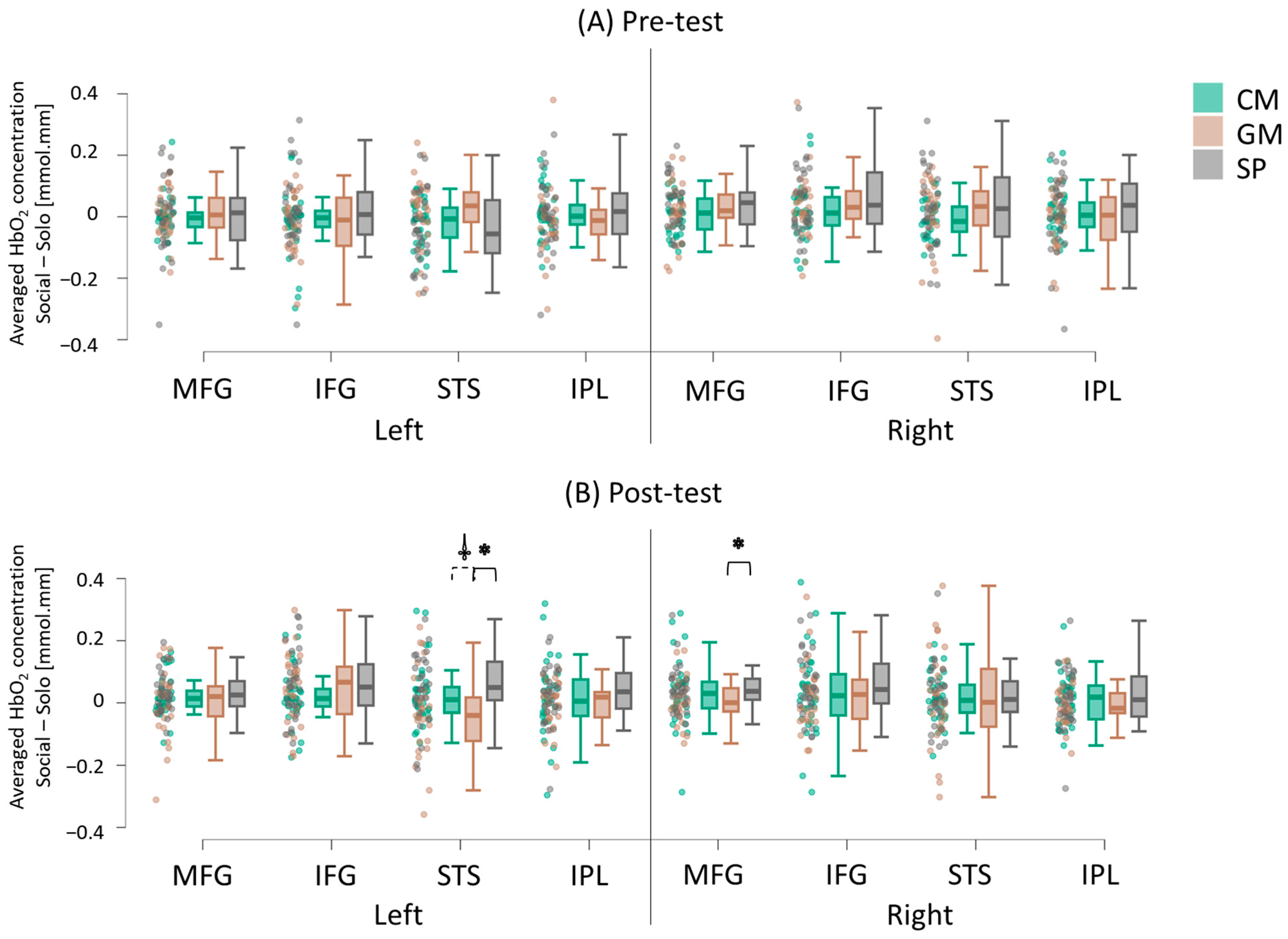
3.2.1. Group Differences
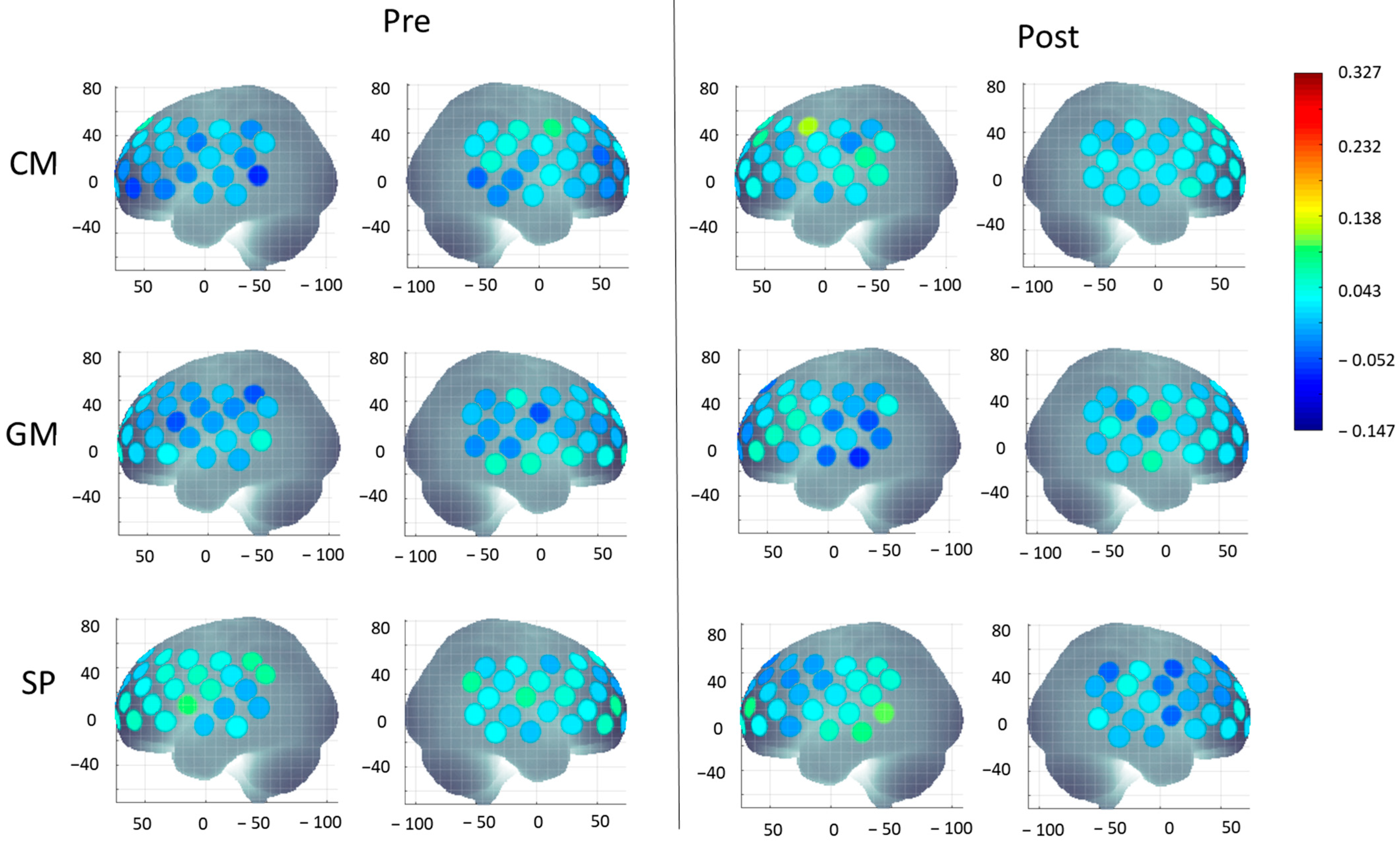
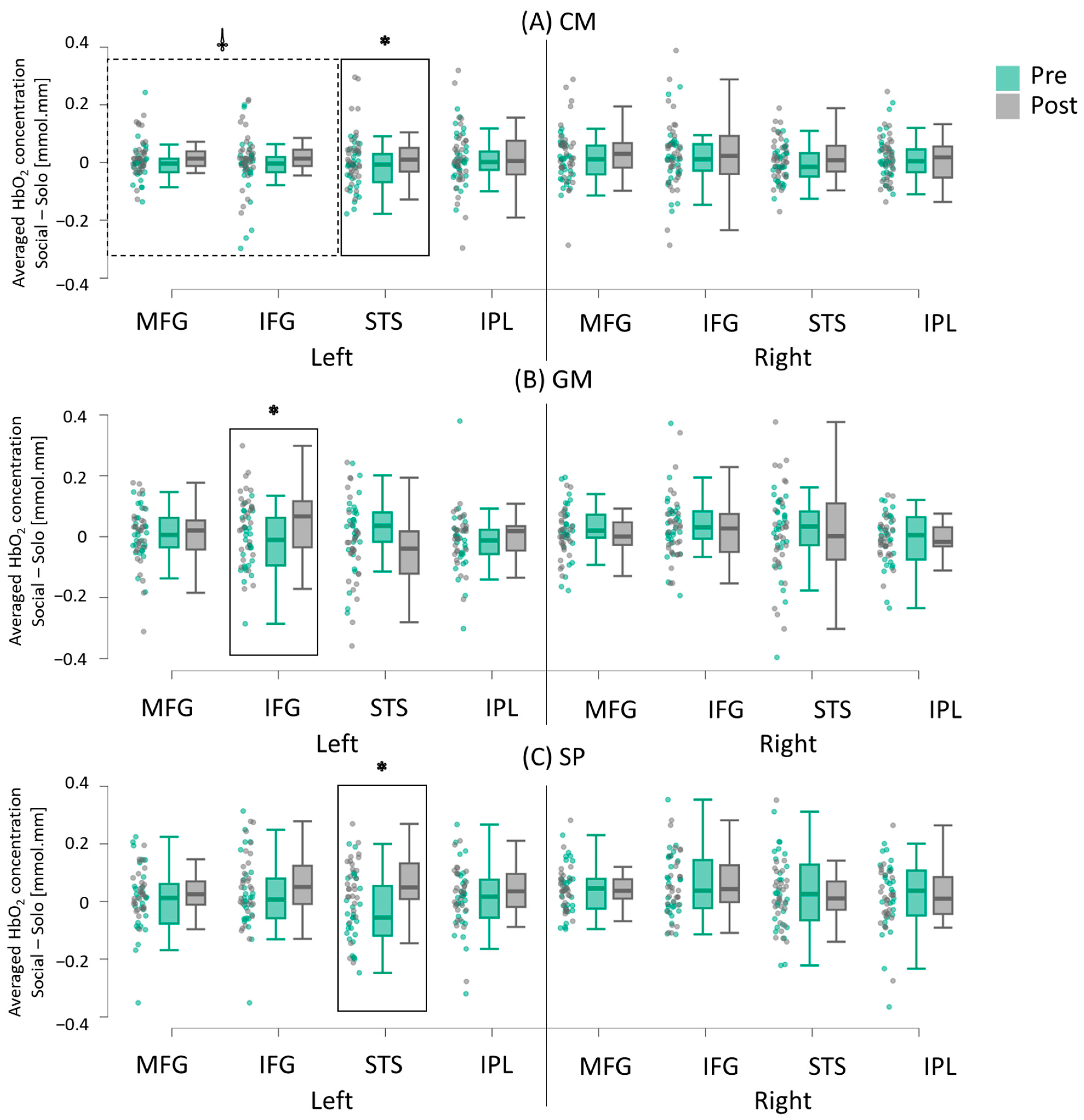
3.2.2. Intervention-Related Differences (Pretest vs. Post-Test)
3.2.3. Hemispheric Differences
3.3. Correlation
3.3.1. Associations Between IPS Performance and Cortical Activation During the Pretest
3.3.2. Associations Between Baseline Adaptive Functioning, Social Responsiveness Measures, and Improvements in IPS Performance
4. Discussion
4.1. Improved IPS After Creative Movement Intervention
4.2. Intervention-Specific Changes in Socially Enhanced Cortical Activation
4.2.1. More Widespread Increase in Socially Enhanced Activation Following CM Intervention
4.2.2. GM Intervention-Related Increase in Socially Enhanced Activation in IFG
4.2.3. SP Intervention-Related Increase in Socially Enhanced Activation in STS
4.3. Increased Left Lateralization Post-Movement Interventions
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Durkin, M.S.; Fitzgerald, R.T.; Furnier, S.M.; Hughes, M.M.; et al. Prevalence and characteristics of Autism Spectrum Disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 Sites, United States, 2020. MMWR Surveill Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Edwards, L.A. A meta-analysis of imitation abilities in individuals with autism spectrum disorders. Autism. Res. 2014, 7, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hofmann, J. Action observation and imitation in autism spectrum disorders: An ALE meta-analysis of fMRI studies. Brain Imaging Behav. 2016, 10, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Su, W.C.; Culotta, M.; Mueller, J.; Tsuzuki, D.; Pelphrey, K.; Bhat, A. Differences in cortical activation patterns during action observation, action execution, and interpersonal synchrony between children with or without autism spectrum disorder (ASD): An fNIRS pilot study. PLoS ONE 2020, 15, e0240301. [Google Scholar] [CrossRef]
- Su, W.C.; Culotta, M.; Tsuzuki, D.; Bhat, A. Movement kinematics and cortical activation in children with and without autism spectrum disorder during sway synchrony tasks: An fNIRS study. Sci. Rep. 2021, 11, 15035. [Google Scholar] [CrossRef] [PubMed]
- Amonkar, N.; Su, W.C.; Bhat, A.N.; Srinivasan, S.M. Effects of creative movement therapies on social communication, behavioral-affective, sensorimotor, cognitive, and functional participation skills of individuals with autism spectrum disorder: A systematic review. Front. Psychiatry 2021, 12, 722874. [Google Scholar] [CrossRef]
- Kaur, M.; Bhat, A. Creative yoga intervention improves motor and imitation skills of children with autism spectrum disorder. Phys. Ther. 2019, 99, 1520–1534. [Google Scholar] [CrossRef]
- Srinivasan, S.M.; Kaur, M.; Park, I.K.; Gifford, T.D.; Marsh, K.L.; Bhat, A.N. The effects of rhythm and robotic interventions on the imitation/praxis, interpersonal synchrony, and motor performance of children with autism spectrum disorder (ASD): A pilot randomized controlled trial. Autism Res. Treat. 2015, 2025, 736516. [Google Scholar] [CrossRef]
- Su, W.C.; Srinivasan, S.; Bhat, A.N. Effects of creative movement, general movement, or seated play interventions on motor performance in children with Autism Spectrum Disorder: A pilot randomized controlled trial. Res. Autism Spectr. Disord. 2025, 119, 102501. [Google Scholar] [CrossRef]
- Fitzpatrick, P.; Frazier, J.A.; Cochran, D.M.; Mitchell, T.; Coleman, C.; Schmidt, R.C. Impairments of social motor synchrony evident in autism spectrum disorder. Front. Psychol. 2016, 7, 1323. [Google Scholar] [CrossRef]
- Fitzpatrick, P.; Romero, V.; Amaral, J.L.; Duncan, A.; Barnard, H.; Richardson, M.J.; Schmidt, R.C. Evaluating the importance of social motor synchronization and motor skill for understanding autism. Autism. Res. 2017, 10, 1687–1699. [Google Scholar] [CrossRef]
- McNaughton, K.A.; Redcay, E. Interpersonal synchrony in autism. Curr. Psychiatry Rep. 2020, 22, 12. [Google Scholar] [CrossRef]
- Bhat, A.N.; Landa, R.J.; Galloway, J.C. Current perspectives on motor functioning in infants, children, and adults with autism spectrum disorders. Phys. Ther. 2011, 91, 1116–1129. [Google Scholar] [CrossRef]
- Federici, A.; Parma, V.; Vicovaro, M.; Radassao, L.; Casartelli, L.; Ronconi, L. Anomalous perception of biological motion in autism: A conceptual review and meta-analysis. Sci. Rep. 2020, 10, 4576. [Google Scholar] [CrossRef]
- Frazier, T.W.; Strauss, M.; Klingemier, E.W.; Zetzer, E.E.; Hardan, A.Y.; Eng, C.; Youngstrom, E.A. A meta-analysis of gaze differences to social and nonsocial information between individuals with and without autism. J. Am. Acad. Child. Adolesc. Psychiatry 2017, 56, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Koehne, S.; Hatri, A.; Cacioppo, J.T.; Dziobek, I. Perceived interpersonal synchrony increases empathy: Insights from autism spectrum disorder. Cognition 2016, 146, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Fonseca, L.; Dotov, D.; Fossion, R.; Froese, T.; Schilbach, L.; Vogeley, K.; Timmermans, B. Multi-scale coordination of distinctive movement patterns during embodied interaction between adults with high-functioning autism and neurotypicals. Front. Psychol. 2019, 9, 2760. [Google Scholar] [CrossRef] [PubMed]
- Shamay-Tsoory, S.G.; Saporta, N.; Marton-Alper, I.Z.; Gvirts, H.Z. Herding brains: A core neural mechanism for social alignment. Trends. Cogn. Sci. 2019, 23, 174–186. [Google Scholar] [CrossRef]
- Demetriou, E.A.; Lampit, A.; Quintana, D.S.; Naismith, S.L.; Song, Y.; Pye, J.E.; Hickie, I.; Guastella, A.J. Autism spectrum disorders: A meta-analysis of executive function. Mol. Psychiatry 2018, 23, 1198–1204. [Google Scholar] [CrossRef]
- Caspers, S.; Zilles, K.; Laird, A.R.; Eickhoff, S.B. ALE meta-analysis of action observation and imitation in the human brain. NeuroImage 2010, 50, 1148–1167. [Google Scholar] [CrossRef]
- Iacoboni, M. Neural mechanisms of imitation. Curr. Opin. Neurobiol. 2005, 15, 632–637. [Google Scholar] [CrossRef]
- Molenberghs, P.; Cunnington, R.; Mattingley, J.B. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neurosci. Biobehav. Rev. 2009, 33, 975–980. [Google Scholar] [CrossRef]
- Ocampo, B.; Kritikos, A.; Cunnington, R. How frontoparietal brain regions mediate imitative and complementary actions: An FMRI study. PLoS ONE 2011, 6, e26945. [Google Scholar] [CrossRef]
- Johnson-Frey, S.H.; Maloof, F.R.; Newman-Norlund, R.; Farrer, C.; Inati, S.; Grafton, S.T. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron 2003, 39, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Koski, L.; Wohlschläger, A.; Bekkering, H.; Woods, R.P.; Dubeau, M.C.; Mazziotta, J.C.; Iacoboni, M. Modulation of motor and premotor activity during imitation of target-directed actions. Cereb. Cortex 2002, 12, 847–855. [Google Scholar] [CrossRef]
- Fontana, A.P.; Kilner, J.M.; Rodrigues, E.C.; Joffily, M.; Nighoghossian, N.; Vargas, C.D.; Sirigu, A. Role of the parietal cortex in predicting incoming actions. NeuroImage 2012, 59, 556–564. [Google Scholar] [CrossRef]
- Molenberghs, P.; Brander, C.; Mattingley, J.B.; Cunnington, R. The role of the superior temporal sulcus and the mirror neuron system in imitation. Hum. Brain Mapp. 2010, 31, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Del Casale, A.; Rapinesi, C.; Kotzalidis, G.D.; De Rossi, P.; Curto, M.; Janiri, D.; Criscuolo, S.; Alessi, M.C.; Ferri, V.R.; De Giorgi, R.; et al. Executive functions in obsessive-compulsive disorder: An activation likelihood estimate meta-analysis of fMRI studies. World. J. Biol. Psychiatry 2016, 17, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Fabbri-Destro, M. Mirror neurons: From discovery to autism. Exp. Brain Res. 2010, 200, 223–237. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Z.; Xiong, X.; Cai, K.; Zhu, L.; Dong, X.; Wang, J.; Zhu, H.; Shi, Y.; Chen, A. Effects of mini-basketball training program on social communication impairment and executive control network in preschool children with autism spectrum disorder. Int. J. Environ. Res. Public Health 2021, 18, 5132. [Google Scholar] [CrossRef]
- Sharda, M.; Tuerk, C.; Chowdhury, R.; Jamey, K.; Foster, N.; Custo-Blanch, M.; Tan, M.; Nadig, A.; Hyde, K. Music improves social communication and auditory motor connectivity in children with autism. Transl. Psychiatry 2018, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Su, W.C.; Amonkar, N.; Cleffi, C.; Srinivasan, S.; Bhat, A. Neural effects of physical activity and movement interventions in individuals with developmental disabilities—A systematic review. Front. Psychiatry 2022, 13, 794652. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Bailey, A.; Lord, C. The social communication questionnaire: Manual. Los Angeles West. Psychol. Serv. 2003, 29, 30. [Google Scholar]
- Sparrow, S.S.; Cicchetti, D.V.; Balla, D.A. Vineland Adaptive Behavior Scales, 2nd ed.; NCS Pearson: San Antonio, TX, USA, 2005. [Google Scholar]
- Constantino, J.N.; Przybeck, T.; Friesen, D.; Todd, R.D. Reciprocal social behavior in children with and without pervasive developmental disorders. J. Dev. Behav. Pediatr. 2000, 21, 2–11. [Google Scholar] [CrossRef]
- Su, W.C.; Cleffi, C.; Srinivasan, S.; Bhat, A. Telehealth versus face-to-face fine motor and social communication interventions for children with Autism Spectrum Disorder: Efficacy, fidelity, acceptability, and feasibility. Am. J. Occup. Ther. 2023, 77, 7706205130. [Google Scholar] [CrossRef]
- Su, W.C.; Cleffi, C.; Srinivasan, S.; Bhat, A. A pilot study comparing the efficacy, fidelity, acceptability, and feasibility of telehealth and face-to-face creative movement interventions in children with Autism Spectrum Disorder. Telemed. Rep. 2024, 5, 67–77. [Google Scholar] [CrossRef]
- Su, W.C.; Cleffi, C.; Srinivasan, S.; Bhat, A.N. Does delivery format matter? A pilot study comparing telehealth versus face-to-face movement interventions for children with Autism Spectrum Disorder. Pediatr. Phys. Ther. 2024, 36, 518–528. [Google Scholar] [CrossRef]
- Srinivasan, S.M.; Bhat, A.N. A review of “music and movement” therapies for children with autism: Embodied interventions for multisystem development. Front. Integr. Neurosci. 2013, 7, 22. [Google Scholar] [CrossRef]
- Sun, Y.; Lamoreau, R.; O’Connell, S.; Horlick, R.; Bazzano, A.N. Yoga and mindfulness interventions for preschool-aged children in educational settings: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 6091. [Google Scholar] [CrossRef]
- Rabinovici, G.D.; Stephens, M.L.; Possin, K.L. Executive dysfunction. Continuum 2015, 21, 646–659. [Google Scholar] [CrossRef]
- Lloyd-Fox, S.; Blasi, A.; Elwell, C.E. Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy. Neurosci. Biobehav. Rev. 2010, 34, 269–284. [Google Scholar] [CrossRef]
- Tsuzuki, D.; Cai, D.S.; Dan, H.; Kyutoku, Y.; Fujita, A.; Watanabe, E.; Dan, I. Stable and convenient spatial registration of stand-alone NIRS data through anchor-based probabilistic registration. Neurosci. Res. 2012, 72, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Dan, H.; Sakamoto, K.; Takeo, K.; Shimizu, K.; Kohno, S.; Oda, I.; Isobe, S.; Suzuki, T.; Kohyama, K.; et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage 2004, 21, 99–111. [Google Scholar] [CrossRef]
- Shattuck, D.W.; Mirza, M.; Adisetiyo, V.; Hojatkashani, C.; Salamon, G.; Narr, K.L.; Poldrack, R.A.; Bilder, R.M.; Toga, A.W. Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage 2008, 39, 1064–1080. [Google Scholar] [CrossRef]
- Sutoko, S.; Sato, H.; Maki, A.; Kiguchi, M.; Hirabayashi, Y.; Atsumori, H.; Obata, A.; Funane, T.; Katura, T. Tutorial on platform for optical topography analysis tools. Neurophotonics 2016, 3, 010801. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, 280–298. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Dan, I. Exploring the false discovery rate in multichannel NIRS. NeuroImage 2006, 33, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V. Distribution theory for glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Pelphrey, K.A.; Mitchell, T.V.; McKeown, M.J.; Goldstein, J.; Allison, T.; McCarthy, G. Brain activity evoked by the perception of human walking: Controlling for meaningful coherent motion. J. Neurosci. 2003, 23, 6819–6825. [Google Scholar] [CrossRef]
- Materna, S.; Dicke, P.W.; Their, P. Dissociable roles of the superior temporal sulcus and the intraparietal sulcus in joint attention: A functional magnetic resonance imaging study. J. Cogn. Neurosci. 2008, 20, 108–119. [Google Scholar] [CrossRef]
- Floris, D.L.; Howells, H. Atypical structural and functional motor networks in autism. Prog. Brain Res. 2018, 238, 207–248. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | CM Group (n = 8) Mean ± SE | GM Group (n = 7) Mean ± SE | SP Group (n = 7) Mean ± SE | Between Group Comparison (p-Value) |
|---|---|---|---|---|
| Age | 9.50 ± 0.96 | 8.42 ± 0.95 | 8.56 ± 0.94 | F(2, 21) = 0.62, p = 0.55 |
| Sex | 6 M, 2 F | 7 M | 6 M, 1 F | X2 (2, N = 22) = 1.99, p = 0.37 |
| Race/Ethnicity | 6 C, 2 AAC | 3C, 3A, 1AA | 5 C, 2 AA | X2 (8, N = 22) = 13.92, p = 0.08 |
| Delivery Method | 5 F2F, 3 TH | 3 F2F, 4 TH | 4 F2F, 3 TH | X2 (2, N = 22) = 0.61, p = 0.74 |
| SCQ | 17.25 ± 2.18 | 12.57 ± 1.66 | 12.29 ± 1.19 | F(2, 21) = 2.56, p = 0.10 |
| VABS-II (SS) | 70.25 ± 3.59 | 77.43 ± 3.45 | 75.89 ± 4.04 | F(2, 21) = 2.25, p = 0.13 |
| VABS-II Communication | 73.50 ± 3.70 | 81.71 ± 4.77 | 82.00 ± 6.00 | F(2, 21) = 2.20, p = 0.14 |
| VABS-II Daily living | 71.75 ± 4.03 a | 80.57 ± 4.44 | 81.11 ± 3.73 | F(2, 21) = 2.68, p = 0.09 |
| VABS-II Socialization | 70.25 ± 3.59 | 80.14 ± 2.87 | 69.11 ± 6.55 | F(2, 21) = 2.53, p = 0.11 |
| SRS (T scores) | 77.88 ± 4.25 | 68.14 ± 2.84 | 73.11 ± 2.05 | F(2, 21) = 2.07, p = 0.15 |
| SCI (T scores) | 74.50 ± 4.02 | 67.14 ± 2.52 | 70.78 ± 2.98 | F(2, 21) = 1.40, p = 0.27 |
| RRB (T scores) | 79.63 ± 3.17 | 68.86 ± 4.79 | 72.00 ± 3.54 | F(2, 21) = 1.94, p = 0.171 |
| r-Values | Solo | Social | ||
|---|---|---|---|---|
| Rhythm | Rhythm | Synchrony | Mirror | |
| Left hemisphere | ||||
| MFG | −0.05 (−0.26 to 0.16) | −0.22 (−0.40 to −0.01) * | −0.29 (−0.47 to −0.09) * | −0.03 (−0.23 to 0.18) |
| IFG | −0.15 (−0.35 to 0.07) | 0.01 (−0.19 to 0.22) | −0.23 (−0.42 to −0.02) * | 0.09 (−0.12 to 0.29) |
| STS | −0.09 (−0.29 to 0.13) | −0.18 (−0.37 to 0.03) | −0.22 (−0.41 to −0.02) * | −0.03 (−0.24 to 0.18) |
| IPL | −0.25 (−0.44 to −0.04) * | −0.13 (−0.33 to 0.08) | −0.20 (−0.39 to 0.01) | −0.04 (−0.25 to 0.17) |
| Right hemisphere | ||||
| MFG | 0.02 (−0.19 to 0.23) | −0.11 (−0.31 to 0.10) | −0.13 (−0.32 to 0.08) | 0.16 (−0.05 to 0.36) |
| IFG | −0.07 (−0.27 to 0.15) | −0.02 (−0.22 to 0.19) | −0.02 (−0.23 to 0.19) | 0.06 (−0.15 to 0.26) |
| STS | −0.10 (−0.30 to 0.12) | −0.01 (−0.22 to 0.20) | −0.10 (−0.30 to 0.11) | 0.15 (−0.06 to 0.35) |
| IPL | −0.13 (−0.33 to 0.08) | −0.05 (−0.26 to 0.16) | 0.04 (−0.17 to 0.24) | 0.14 (−0.07 to 0.33) |
| r-Values | Solo | Social | ||
|---|---|---|---|---|
| Δ Rhythm | Δ Rhythm | Δ Synchrony | Δ Mirror | |
| CM Group | ||||
| VABS (SS) | 0.45 (0.12 to 0.68) * | −0.04 (−0.38 to 0.31) | −0.13 (−0.46 to 0.22) | −0.04 (−0.38 to 0.31) |
| SRS (T score) | −0.33 (−0.61 to 0.01) | 0.10 (−0.25 to 0.43) | 0.18 (−0.18 to 0.49) | −0.17 (−0.49 to 0.18) |
| GM Group | ||||
| VABS (SS) | −0.10 (−0.45 to 0.27) | −0.04 (−0.40 to 0.33) | 0.03 (−0.34 to 0.39) | −0.24 (−0.56 to 0.14) |
| SRS (T score) | −0.07 (−043 to 0.30) | 0.08 (−0.30 to 0.43) | −0.47 (−0.72 to −0.13) * | 0.03 (−0.34 to 0.39) |
| SP Group | ||||
| VABS (SS) | −0.05 (−0.41 to 0.33) | −0.23 (−0.55 to 0.16) | −0.31 (−0.61 to 0.08) | −0.12 (−0.47 to 0.27) |
| SRS (T score) | 0.42 (0.06 to 0.69) * | −0.13 (−0.48 to 0.26) | 0.14 (−0.25 to 0.49) | 0.24 (−0.15 to 0.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.-C.; Tsuzuki, D.; Srinivasan, S.; Bhat, A. Neural Effects of Creative Movement, General Movement, and Sedentary Play Interventions on Interpersonal Synchrony in Children with Autism Spectrum Disorder: A Preliminary fNIRS Study. Brain Sci. 2025, 15, 683. https://doi.org/10.3390/brainsci15070683
Su W-C, Tsuzuki D, Srinivasan S, Bhat A. Neural Effects of Creative Movement, General Movement, and Sedentary Play Interventions on Interpersonal Synchrony in Children with Autism Spectrum Disorder: A Preliminary fNIRS Study. Brain Sciences. 2025; 15(7):683. https://doi.org/10.3390/brainsci15070683
Chicago/Turabian StyleSu, Wan-Chun, Daisuke Tsuzuki, Sudha Srinivasan, and Anjana Bhat. 2025. "Neural Effects of Creative Movement, General Movement, and Sedentary Play Interventions on Interpersonal Synchrony in Children with Autism Spectrum Disorder: A Preliminary fNIRS Study" Brain Sciences 15, no. 7: 683. https://doi.org/10.3390/brainsci15070683
APA StyleSu, W.-C., Tsuzuki, D., Srinivasan, S., & Bhat, A. (2025). Neural Effects of Creative Movement, General Movement, and Sedentary Play Interventions on Interpersonal Synchrony in Children with Autism Spectrum Disorder: A Preliminary fNIRS Study. Brain Sciences, 15(7), 683. https://doi.org/10.3390/brainsci15070683







