Hypoxia’s Impact on Hippocampal Functional Connectivity: Insights from Resting-State fMRI Studies
Abstract
:1. Introduction
1.1. Background
1.2. Scope of Review
- Identify the RSNs involved in the hippocampal networks during rest.
- Explore how rs-fMRI reveals changes in hippocampal FC during hypoxia.
- Compare the effects of acute and chronic hypoxia on brain FC, examining whether they produce distinct or shared patterns of network changes.
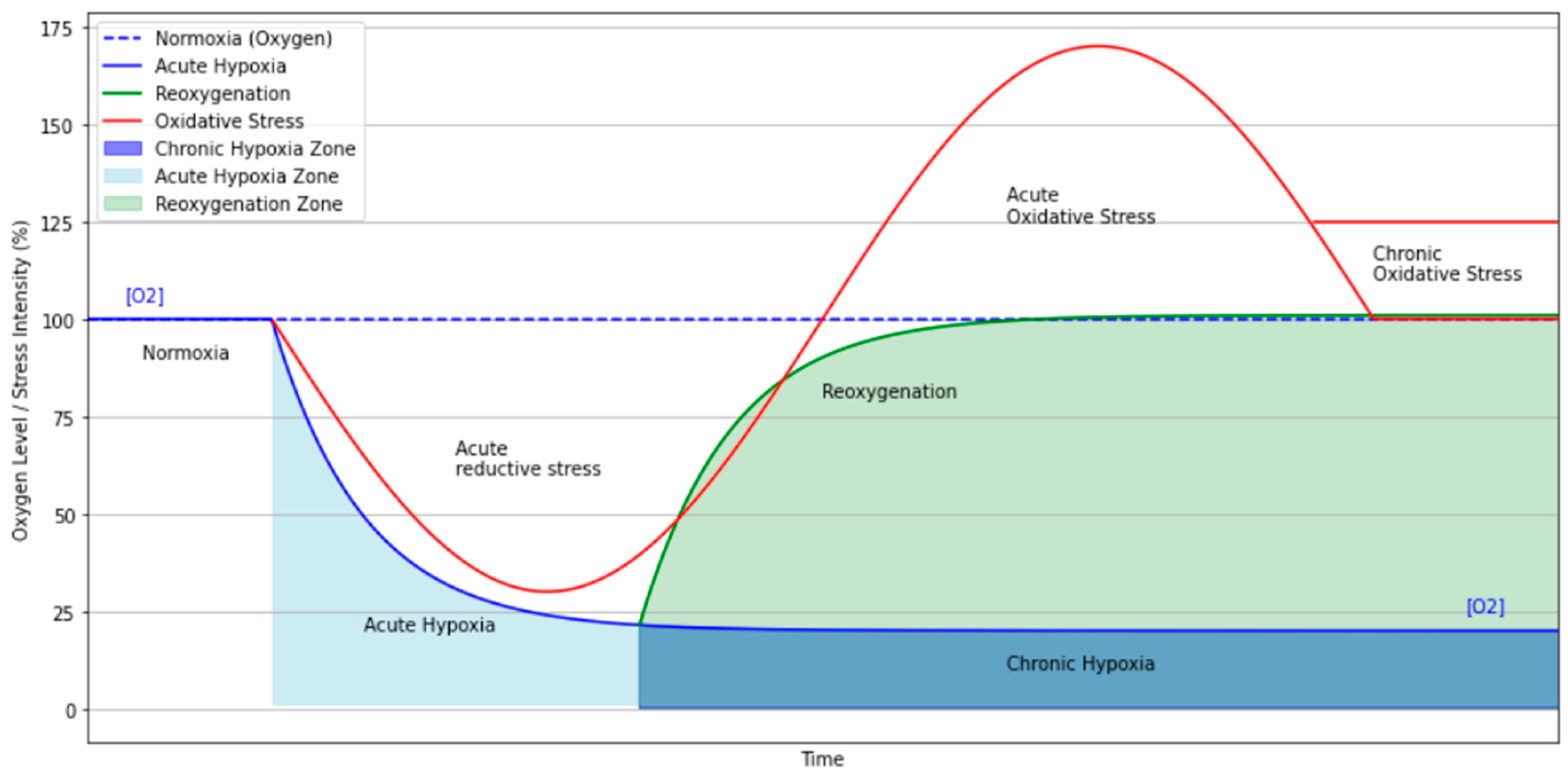
2. Physiological Basis of Hypoxia in the Context of Resting-State fMRI
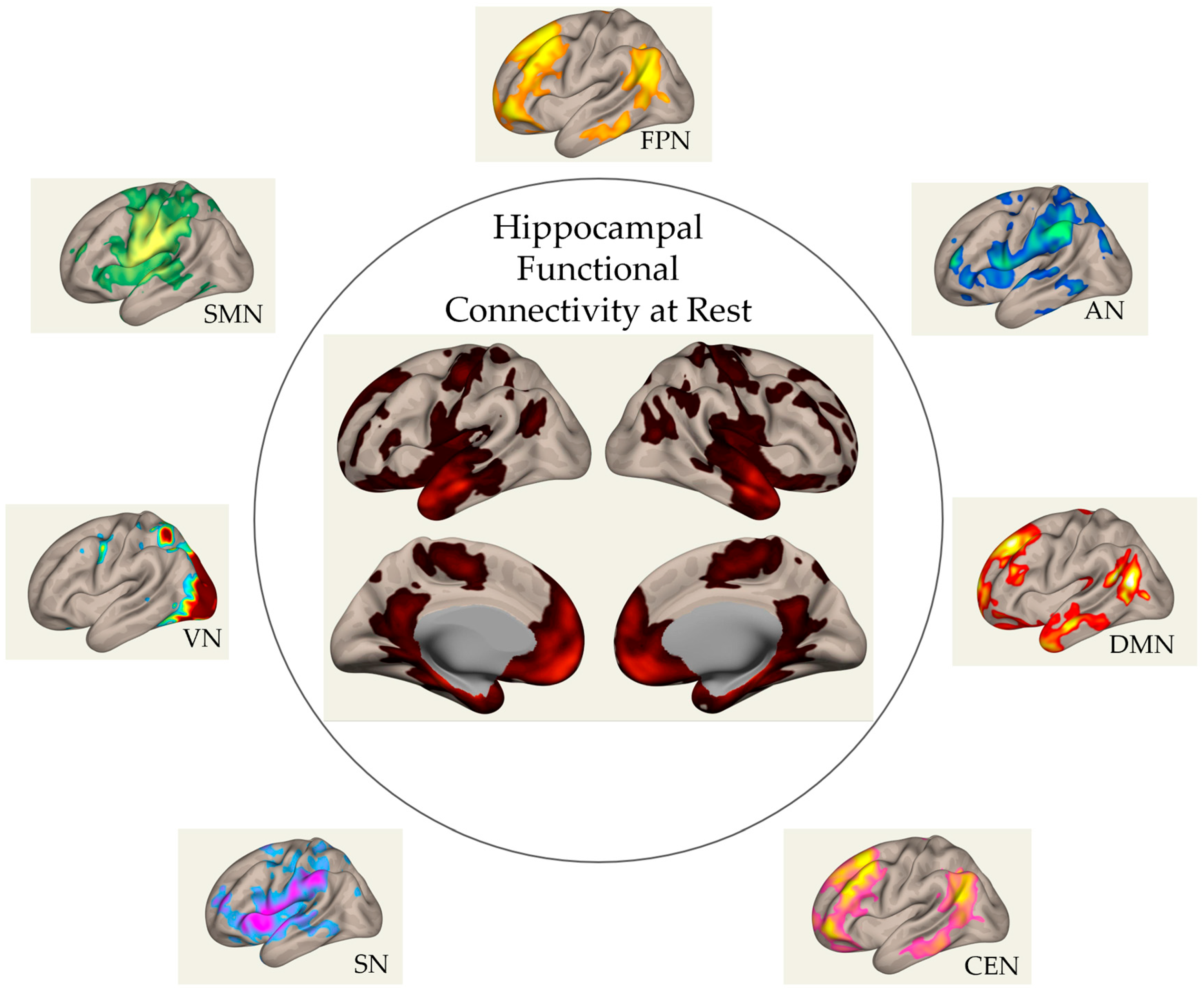
3. Hippocampal Functional Networks at Rest
4. Rs-fMRI in Acute Hypoxia
4.1. Cerebral Adaptations to Hypoxic Environments
4.2. Cerebral Adaptations to Hypoxia-Induced Pathological Conditions
5. Rs-fMRI in Chronic Hypoxia
5.1. Cerebral Adaptations to Chronic Hypoxic Environments
5.2. Cerebral Adaptations to Hypoxia Induced by Pathological Conditions
6. Acute and Chronic Hypoxia Patterns
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHI | Apnea–Hypopnea Index |
| ALFF | Amplitude of Low-Frequency Fluctuations |
| AMS | Acute Mountain Sickness |
| ASL | Arterial Spin Labeling |
| ATP | Adenosine Triphosphate |
| BH | Breath Holding |
| BOLD | Blood-Oxygen-Level Dependent |
| CEN | Central Executive Network |
| CMRO2 | Cerebral Metabolic Rate of Oxygen |
| CMS | Chronic Mountain Sickness |
| DAN | Dorsal Attention Network |
| DCM | Dynamic Causal Modeling |
| dFC | Dynamic Functional Connectivity |
| DMN | Default Mode Network |
| EPI | Echo-Planar Imaging |
| fALFF | Fractional Amplitude of Low-Frequency Fluctuation |
| FC | Functional Connectivity |
| FCD | Functional Connectivity Density |
| FPN | Fronto-Parietal Network |
| HA | High Altitude |
| HIE | Hypoxic–Ischemic Encephalopathy |
| ICA | Independent Component Analysis |
| MCI | Mild Cognitive Impairment |
| ODI | Oxygen Desaturation Index |
| OSA | Obstructive Sleep Apnea |
| PAIR | Presaturation with Inversion Recovery |
| ReHo | Regional Homogeneity |
| ROS | Reactive Oxygen Species |
| rs-fMRI | Resting-State Dunctional Magnetic Resonance Imaging |
| RSN | Resting-State Network |
| SMN | Sensory–Motor Network |
| SN | Salience Network |
| VAN | Ventral Attention Network |
| VN | Visual Network |
References
- West, J.B. Respiratory Physiology: The Essentials; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Davis, C.; Hackett, P. Advances in the prevention and treatment of High-Altitude Illness. Emerg. Med. Clin. 2017, 35, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins Basic Pathology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factors Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef]
- Wu, Y.W.; Tang, C.Y.; Ng, J.; Wong, E.; Carpenter, D.; Tao, X. Effects of hyperoxia on resting state functional magnetic resonance imaging. NeuroReport 2014, 25, 1186–1190. [Google Scholar] [CrossRef]
- Kawasaki, K.; Traynelis, S.F.; Dingledine, R. Different responses of CA1 and CA3 regions to hypoxia in rat hippocampal slice. J. Neurophysiol 1990, 63, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Hencz, A.J.; Magony, A.; Thomas, C.; Kovacs, K.; Szilagy, G.; Pal, J.; Sik, A.B. Sort-term hyperoxia-induced functional and morphological changes in rat hippocampus. Front. Cell. Neurosci. 2024, 18, 1376577. [Google Scholar] [CrossRef]
- Lana, D.; Ugolini, F.; Giovannini, M.G. An Overview on the Differential Interplay Among Neurons–Astrocytes–Microglia in CA1 and CA3 Hippocampus in Hypoxia/Ischemia. Front. Cell. Neurosci. 2020, 14, 585833. [Google Scholar] [CrossRef] [PubMed]
- McClelland, J.L. Role of the hippocampus in Learning and memory: A Computational Analysis. In Brain and Values, 1st ed.; Pribram, K.H., Ed.; Psychology Press: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Zhu, Y. Emotion Regulation of Hippocampus Using Real-Time fMRI Neurofeedback in Healthy Human. Front. Hum. Neurosci. 2019, 13, 242. [Google Scholar] [CrossRef]
- Wang, Z.-X. Changes in Hippocampus and Amygdala Volume with Hypoxic Stress Related to Cardiorespiratory Fitness under a High-Altitude Environment. Brain Sci. 2022, 12, 359. [Google Scholar] [CrossRef] [PubMed]
- Hencz, A.; Magony, A.; Thomas, C.; Kovacs, K.; Szilagyi, G.; Pal, J.; Sik, A. Mild hypoxia-induced structural and functional changes of the hippocampal network. Front. Cell. Neurosci. 2023, 17, 1277375. [Google Scholar] [CrossRef]
- Aboouf, M.A.; Thiersch, M.; Soliz, J.; Gassmann, M.; Schneider Gasser, E.M. The Brain at High Altitude: From Molecular Signaling to Cognitive Performance. Int. J. Mol. Sci. 2023, 24, 10179. [Google Scholar] [CrossRef]
- Cui, C. Cerebral Hypoxia-Induced Molecular Alterations and Their Impact on the Physiology of Neurons and Dendritic Spines: A Comprehensive Review. Cell. Mol. Neurobiol. 2024, 44, 58. [Google Scholar] [CrossRef]
- Drew, P.J. Vascular and neural basis of the BOLD signal. Curr. Opin. Neurobiol. 2019, 58, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-G.; Ogawa, S. Biophysical and Physiological Origins of Blood Oxygenation Level-Dependent fMRI Signals. J. Cereb. Blood Flow Metab. 2012, 32, 1188–1206. [Google Scholar] [CrossRef] [PubMed]
- Uludag, K. Physiology and Physics of the fMRI Signal. In fMRI: From Nuclear Spins to Brain Functions; Uludag, K., Ugurbil, K., Berliner, L., Eds.; Biological Magnetic Resonance; Springer: Boston, MA, USA, 2015. [Google Scholar] [CrossRef]
- Bandettini, P.A. Twenty years of functional MRI: The science and the stories. NeuroImage 2012, 62, 575–588. [Google Scholar] [CrossRef]
- Yousaf, T.; Dervenoulas, G.; Politis, M. Chapter Two—Advances in MRI Methodology. In Imaging in Movement Disorders: Imaging Methodology and Applications in Parkinson’s Disease; Politis, M., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 141, pp. 31–76. [Google Scholar] [CrossRef]
- Bijsterbosch, J.; Smith, S.M.; Beckmann, C. An Introduction to Resting State FMRI Functional Connectivity; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Lv, H. Resting-State Functional MRI: Everything That Nonexperts Have Always Wanted to Know. Am. J. Neuroradiol. 2018, 39, 1390–1399. [Google Scholar] [CrossRef]
- Liu, J. Impaired brain networks functional connectivity after acute mild hypoxia. Medicine 2022, 101, e30485. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Zuo, X.-N.; Xing, X.-X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: A systems neuroscience perspective. Neurosci. Biobehav. Rev. 2014, 45, 100–118. [Google Scholar] [CrossRef]
- Smitha, K.A.; Akhil Raja, K.; Arun, K.M.; Rajesh, P.G.; Thomas, B.; Kapilamoorthy, T.R.; Kesavadas, C. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol. J. 2017, 30, 305–317. [Google Scholar] [CrossRef]
- Lurie, D.J.; Kessler, D.; Basset, D.S.; Betzel, R.F.; Breakspear, M.; Kheilholz, S.; Kucyi, A.; Liégeois, R.; Lindquist, M.A.; McIntosh, A.R.; et al. Questions and controversies in the study of time-varying functional connectivity in resting fMRI. Netw. Neurosci. 2020, 4, 30–69. [Google Scholar] [CrossRef]
- Lee, T.-W. Independent Component Analysis. In Independent Component Analysis: Theory and Applications; Lee, T.-W., Ed.; Springer: Boston, MA, USA, 1998; pp. 27–66. [Google Scholar] [CrossRef]
- Yang, H. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. NeuroImage 2007, 36, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, D.; Volkow, N.D. Functional connectivity density mapping. Proc. Natl. Acad. Sci. USA 2010, 107, 9885–9890. [Google Scholar] [CrossRef]
- Friston, K.J.; Kahan, J.; Biswal, B.; Razi, A. A DCM for resting state fMRI. NeuroImage 2014, 94, 396–407. [Google Scholar] [CrossRef]
- Medaglia, J.D. Graph Theoretic Analysis of Resting Stae Functional MR Imaging. Neuroimaging Clin. N. Am. 2017, 27, 593–607. [Google Scholar] [CrossRef]
- Foltyn, J.; Ploszczyca, K.; Czuba, M.; Niemaszyk, A.; Langfort, J.; Gajda, R. Effects of Normobaric Hypoxia of Varying Severity on Metabolic and Hormonal Responses Following Resistance Exercise in Men and Women. J. Clin. Med. 2025, 14, 1514. [Google Scholar] [CrossRef] [PubMed]
- Zani, A.; Dishi, Y.; Proverbio, A.M. From oxygen shortage to neurocognitive challenges: Behavioral patterns and imaging insights. Front. Cogn. 2024, 3, 1468306. [Google Scholar] [CrossRef]
- Dmytriv, T.R.; Duve, K.V.; Storey, K.B.; Lushchak, V.I. Vicious cycle of oxidative stress and neuroinflammation in pathophysiology of chronic vascular encephalopathy. Front. Physiol. 2024, 15, 1443604. [Google Scholar] [CrossRef] [PubMed]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef]
- Turner, C.E.; Barker-Collo, S.L.; Connell, C.J.W.; Gant, N. Acute hypoxic gas breathing severely impairs cognition and task learning in humans. Physiol. Behav. 2015, 142, 104–110. [Google Scholar] [CrossRef]
- Damgaard, V.; Mariegaard, J.; Lindhardsen, J.M.; Ehrenreich, H.; Miskowiak, K.W. Neuroprotective Effects of Moderate Hypoxia: A Systematic Review. Brain Sci. 2023, 13, 1648. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J.; Barwood, M.; Costello, J.; Corbett, J. Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci. Biobehav. Rev. 2017, 74, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Young, J.M.; Williams, D.R.; Thompson, A.A.R. Thin Air, Thick Vessels: Historical and Current Perspectives on Hypoxic Pulmonary Hypertension. Front. Med. 2019, 6, 93. [Google Scholar] [CrossRef]
- Chen, X. Combined fractional anisotropy and subcortical volumetric abnormalities in healthy immigrants to high altitude: A longitudinal study. Hum. Brain Mapp. 2019, 40, 4202–4212. [Google Scholar] [CrossRef] [PubMed]
- Li, G. Chronic hypoxia leads to cognitive impairment by promoting HIF-2α-mediated ceramide catabolism and alpha-synuclein hyperphosphorylation. Cell Death Discov. 2022, 8, 473. [Google Scholar] [CrossRef]
- Lei, L. HIF-1α Causes LCMT1/PP2A Deficiency and Mediates Tau Hyperphosphorylation and Cognitive Dysfunction during Chronic Hypoxia. Int. J. Mol. Sci. 2022, 23, 16140. [Google Scholar] [CrossRef]
- Allsopp, G.L.; Addinsall, A.B.; Hoffmann, S.M.; Russell, A.P.; Wright, C.R. Hormonal and metabolic responses of older adults to resistance training in normobaric hypoxia. Eur. J. Appl. Physiol. 2022, 122, 1007–1017. [Google Scholar] [CrossRef]
- Seitzman, B.A.; Snyder, A.Z.; Leuthardt, E.C.; Shimony, J.S. The State of Resting State Networks. Top. Magn. Reson. Imaging 2019, 28, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Menon, V. 20 years of the default mode network: A review and synthesis. Neuron 2023, 111, 2469–2487. [Google Scholar] [CrossRef]
- Smallwood, J.; Bernhardt, B.C.; Leech, R.; Bzdok, D.; Jefferies, E.; Margulies, D.S. The default mode network in congnition: A topographical perspective. Nat. Rev. Neurosci. 2021, 22, 503–513. [Google Scholar] [CrossRef]
- Hughes, C.; Setton, R.; Mwilambwe-Tshilobo, L.; Baracchini, G.; Tuner, G.R.; Spreng, N. Precision mapping of the default mode network reveals common and distinct (inter) activity for autobiographical memory and theory mind. J. Neurophysiol. 2024, 132, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Ezama, L.; Hernández-Cabrera, J.A.; Seoane, S.; Pereda, E.; Janssen, N. Functional connectivity of the hippocampus and its subfields in resting-state networks. Eur. J. Neurosci. 2021, 53, 3378–3393. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.J.; Reilly, W.; Dimsdale-Zucker, H.R.; Mizrak, E.; Reagh, Z.; Ranganath, C. Intrinsic connectivity reveals functionally distinct cortico-hippocampal networks in the human brain. PLoS Biol. 2021, 19, e3001275. [Google Scholar] [CrossRef]
- Danieli, K.; Guyon, A.; Bethus, I. Episodic Memory formation: A review of complex Hippocampus input pathways. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 126, 110–757. [Google Scholar] [CrossRef]
- Gordon, E.M.; Laumann, T.O.; Gilmore, A.W.; Newbold, D.J.; Greene, D.J.; Berg, J.J.; Ortega, M.; Hoyt-Drazen, C.; Gratton, C.; Sun, H.; et al. Precision Functional Mapping of Individual Human Brains. Neuron 2017, 95, 791–807. [Google Scholar] [CrossRef]
- Uddin, L.Q. Salience Network of the Human Brain; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Andreano, J.M.; Touroutoglou, A.; Dickerson, B.C.; Barrett, L.F. Resting connectivity between salience nodes predicts recognition memory. Soc. Cogn. Affect. Neurosci. 2017, 12, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zou, Q.; He, Y.; Yang, Y. Topologically Reorganized Connectivity Architecture of Default-Mode, Executive-Control, and Salience Networks across Working Memory Task Loads. Cereb. Cortex 2016, 26, 1501–1511. [Google Scholar] [CrossRef]
- Fang, X. Resting-State Coupling between Core Regions within the Central-Executive and Salience Networks Contributes to Working Memory Performance. Front. Behav. Neurosci. 2016, 10, 27. [Google Scholar] [CrossRef]
- Machner, B. Resting-State Functional Connectivity in the Dorsal Attention Network Relates to Behavioral Performance in Spatial Attention Tasks and May Show Task-Related Adaptation. Front. Hum. Neurosci. 2022, 15, 757128. [Google Scholar] [CrossRef]
- Bernard, F.; Lemee, J.-M.; Mazerand, E.; Leiber, L.-M.; Menei, P.; Ter Minassian, A. The ventral attention network: The mirror of the language network in the right brain hemisphere. J. Anat. 2020, 237, 632–642. [Google Scholar] [CrossRef]
- Vossel, S.; Geng, J.J.; Fink, G.R. Dorsal and Ventral Attention Systems: Distinct Neural Circuits but Collaborative Roles. Neuroscientist 2014, 20, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Dunne, L.; Opitz, B. Attention control processes that prioritise task execution may come at the expense of incidental memory encoding. Brain Cogn. 2020, 144, 105–602. [Google Scholar] [CrossRef] [PubMed]
- Aly, M.; Turk-Browne, N.B. Attention Stabilizes Representations in the Human Hippocampus. Cereb. Cortex 2016, 26, 783–796. [Google Scholar] [CrossRef]
- van Ede, F.; Board, A.G.; Nobre, A.C. Goal-directed and stimulus-driven selection of internal representations. Proc. Natl. Acad. Sci. USA 2020, 117, 24590–24598. [Google Scholar] [CrossRef]
- Lee, M.H.; Smyser, C.D.; Shimony, J.S. Resting-State fMRI: A Review of Methods and Clinical Applications. Am. J. Neuroradiol. 2013, 34, 1866–1872. [Google Scholar] [CrossRef]
- Kassab, R.; Alexandre, F. Pattern separation in the hippocampus: Distinct circuits under different conditions. Brain Struct. Funct. 2018, 223, 2785–2805. [Google Scholar] [CrossRef]
- Pena, E.; El Alam, S.; Siques, P.; Brito, J. Oxidative Stress and Diseases Associated with High-Altitude Exposure. Antioxidants 2022, 11, 267. [Google Scholar] [CrossRef]
- Ramírez-delaCruz, M.; Bravo-Sánchez, A.; Sánchez-Infante, J.; Abián, P.; Abián-Vicén, J. Effects of Acute Hypoxic Exposure in Simulated Altitude in Healthy Adults on Cognitive Performance: A Systematic Review and Meta-Analysis. Biology 2024, 13, 835. [Google Scholar] [CrossRef] [PubMed]
- Davranche, K.; Casini, L.; Arnal, P.J.; Rupp, T.; Perrey, S.; Verges, S. Cognitive functions and cerebral oxygenation changes during acute and prolonged hypoxic exposure. Physiol. Behav. 2016, 164, 189–197. [Google Scholar] [CrossRef]
- Critchley, H.D. Slow Breathing and Hypoxic Challenge: Cardiorespiratory Consequences and Their Central Neural Substrates. PLoS ONE 2015, 10, e0127082. [Google Scholar] [CrossRef]
- ADAMSON, M.M. Pilot Expertise and Hippocampal Size: Associations with Longitudinal Flight Simulator Performance. Aviat. Space Environ. Med. 2012, 83, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Altered Default Mode Network Dynamics in Civil Aviation Pilots. Front. Neurosci. 2020, 13, 1406. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, G.; Li, M.; Yin, M.; Chen, H. Neuron loss and dysfunctionality in hippocampus explain aircraft noise induced working memory impairment: A resting-state fMRI study on military pilots. Biosci. Trends 2019, 13, 430–440. [Google Scholar] [CrossRef]
- Liu, J. Changes in resting-state brain function of pilots after hypoxic exposure based on methods for fALFF and ReHo analysis. Med. J. Chin. Peoples Lib. Army 2015, 40, 507–512. [Google Scholar]
- Xu, K. Research on brain functions related to visual information processing and body coordination function of pilots based on the low-frequency amplitude method. Front. Hum. Neurosci. 2023, 17, 796526. [Google Scholar] [CrossRef] [PubMed]
- Annen, J. Mapping the functional brain state of a world champion freediver in static dry apnea. Brain Struct. Funct. 2021, 226, 2675–2688. [Google Scholar] [CrossRef]
- Song, R.; Tao, G.; Guo, F.; Ma, H.; Zhang, J.; Wang, Y. The change of attention network functions and physiological adaptation during high-altitude hypoxia and reoxygenation. Physiol. Behav. 2023, 268, 114240. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.W. Effects of cognitive training under hypoxia on cognitive proficiency and neuroplasticity in remitted patients with mood disorders and healthy individuals: ALTIBRAIN study protocol for a randomized controlled trial. Trials 2024, 25, 648. [Google Scholar] [CrossRef]
- Zhang, G. Intermittent hypoxia training effectively protects against cognitive decline caused by acute hypoxia exposure. Pflüg. Arch.—Eur. J. Physiol. 2024, 476, 197–210. [Google Scholar] [CrossRef]
- Wang, L.; Sang, L.Q.; Cui, Y.; Li, P.Y.; Qiao, L.; Wang, Q.N.; Zhao, W.Q.; Hu, Q.; Zhang, N.J.; Zhang, Y.; et al. Effects of acute high-altitude exposure on working memory: A functional near-infrared spectroscopy study. Brain Behav. 2022, 12, e2776. [Google Scholar] [CrossRef]
- Zhang, X. Neuroplasticity of visual brain network induced by hypoxia. Cereb. Cortex 2024, 34, bhae198. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, P.; Kumari, P.; Sharma, N.K. Introduction to High Altitude and Hypoxia. In High Altitude Sickness—Solutions from Genomics, Proteomics and Antioxidant Interventions; Sharma, N.K., Arya, A., Eds.; Springer Nature: Singapore, 2022; pp. 1–17. [Google Scholar] [CrossRef]
- Zhang, W. Investigating Sea-Level Brain Predictors for Acute Mountain Sickness: A Multimodal MRI Study before and after High-Altitude Exposure. Am. J. Neuroradiol. 2024, 45, 809–818. [Google Scholar] [CrossRef]
- Limmer, M.; Platen, P. The influence of hypoxia and prolonged exercise on attentional performance at high and extreme altitudes: A pilot study. PLoS ONE 2018, 13, 0205285. [Google Scholar] [CrossRef]
- Li, H.-X. Resting-state network complexity and magnitude changes in neonates with severe hypoxic ischemic encephalopathy. Neural Regen. Res. 2019, 14, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Changes of Functional Brain Network in Neonates with Different Degrees of Hypoxic-Ischemic Encephalopathy. Brain Connect. 2023, 13, 427–435. [Google Scholar] [CrossRef]
- Jiang, L. Alterations in motor functional connectivity in Neonatal Hypoxic Ischemic Encephalopathy. Brain Inj. 2022, 36, 287–294. [Google Scholar] [CrossRef]
- Boerwinkle, V.L. Association of network connectivity via resting state functional MRI with consciousness, mortality, and outcomes in neonatal acute brain injury. NeuroImage Clin. 2022, 34, 102962. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.P.C.; Goodfellow, M.; Chakkarapani, E.; Brooks, J.C.W. Resting-state functional connectivity in children cooled for neonatal encephalopathy. Brain Commun. 2024, 6, fcae154. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-B. Neuroplasticity for spontaneous functional recovery after neonatal hypoxic ischemic brain injury in rats observed by functional MRI and diffusion tensor imaging. NeuroImage 2016, 126, 140–150. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Y.; Liu, P.-F.; Yue, Y.-L.; Guo, J.-S.; Wang, Z.-C. Abnormal brain activity in rats with sustained hypobaric hypoxia exposure: A resting-state functional magnetic resonance imaging study. Chin. Med. J. 2019, 132, 2621–2627. [Google Scholar] [CrossRef]
- Miller, J. Lateralized hippocampal oscillations underlie distinct aspects of human spatial memory and navigation. Nat. Commun. 2018, 9, 2423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Consistent differences in brain structure and functional connectivity in high-altitude native Tibetans and immigrants. Brain Imaging Behav. 2023, 17, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Hippocampal adaptation to high altitude: A neuroanatomic profile of hippocampal subfields in Tibetans and acclimatized Han Chinese residents. Front. Neuroanat. 2022, 16, 999033. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y. Effects of Long-Term Exposure to High Altitude Hypoxia on Cognitive Function and Its Mechanism: A Narrative Review. Brain Sci. 2022, 12, 808. [Google Scholar] [CrossRef]
- Su, R. The effects of long-term high-altitude exposure on cognition: A meta-analysis. Neurosci. Biobehav. Rev. 2024, 161, 105682. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, J.; Gong, Q.; Weng, X. Prolonged high-altitude residence impacts verbal working memory: An fMRI study. Exp. Brain Res. 2011, 208, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z. Alteration in topological properties of brain functional network after 2-year high altitude exposure: A panel study. Brain Behav. 2020, 10, 01656. [Google Scholar] [CrossRef]
- Chen, X. Cognitive and neuroimaging changes in healthy immigrants upon relocation to a high altitude: A panel study. Hum. Brain Mapp. 2017, 38, 3865–3877. [Google Scholar] [CrossRef]
- Lehn, H.; Steffenach, H.-A.; van Strien, N.M.; Veltman, D.J.; Witter, M.P.; Håberg, A.K. A Specific Role of the Human Hippocampus in Recall of Temporal Sequences. J. Neurosci. 2009, 29, 3475–3484. [Google Scholar] [CrossRef]
- Kumaran, D.; Maguire, E.A. Match–Mismatch Processes Underlie Human Hippocampal Responses to Associative Novelty. J. Neurosci. 2007, 27, 8517–8524. [Google Scholar] [CrossRef]
- Maguire, E.A.; Frith, C.D. Lateral Asymmetry in the Hippocampal Response to the Remoteness of Autobiographical Memories. J. Neurosci. 2003, 23, 5302–5307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Resting-State Neuronal Activity and Functional Connectivity Changes in the Visual Cortex after High Altitude Exposure: A Longitudinal Study. Brain Sci. 2022, 12, 724. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Altered resting-state networks may explain the executive impairment in young health immigrants into high-altitude area. Brain Imaging Behav. 2021, 15, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Zhang, W.; Liu, J.; Ji, W. Effects of Chronic Hypoxic Environment on Cognitive Function and Neuroimaging Measures in a High-Altitude Population. Front. Aging Neurosci. 2022, 14, 788322. [Google Scholar] [CrossRef]
- Zhang, J. Adaptive Modulation of Adult Brain Gray and White Matter to High Altitude: Structural MRI Studies. PLoS ONE 2013, 8, 68621. [Google Scholar] [CrossRef]
- Zhang, J. Alteration of Spontaneous Brain Activity After Hypoxia-Reoxygenation: A Resting-State fMRI Study. High Alt. Med. Biol. 2017, 18, 20–26. [Google Scholar] [CrossRef]
- Zhong, M.; Zeng, H.; Wang, D.; Li, J.; Duan, X.; Li, Y. Structure and activity alteration in adult highland residents’ cerebrum: Voxel-based morphometry and amplitude of low-frequency fluctuation study. Front. Neurosci. 2022, 16, 1035308. [Google Scholar] [CrossRef]
- Zhang, X. Brain Structural and Functional Alterations in Native Tibetans Living at High Altitude. Neuroscience 2023, 520, 134–143. [Google Scholar] [CrossRef]
- Chen, J. Increased Intraregional Synchronized Neural Activity in Adult Brain after Prolonged Adaptation to High-Altitude Hypoxia: A Resting-State fMRI Study. High Alt. Med. Biol. 2016, 17, 16–24. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, S.; Ge, R.-L.; Bao, H.; Mou, Y.; Ji, W. Brain-aging related protein expression and imaging characteristics of mice exposed to chronic hypoxia at high altitude. Front. Aging Neurosci. 2023, 15, 1268230. [Google Scholar] [CrossRef]
- Cramer, N.P. Neuronal and vascular deficits following chronic adaptation to high altitude. Exp. Neurol. 2019, 311, 293–304. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.-X.; Huang, S.; Hu, Y.-H.; Wang, H.; Chen, X. Effects of long-term exposure to high altitude on brain structure in healthy people: An MRI-based systematic review and meta-analysis. Front. Psychiatry. 2023, 14, 1196113. [Google Scholar] [CrossRef] [PubMed]
- Veasey, S.C.; Rosen, I.M. Obstructive Sleep Apnea in Adults. N. Engl. J. Med. 2019, 380, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Bao, J. Elucidating the association of obstructive sleep apnea with brain structure and cognitive performance. BMC Psychiatry 2024, 24, 338. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T. Functional connectivity in default mode network correlates with severity of hypoxemia in obstructive sleep apnea. Brain Behav. 2020, 10, 01889. [Google Scholar] [CrossRef]
- Kang, D. Brain functional changes in tibetan with obstructive sleep apnea hypopnea syndrome: A resting state fMRI study. Medicine 2020, 99, 18957. [Google Scholar] [CrossRef]
- Qin, Z.; Kang, D.; Feng, X.; Kong, D.; Wang, F.; Bao, H. Resting-state functional magnetic resonance imaging of high altitude patients with obstructive sleep apnoea hypopnoea syndrome. Sci. Rep. 2020, 10, 15546. [Google Scholar] [CrossRef]
- Tung, A.; Takase, L.; Fornal, C.; Jacobs, B. Effects of sleep deprivation and recovery sleep upon cell proliferation in adult rat dentate gyrus. Neuroscience 2005, 134, 721–723. [Google Scholar] [CrossRef]
- Li, K. Dynamic regional homogeneity alterations and cognitive impairment in patients with moderate and severe obstructive sleep apnea. Front. Neurosci. 2022, 16, 940721. [Google Scholar] [CrossRef]
- Huang, L. Abnormal dynamic functional connectivity in the hippocampal subregions of patients with untreated moderate-to-severe obstructive sleep apnea. Sleep Med. 2023, 112, 273–281. [Google Scholar] [CrossRef]
- He, Y.; Shen, J.; Wang, X.; Wu, Q.; Liu, J.; Ji, Y. Preliminary study on brain resting-state networks and cognitive impairments of patients with obstructive sleep apnea–hypopnea syndrome. BMC Neurol. 2022, 22, 456. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Cha, J.; Joo, E.Y.; Kim, H. Altered cerebrocerebellar functional connectivity in patients with obstructive sleep apnea and its association with cognitive function. Sleep 2022, 45, zsab209. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y. Inherent regional brain activity changes in male obstructive sleep apnea with mild cognitive impairment: A resting-state magnetic resonance study. Front. Aging Neurosci. 2022, 14, 1022628. [Google Scholar] [CrossRef]
- Chen, L.-T. Disrupted small-world brain functional network topology in male patients with severe obstructive sleep apnea revealed by resting-state fMRI. Neuropsychiatr. Dis. Treat. 2017, 13, 1471–1482. [Google Scholar] [CrossRef]
- Peng, D.-C.; Dai, X.-J.; Gong, H.-H.; Li, H.-J.; Nie, X.; Zhang, W. Altered intrinsic regional brain activity in male patients with severe obstructive sleep apnea: A resting-state functional magnetic resonance imaging study. Neuropsychiatr. Dis. Treat. 2014, 10, 1819–1829. [Google Scholar]
- Naismith, S.L. Nocturnal Hypoxemia Is Associated with Altered Parahippocampal Functional Brain Connectivity in Older Adults at Risk for Dementia. J. Alzheimer’s Dis. 2020, 73, 571–584. [Google Scholar] [CrossRef]
- Bai, J. Altered Spontaneous Brain Activity Related to Neurologic and Sleep Dysfunction in Children With Obstructive Sleep Apnea Syndrome. Front. Neurosci. 2021, 15, 595412. [Google Scholar] [CrossRef]
- Bao, H.; He, X.; Wang, F.; Kang, D. Study of Brain Structure and Function in Chronic Mountain Sickness Based on fMRI. Front. Neurol. 2021, 12, 763835. [Google Scholar] [CrossRef] [PubMed]
- Davenport, P.W.; Vovk, A. Cortical and subcortical central neural pathways in respiratory sensations. Respir. Physiol. Neurobiol. 2009, 167, 72–86. [Google Scholar] [CrossRef]
- Harper, R.M. fMRI responses to cold pressor challenges in control and obstructive sleep apnea subjects. J. Appl. Physiol. 2003, 94, 1583–1595. [Google Scholar] [CrossRef]
- Macey, K.E. fMRI signal changes in response to forced expiratory loading in congenital central hypoventilation syndrome. J. Appl. Physiol. 2004, 97, 1897–1907. [Google Scholar] [CrossRef]
- Guan, L.; Ge, R.; Ma, S. Impact of hypoxia on the hippocampus: A review. Medicine 2025, 104, e41479. [Google Scholar] [CrossRef]
- Yu, J.J.; Non, A.L.; Heinrich, E.C.; Gu, W.; Alcock, J.; Moya, E.A.; Lawrence, E.S.; Tift, M.S.; O’Brien, K.A.; Storz, J.F.; et al. Time Domains of Hypoxia Responses and -Omics Insights. Front. Physiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.S.; Holmes, P.A.; Koutroumanidis, M.A. Hypoxic-ischaemic brain injury. Pract. Neurol. 2011, 11, 4–18. [Google Scholar] [CrossRef]
- Kolisnyk, M. Predicting neurologic recovery after severe acute brain injury using resting-state networks. J. Neurol. 2023, 270, 6071–6080. [Google Scholar] [CrossRef] [PubMed]
- Cole, D.M.; Smith, S.M.; Beckmann, C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010, 4, 1459. [Google Scholar] [CrossRef]
- Duncan, N.W.; Northoff, G. Overview of potential procedural and participant-related confounds for neuroimaging of the resting state. J. Psychiatry Neurosci. 2013, 38, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Le, L.N.N. Cortical oxygen extraction fraction using quantitative BOLD MRI and cerebral blood flow during vasodilation. Front. Physiol. 2023, 14, 1231793. [Google Scholar] [CrossRef]
- Deckers, P.T. Hemodynamic and metabolic changes during hypercapnia with normoxia and hyperoxia using pCASL and TRUST MRI in healthy adults. J. Cereb. Blood Flow Metab. 2022, 42, 861–875. [Google Scholar] [CrossRef]
- Chen, J.J.; Gauthier, C.J. The Role of Cerebrovascular-Reactivity Mapping in Functional MRI: Calibrated fMRI and Resting-State fMRI. Front. Physiol. 2021, 12, 657362. [Google Scholar] [CrossRef]
- Kurban, D.; Ivanov, D.; Kashyap, S.; Huber, L.; Liberman, G.; Poser, B.A. Concurrent CBF and BOLD FMRI with dual-echo spiral simultaneous multi-slice acquisitions at 7T. NeuroImage 2022, 247, 118820. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Nencka, A.S.; Lebel, R.M.; et Wang, Y. Multiband multi-echo imaging of simultaneous oxygenation and flow timeseries for resting state connectivity. PLoS ONE 2017, 12, 0169253. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Nencka, A.S.; Wang, Y. Multiband multi-echo simultaneous ASL/BOLD for task-induced functional MRI. PLoS ONE 2018, 13, 0190427. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; In, M.H.; Jo, H.J.; Halverson, M.; Meyer, N.K. Improved Resting-State Functional MRI Using Multi-Echo Echo-Planar Imaging on a Compact 3T MRI Scanner with High-Performance Gradients. Sensors 2023, 23, 4329. [Google Scholar] [CrossRef]



| Hypoxia’s Type | Key Findings | Affected Networks |
|---|---|---|
| Acute Hypoxia (e.g., High Altitude, Aviation) | Decreased FC between the hippocampus and prefrontal cortex/DMN: impaired attention and memory. | DMN, FPN, DAN, VAN, Visual, Prefrontal |
| Disrupted FC in DAN, VAN, and FPN: longer reaction times. | ||
| Controlled breathing improves cerebral oxygenation. | ||
| Aviation-related hypoxia alters hippocampal volume and DMN connectivity. | ||
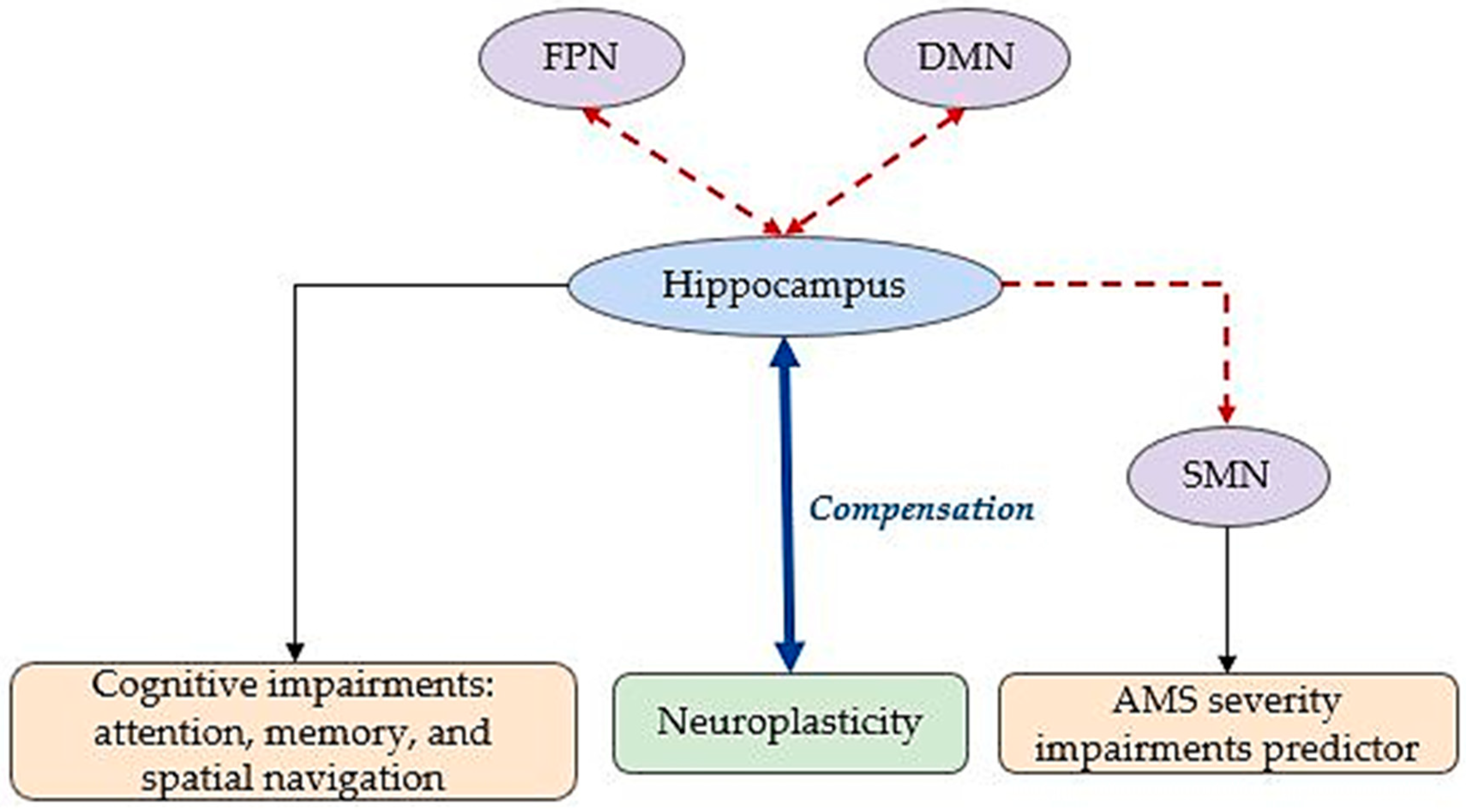
| Pathological Acute Hypoxia (e.g., HIE, AMS) | FC impairments in DMN, SMN, and FPN, with SMN as a key predictor of AMS severity. | DMN, SMN, FPN, Motor, Temporal |
| HIE shows decreased local efficiency and hippocampal connectivity. | ||
| Compensatory increases in FC among motor, frontal, and parietal areas: neuroplasticity observed in recovery cases. | ||
| Chronic Environmental Hypoxia (e.g., High Altitude) | Reduced hippocampal FC with memory networks: cognitive decline in memory and spatial navigation. | DMN, FPN, SMN, Visual, Memory Networks |
| FC disruptions in visual network and SMN: adaptation varies across populations. | ||
| Animal models show hippocampal damage and mitochondrial impairment. | ||
| Chronic Pathological Hypoxia (e.g., OSA, CMS) | Altered FC in DMN, DAN, VAN, and SN; hippocampal dysfunction linked to intermittent hypoxia and sleep fragmentation: cognitive impairments in attention, memory, and executive functions. | DMN, VAN, DAN, SN, Frontal, Para-Hippocampal Gyrus |
| Topological disruptions in network efficiency. | ||
| Para-hippocampal activity changes in CMS; observed effects in adults, children, and animal models. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micaux, J.; Troudi Habibi, A.; Mauconduit, F.; Noulhiane, M. Hypoxia’s Impact on Hippocampal Functional Connectivity: Insights from Resting-State fMRI Studies. Brain Sci. 2025, 15, 643. https://doi.org/10.3390/brainsci15060643
Micaux J, Troudi Habibi A, Mauconduit F, Noulhiane M. Hypoxia’s Impact on Hippocampal Functional Connectivity: Insights from Resting-State fMRI Studies. Brain Sciences. 2025; 15(6):643. https://doi.org/10.3390/brainsci15060643
Chicago/Turabian StyleMicaux, Julia, Abir Troudi Habibi, Franck Mauconduit, and Marion Noulhiane. 2025. "Hypoxia’s Impact on Hippocampal Functional Connectivity: Insights from Resting-State fMRI Studies" Brain Sciences 15, no. 6: 643. https://doi.org/10.3390/brainsci15060643
APA StyleMicaux, J., Troudi Habibi, A., Mauconduit, F., & Noulhiane, M. (2025). Hypoxia’s Impact on Hippocampal Functional Connectivity: Insights from Resting-State fMRI Studies. Brain Sciences, 15(6), 643. https://doi.org/10.3390/brainsci15060643







