Abstract
Aims: Cue exposure therapy (CET) is a promising treatment approach for cocaine substance use disorder (SUD). CET specifically targets the psychological and physiological responses elicited by drug-related cues, aiming to reduce their motivational impact. To advance understanding of CET for cocaine treatment, this systematic review aims to categorise the range of cocaine cues used in research. Methods: A systematic review of the existing literature with searches conducted on PubMed and Web of Science bibliographic databases with no time constraints in August 2024 (PROSPERO: CRD42024554361). Three reviewers were independently involved in the screening, review and data extraction process, in line with PRISMA guidelines. Data extracted included participant demographics, study design, data on the cocaine cue task, and examples (if provided). Each study was appraised and received a quality score. The secondary outcome was to summarise examples for each category type identified. The data are presented as a narrative synthesis. Results: 3600 articles were identified and screened. 235 articles were included in the analysis. Cues identified included images, paraphernalia, drug-related words, cocaine smell, auditory stimuli presented via audiotapes, video recordings, scripts, and virtual reality environments, often combining multiple modalities. Included studies recruited cocaine-dependent individuals, recreational users, polydrug users, and non-cocaine-using controls. The sample sizes of the studies ranged from a single case study to a study including 1974 participants. Conclusions: This review found that studies employed a wide range of cue categories, but detailed examples were often lacking, limiting replication. The number and combination of cues varied: some studies used only cocaine-related images, while others included images, videos, physical items, and audiotapes. The level of immersion and personalisation also differed considerably. All studies used cocaine-specific cues, most commonly images or representations of cocaine substance, cocaine use or drug paraphernalia, drug preparation items, or conversations of cocaine use and its effects. The overall quality of the included studies was deemed good, with all adhering to standard research norms. While this review highlights the breath of cue types used in the literature, further research should focus on enhancing cue exposure techniques by incorporating more immersive and personalised stimuli, and by providing clearer documentation of cue characteristics to support replication and clinical translation.
1. Background
Cocaine dependence is complex and difficult to treat, with cocaine use and related deaths in the UK, and globally, rising from previous years [1,2,3] and the global supply of cocaine at record levels [4]. There were 1118 deaths involving cocaine registered in 2023, which is 30.5% higher than in 2022 [5]. Current treatment for cocaine dependence emphasises psychological interventions such as cognitive-behavioural therapy (CBT), contingency management (CM), motivational interviewing (MI), and peer-support approaches to address behaviour change and prevent relapse [2,3,4,5,6,7]. However, treatment outcomes are often hindered by low engagement and high relapse rates, where cravings play a significant role in impeding adherence and long-term effectiveness [2,3,4,5,6]. Engagement challenges are further exacerbated by heightened reactivity to cocaine cues, triggers, and withdrawal symptoms [2].
Unlike opioid use disorder, for which effective pharmacotherapies and substitution treatments such as methadone and buprenorphine exist, there are no approved pharmacological treatments for cocaine dependence [3]. While research has explored various medications to support cocaine treatment, a systematic review concluded that medication alone was not effective for treating cocaine dependence [8]. Even though combining medication with psychosocial treatments has shown potential for reducing dropout rates, it does not significantly improve cocaine abstinence rates following treatment completion [9,10]. A critical challenge in treating cocaine dependence remains and can be addressed by reducing cravings, which is an important factor influencing relapse [2].
One promising alternative treatment approach under investigation is cue exposure therapy (CET) [6]. CET specifically targets the psychological and physiological responses elicited by drug-related cues, aiming to reduce their motivational impact. By repeatedly exposing substance-dependent individuals to these cues in a controlled environment without subsequent drug use, CET weakens the learned associations between cues and drug-related behaviours through a process known as extinction [7].
The theoretical foundation of CET is rooted in the classical conditioning model of learning, where drug-related cues become associated with drug use through repeated pairings [8]. When these cues are repeatedly presented without reinforcement (i.e., drug use), the conditioned responses, such as craving, diminish over time. In the context of SUD treatment, CET sessions involve repeated, unreinforced exposure to items, places, or actions the patient associates with drug use, in order to extinguish their previously conditioned response. By breaking the link between cue encounters and subsequent drug use, CET offers a targeted strategy for addressing drug-related triggers and reducing relapse rates [7].
Previous studies in addiction have used CET with various substance use disorders (SUD), including alcohol, nicotine, cocaine, and opiates [6,9,10,11,12,13,14,15,16,17,18]. There is some evidence for the effectiveness of CET for alcohol dependence [11,13,19,20,21] and there are emerging literature on CET for cocaine dependence [6,15,22]. CET has the potential to reduce craving and cue reactivity and, hence, reduce the risk of relapse of cocaine use [19,20], but more research is needed. There has been limited publications comparing the difference between exposure to standardised vs. personalised cues within CET substance treatment [23,24]. There is still a gap in knowledge of CET within substance use treatment of large multi-site RCTs comparing multiple cue types and investigating individual difference impact. Evidence suggests that, for successful extinction to occur, cue exposure and extinction must occur across multiple contexts to increase extinction generalisability [9], suggesting that greater variety and context provided for cues would improve treatment outcomes.
Although previous meta-analyses have shown the limited efficacy of CET for SUD treatment [9,25], this may be due to methodological limitations in CET research [26]. Many studies have presented cues individually within abstract contexts, for example, a bottle of beer on a desk in a clinic. This approach may limit generalisability to real-world drug use settings beyond the clinical environment. However, CET for SUD treatment can now take advantage of technological advances. CET research could now explore new ways to implement cues through exposure to more salient, realistic, and personally meaningful stimuli [19].
Devices such as virtual reality (VR) headsets and wearable devices enable the recording of biometric data, including heart rate variability, galvanic skin response, eye gaze, and body movements, which are biomarkers linked to substance craving. These technologies provide new ways to present multiple cues across various contexts, potentially enhancing generalisation beyond the treatment environment. These innovations should increase the generalisation of treatment effects beyond the clinical setting [26]. Notably, VR has already been successfully implemented in research to elicit and reduce alcohol cravings [26].
To advance the development of paradigms for cocaine CET, it is essential to gain a greater understanding of the types of cues that elicit craving responses, how these can be presented, and how these cues can be adapted or enhanced using emerging technologies. However, existing research on cocaine-related cues has largely overlooked the use of personalised and immersive cues in experimental studies, which are essential for improving ecological validity and replicating real-world drug-use contexts. While reviews and meta-analyses have examined CET in SUD [9,20,25], to our knowledge, no prior review has specifically focused on cocaine cues.
This paper aims to address this gap by providing a comprehensive review of cocaine cues utilised in previous cue-exposure studies. By critically evaluating these cues, we seek to inform the design and development of more effective, personalised and immersive VR-enhanced CET interventions, to reduce relapse rates and improve treatment outcomes for individuals with cocaine dependence. This review will focus on cue categories and examples within each category, including cues, situations, sense of presence and ecological validity (realism). The objectives for this review are to (1) identify the categories of cocaine craving cues used in previous research. This will include categories such as, visual cues or auditory cues, examples of cues used, how they were delivered and if they were delivered in combination with other cue types; (2) examine the range of cues utilised within each category; and (3) assess the risk of bias and quality of evidence in the included studies.
2. Methods
This review has been conducted according to the PRISMA 2020 guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [27]. This protocol has been registered at the International Prospective Register of Systematic Review (PROSPERO: CRD42024554361). This review was initially registered as a Rapid Review on PROSPERO; however, considering the scope and methods ultimately applied, we have revised this to a systematic review.
2.1. Inclusion Criteria
Studies meeting the following criteria were included: full text, original studies published in peer-reviewed journals, in English and investigating, reporting or including the use of cocaine cues. There were no restrictions on publication year or participant clinical and demographic characteristics. Cocaine-dependent individuals, recreational users, polydrug users, and non-cocaine-using controls were included and not restricted by the severity of cocaine dependence or comorbidities to provide a comprehensive understanding of cue reactivity across diverse groups. Conference abstracts, dissertations, and grey literature were not considered.
2.2. Information Sources
The bibliographic databases used were PubMed and Web of Science. Searches were carried out on the 2 August 2024. The search terms were adapted for use with each bibliographic database in combination with their specific filters (Table 1). The searches were supplemented by cross-checking the reference lists of key publications, related reviews, and included papers. The search terms and information sources were collaboratively developed and refined in consultation with the 15-member project team.

Table 1.
Search terms.
All papers identified in the search strategy were exported into the citation management system, Rayyan [28], and were screened at the title and/or abstract level to identify studies that potentially met the inclusion criteria (EB). From this list, the full text was retrieved and assessed independently by EB and NL, and any doubts were discussed between the first and second reviewers (EB and NL). Any disagreement between the first and second reviewers was discussed with a third reviewer (PD). Hand searching for additional papers also occurred within the already identified papers. A data extraction table was created and pilot-tested with the first five included studies and refined as necessary. EB extracted the data independently, and NL conducted entry checks for accuracy.
2.3. Outcomes
The primary outcome of this review was to identify categories of cocaine craving cues. The secondary outcome of this review was to identify the range of cues utilised within each category. Data extracted included study characteristics: country, publication year, source of funding, author conflicts of interest, study aims, population health status, type of cocaine, number of participants, study design, type of cues, examples, results, and any study limitations relating to cocaine cues.
2.4. Risk of Bias in Individual Studies
Study quality was assessed using an adapted eight-item framework [29], assessing study design, sample representativeness, measurement reliability, outcome validity, confounding control, statistical analysis, attrition and follow-up, with details provided in the Supplementary Materials. The scoring system awarded a maximum of one point for each of the eight criteria (maximum score of eight). Scores for attrition rates were adapted by allowing an incremental 0.5 points for discussion of attrition rates and an additional 0.5 points for having a follow-up rate greater than 50% [30]. The first and second reviewers (EB and NL) scored the articles independently and discussed any queries. The purpose of this quality appraisal was to ensure adherence to research standards and were not unevaluated reports of clinical innovations. The aim of this quality appraisal was not to exclude papers with lower scores but to explore all studies using cocaine cues and the range of methods and outcomes studied.
2.5. Data Synthesis and Analysis
Findings from the included studies are presented in a narrative synthesis. Information is included on the type of intervention (individual behaviour change, health service use), population characteristics, clinical or non-clinical population, the type of outcome and intervention content. The narrative synthesis of results on the primary outcomes, categories of cocaine craving cues, and subsequent examples are grouped by senses: Visual, tactile, auditory, gustatory and olfactory. Information on the extent of personalisation of cues and immersiveness was also identified. This review aimed to categorise and summarise examples of cocaine cues. Therefore, a meta-analysis was not appropriate within the scope of this review.
3. Results
After duplications were removed, 3600 papers were screened; of these, 3113 were excluded at the title/abstract screening, and 487 full-text papers were assessed. A total of 267 papers were then excluded. There were 28 additional papers identified by hand searching; of these, 15 were included. The final sample included 235 publications (Figure 1).
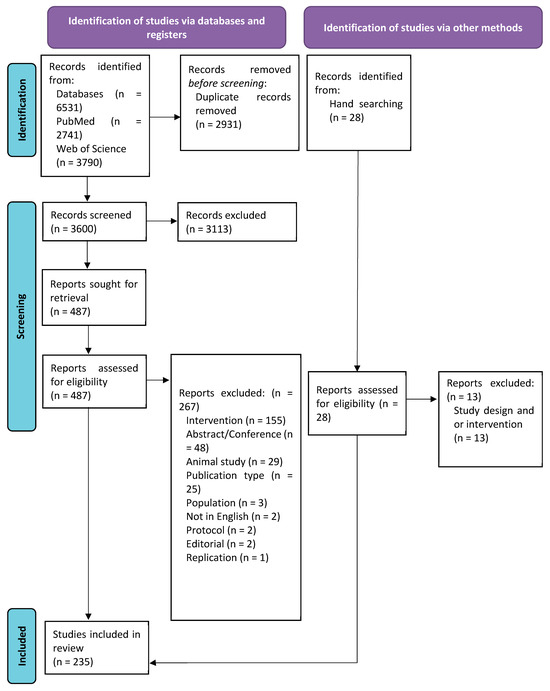
Figure 1.
PRISMA flow diagram.
3.1. Study Characteristics
Of the 235 included papers, most were conducted in the United States (US) (84.9%) (Table 2). The first paper was published in 1987, and over half of the studies have been published since 2010 (59.6%). Most included participants not currently in treatment and without a clinical SUD diagnosis (60.4%). The majority reported including cocaine users (85.1%), with only 30 (12.8%) specifying the type of cocaine used (powder cocaine, crack or combination with or without opioids). There were 234 quantitative studies, of which 59 were randomised controlled trials [22,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88] and one qualitative study [89,90]. A summary of all included studies is provided in the Supplementary Materials.

Table 2.
Study characteristics.
A variety of cue types were used. These are reported below with example in categories of sense types: visual (n = 200 studies), auditory (n = 70 studies), tactile (n = 52 studies), olfactory (n = 5 studies) and gustatory (n = 4 studies). The most commonly used cue was a still image (83 studies, 25.7%), and the second videos (73 studies, 24.5%), the least common were VR cocaine experience (3 studies, 0.9%) and drug memory recall (1 study, 0.3%). Many studies used only one type of cue (166 studies, 70.6%) of either images only, video only, script only or words only. But other studies used combinations of two or more cue types (Table 2). Of those who report including cocaine powder and crack users or crack users only, many (20/25, 80%) reports using specific crack-related videos, images, paraphernalia, or participant-identified scenarios for guided scripts [14,39,87,88,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106].
3.2. Quality Assessment
Study quality scores ranged from 4.5 to 8.0 (Mean: 6.85, Median: 7, Mode: 6.5) out of a possible 8. Only one study scored 4.5, and this was a case study [107]. Overall, the descriptive quality of the included studies was deemed good; all conformed to standard research norms and were not unevaluated reports or clinical innovations.
3.3. Narrative Findings
A range of sensory cues were used to trigger cocaine cravings, including visual, auditory, tactile, olfactory and gustatory cues. The following subsections provide a detailed analysis of how each sensory modality was used to trigger craving responses.
Of the 235 studies, a total of 33 (14.0%) included an example of the cue, either an image, a still image of the video, or a passage from the audio or guided script [6,35,46,50,62,70,75,106,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132].
3.3.1. Visual
There were 200 studies included that incorporated visual cues. These included cocaine-related videos, images, items and VR. Most studies used cocaine-related images (n = 83) [14,22,35,36,43,44,46,47,48,49,50,53,62,65,67,82,91,92,94,99,104,105,107,109,110,111,114,115,118,119,120,122,123,124,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175], or videos (n = 79 studies) [31,32,33,34,37,56,57,58,61,69,70,72,73,75,76,77,79,80,81,83,85,86,87,88,91,96,97,98,100,102,103,112,125,162,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220]. A total of 52 studies [34,39,40,44,51,54,57,66,68,75,76,79,80,81,83,84,85,86,87,88,96,100,101,102,103,133,134,163,176,177,178,179,180,181,182,183,184,186,187,188,189,190,191,192,193,194,221,222,223,224,225,226] had cocaine-use-related items that participants could look at, touch, or use in a drug preparation or drug use task. Cocaine-related items included crack pipes/stems, simulated powder cocaine, simulated crack rocks, lighters, banknotes, mirrors, straws, and razor blades. In studies that recruited powder or crack cocaine users, the paraphernalia often corresponded to their preferred method of administration, for example, small plastic bags and banknotes for powder cocaine users and crack pipes and lighters for crack users.
Eight studies [34,87,88,96,180,186,192,193] involved a drug preparation task in which participants were asked to repeatedly prepare lines of powder using paraphernalia (e.g., a razor blade) or to prepare a pipe or spoon with rocks. Four studies included a cocaine drug use task [101,133,193,225], while two involved placebo drug use [84,133]. These tasks required participants to view drug preparation items and cocaine or placebo cocaine; while noted here, further details on these tasks will be provided in the following sections. Additionally, three studies used VR environments and headsets to immerse participants in drug-related scenarios [6,106,108].
Examples of images include drug paraphernalia, such as crack pipes, mirrors, razor blades, straws, rolled-up money, lighters, vials, scrapers, rolling paper, plastic bags, injecting equipment, and glass stems. Images also depicted individuals using cocaine, either snorting or injecting, as well as people buying, using, or becoming intoxicated. Many images featured cocaine with general themes of drug preparation rituals, such as lines of powder next to a credit card, crack rocks next to a pipe or preparing a syringe for injection.
Videos often showed adults purchasing, using, and feeling the effects of cocaine via different administrations (snorting, smoking, injecting). Some videos featured individuals talking about their use and the rush or sensation they experienced afterwards. Studies often noted that these videos contained drug-related themes and scenes where individuals engaged in various drug-related tasks (purchasing, using, or discussing drug use). While some studies noted that videos showed drug use in various environments [81,111], very few provided detailed descriptions of the environments where drug use or purchases took place. Additionally, some videos included scenes of stress and the effects of using cocaine.
In addition to the above, there were also tasks involving viewing drug-related words, for example, when completing a Stroop task. A total of 27 studies described cues involving words or a Stroop task [63,64,78,119,130,149,168,175,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245], where drug-related words were presented on a computer screen. Examples of cocaine-related words included cocaine, coke, base, high, flash, blow, pipe, and dope. Neutral words such as household items (sofa, oven, kitchen, table, book), environmental terms (north, south, east, west), and food-related words (fruit and vegetables), were often used as control conditions.
There were also some studies which did not include specific examples but referred to ‘cocaine cues’, ‘cues of images containing cocaine themes’, ‘cocaine video’ or ‘cocaine words’, and the full description of the cue was not always reported [36,49,50,58,62,64,70,82,94,107,119,128,131,139,144,154,156,166,170,174,175,184,211,219,229,230,231,233,234,237,240,241,242,243,244,245].
There was a lack of clarity on how these cues were decided or where they were sourced from. One study reported the use of cocaine scenes taken from television programmes [185]. Four studies reported using images from participants’ real drug use locations. These images were created by researchers [43,57,72] or, in one study, created by the participants themselves [53].
In studies using VR, participants were exposed to computer-generated VR environments designed to simulate cocaine-related scenarios [6,106,108]. For example, in one study [6], the participant is placed in an apartment setting when friends arrive and start talking about and using cocaine on a coffee table. The participant is offered cocaine and, at least once during the scene, uses VR hand controllers to simulate the handling and touching of virtual cocaine paraphernalia, such as a steel spoon, paper tubes, glass pipes, or syringes. Participants could also virtually prepare and self-administer cocaine within the VR environment.
3.3.2. Auditory
Seventy studies incorporated auditory cues [5,11,31,35,38,39,42,48,49,50,52,54,56,57,68,71,72,77,81,82,83,85,86,94,98,102,103,107,108,117,152,166,167,169,170,173,176,178,179,180,182,183,184,185,188,190,195,205,210,229,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258]. All 70 studies used at least one of either audiotapes, audio within videos, guided imagery scripts, or audio in VR. One of these 70 studies [166] reported using audio cues but did not specify the content or delivery of the cue.
In addition, there are also three studies which referred to script development and rehearsal of a drug experience, but it is unclear if this was then listened to [96,179,259].
A total of 13 studies used audiotapes to elicit cocaine cravings [11,34,52,53,85,116,180,186,188,190,192,194,199].
One study [199] was a 45 min long and depicted a variety of pleasurable experiences from cocaine use. Other involved individuals talking about cocaine use [12], relapse-risk situations [34,52,192], and a discussion among a group of cocaine users concerning the effects of smoking crack [34]. Similarly, five studies incorporated audiotapes depending on the route of administration (intravenous or smoking), and participants listened to patients discussing the effects of cocaine [85,186,188,190,194]. Another study had a five-minute audio recording of the participant describing craving experiences and other sounds recalled in the craving experience, such as traffic or music playing [53].
Twelve studies reported including an audio component to a video cue. Most of the videos (n = 11) included audio recordings of actors [57,86,87,88,103,125,183,197,203,212,217], and one had audio of the participant’s friend using cocaine [193]. Videos showed actors engaging in cocaine-related activities [217]; examples included an actor describing cocaine use [203], a five-minute video depicting actors purchasing and smoking crack cocaine [86,88,103], seven-minute video of actors engaging in cocaine-related activities [183], a 30 s video, of an actor purchasing, preparing, ingesting and feeling intoxicated from powder cocaine [125] and a five [57] or 25 min video, also simulating, purchasing, preparation, and smoking of crack cocaine [197]. Another example of a 3–4.5 min video that used audio from an actor spoke about perceived injustices, described the desire to get ‘high’, prepared and used fake crack cocaine, commented on being dissatisfied by the experience and teased the viewer about wanting to get ‘high’ [212]. Finally, one 60 min video had audio of the participant’s friend using cocaine [193].
Forty-one of the 45 studies which used guided imagery scripts also reported the use of listening to these to elicit cocaine cravings [38,41,42,45,51,55,60,71,74,75,80,84,89,93,95,100,113,117,121,126,135,165,178,189,246,247,248,249,250,251,252,253,254,255,256,257,258,260,261,262,263]. These were mainly delivered via a recorded audiotape of the developed guided image script; however, one study mentioned that it was read by the therapist [84], and another where the participants were instructed to think about the drug scenario through guided imagery [102]. The other three studies that used guided imagery were unclear on whether this was listened to [96,179,259]. Some described a situation where the participant had previously craved cocaine which resulted in cocaine use [38,41,55,250,251,252,253,254,255]. Examples of the guided imagery scripts include first-person and present tense guided imagery lasting approximately one minute [117]; the content of the imagery was a typical cocaine-use experience. Another imagery exercise lasted one minute and was customised depending on the route of administration and described the urge to use cocaine [71]. In some instances, the cocaine-related script was personalised [74] by including people, places, and objects [256,257]. Other forms of personalising a script included emotional and sensory cocaine-related cues and drawing upon participants’ personal drug experiences [258]. Another study [75] asked participants to create four personalised imagery scripts: past–positive, a pleasurable event from before initiation to cocaine use; past–negative, a ‘worst time’ aversive cocaine craving and use; future–positive, simulation of a ‘wished for’ event if they recovered from cocaine use disorder; future–negative, a ‘most feared’ event if cocaine use disorder worsened. Each audio lasted approximately five minutes. Other examples of a cocaine-related script were a pleasurable scenario, with associated senses [42,96,100,261] or a time when they had anticipatory excitement for cocaine [45,55,121]; one study focused on the physiologic sensations [247].
The videos, script, and audio cues included in this section include all the studies which refer to audio within these cues, either within the video or audiotape used or mention the script being read aloud.
Two studies incorporated auditory elements in VR. One study used a Meta Quest 2 VR headset, a device capable of spatial audio positioning, which was reported to enhance the sense of 360-degree immersion [6]. The other study used a VR system equipped with a VFX3D HMD, integrated stereo headphones, and a floor platform designed to enhance auditory stimuli with vibrational feedback. For instance, during a police raid scene, the floor vibrated to simulate the impact of car doors slamming or the forceful opening of doors to a crack house. The audio was set in a typical environment such as a crack house [106].
In addition to audio cues being used to elicit cocaine craving, another aspect of the audio cues in some studies was to elicit anticipation of cocaine. Anticipation-induced craving was elicited through audio scripts or direct verbal communication from the researchers about the future use of cocaine. There were five studies which reported using anticipation as a cocaine cue [89,121,226,246,260]. Four studies used anticipation in the form of an imagery script [89,121,246,260]. These scripts included memories and actions associated with anticipation of drug use [121], and describing recent situations that involved anticipatory excitement for wanting cocaine [246], and led to subsequent cocaine use [89,260]. One study used anticipation in the form of being told they were able to self-administer cocaine use (via insufflation) after the experimental procedures were completed [226].
3.3.3. Tactile
A total of 52 studies incorporated tactile elements into the cocaine cue exposure [34,39,40,44,51,54,57,66,68,75,76,79,80,81,83,84,85,86,87,88,96,100,101,102,103,133,134,163,176,177,178,179,180,181,182,183,184,186,187,188,189,190,191,192,193,194,221,222,223,224,225,226]. This was achieved by providing paraphernalia and drug-related items for participants to handle, to prepare, or use, in placebo or drug-related tasks. Drug preparation tasks involved providing participants with paraphernalia and instructing them to complete a preparation task (n = 8) [34,87,88,96,180,186,192,193] or a drug use task involving cocaine or cocaine placebo (n = 4) [84,101,133,193].
Participants could touch various items, including simulated cocaine (in powder or rock form) and tools associated with drug preparation, for example, ‘dime bags’, a small bag of simulated crack or powder cocaine, crack pipe, lighter, money (typically $5 or $10 bills), mirror, straw, razor blade, rolling paper, lactose, rock candy, butane lighter, or a glass stem.
In drug preparation tasks, participants were asked to go beyond merely touching the items by performing the actions typically performed before drug use. For example, they were instructed to use a razor blade to divide the powder into lines multiple times, hold a straw, touch and smell the crystal and put them in a pipe.
3.3.4. Olfactory
There were five studies included in this review that incorporated smell into the cocaine cue exposure [84,87,88,103,189]. All studies included an olfactory cocaine cue alongside other cues. Four studies involved smelling a recently used crack pipe [87,88,103,189] and the other study used a commercially available cocaine aroma and drops of this were placed in the participants’ hands to try to recreate the smell of cocaine [84].
In addition to these five studies that mention smell as a cue, other studies may have had participants also experiencing cocaine-related smells through cocaine drug use tasks, as described in the below Section 3.3.5.
3.3.5. Gustatory
Four studies incorporated taste into cocaine cue exposure [114,182,219,220]. Both studies only recruited participants who used crack cocaine and asked them to smoke cocaine. Within two studies [219,220], participants were blindfolded to remove any visual cues and then given either a cocaine base formula or a placebo in a glass stem. Participants were then asked to inhale the substance with their usual smoking practices. In the third study [133], participants were also asked to smoke cocaine base or a placebo, through a glass mouthpiece silicone oil bath. The fourth study involved participants using cocaine [182] and consuming lines of powder cocaine.
4. Discussion
Cue exposure and its therapeutic applications can enhance treatment outcomes for individuals with addiction. This review highlights that cue exposure is widely used in cocaine use research, allowing us to identify and categorise different types of cocaine craving cues. Notably, this is the first review to systematically explore and classify these cues. Our findings have the potential to enhance the use of cues in experimental and clinical research, ultimately contributing to more effective treatment strategies.
4.1. Types of Cocaine Cues and Their Use in Research
A variety of cue types were used either individually or in combination, including images, videos, physical items, scripted/guided imagery, words, audiotapes, drug preparation or drug use, VR, and drug memory recall. Images were the most used cues [83 studies) [14,22,35,36,43,44,46,47,48,49,50,53,62,65,67,82,91,92,94,99,104,105,107,109,110,111,114,115,118,119,120,122,123,124,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175], followed by videos (79 studies) [31,32,33,34,37,56,57,58,61,69,70,72,73,75,76,77,79,80,81,83,85,86,87,88,91,96,97,98,100,102,103,112,125,162,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220]. In contrast, VR (3 studies) [6,106,108] and drug memory recall were the least used cues (one study) [51].
Most studies used only one cue (183 studies, 77.9%), while 52 studies (22.1%) incorporated a combination of two, three, or four cues. All studies included at least one of the following: cocaine images, videos, references to cocaine drug use in audiotapes, and physical drug-related items.
The level of immersion varied across studies, depending on the number of cues used and how many senses were involved during the cue exposure. Some studies used one type of cue, e.g., images of cocaine, drug paraphernalia, or people using cocaine. Others combined multiple sensory cues, such as images or videos paired with audio recordings of drug-related conversations, the smell of cocaine, or tactile interaction with paraphernalia. VR [6,106,108] was used in a few studies to create immersive drug-related environments while maintaining the safety of the research laboratory.
4.2. The Role of Personalisation and Ecological Validity
Most studies were conducted in laboratory settings, where participants attended sessions and were presented with standardised cues. While this approach ensures uniformity and replicability, it may not fully capture the complexity of real-world drug experiences. A few studies extended data collection to participants’ real-world settings, allowing them to document their own cues [43,53,72]. Incorporating real-world settings and participant-identified cues could capture more personalised and non-drug-specific cues. For instance, a particular song linked to drug use, someone they have used cocaine with, a specific bag used to carry drugs, a street corner associated with purchases, or the type of phone used by a dealer could serve as intensely personal cues.
There was also variation in the degree of personalisation used in different studies. Many used stock images of cocaine, paraphernalia, and drug use [122,164,168,171] while other used images from media/films portraying drug use [185]. A small number of studies attempted to personalise cues by incorporating images of local areas where participants had previously used cocaine [43,57,72], asking participants to provide personal images [53], or featuring a friend of the participant in a cocaine-use video [193]. Some studies also personalised the guided imagery scripts with the participants’ input [74,75,256,257,258].
Despite the importance of ecological validity, none of the reviewed studies explicitly mentioned incorporating the sense of presence or realism in cue design. However, five studies did include anticipation as a cocaine cue [89,121,226,246,260], using imagery scripts or telling participants they would have access to cocaine after the experiment.
4.3. The Importance of Multi-Sensory Cues
Cocaine craving cues engage multiple sensory modalities [264]; yet, most studies focused primarily on visual drug-related stimuli. This is supported by research showing that the brain regions associated with cravings are activated by visual drug-related cues [137,265]. However, other senses, particularly olfaction, remain underexplored in cocaine-related studies despite the evidence suggesting a strong association between smell, craving and addiction [266,267]. The under-utilisation of olfactory cues may limit the vividness and personal relevance of the cues, potentially reducing their effectiveness in eliciting realistic craving responses.
4.4. Cue Exposure Therapy (CET) and Future Directions
While there was a range of aims and reasons for displaying or delivering cocaine cues within these included studies, only two specifically examined its application in CET [22,66]. In both studies, participants were randomised to receive d-cycloserine (DCS) or a placebo and completed cue reactivity tasks. The findings indicated that CET reduced brain activation to drug cues within various brain regions and that extinction to drug cues occurred in both the DCS and placebo groups between cue exposures, but that DCS did not facilitate learning extinction.
CET is an area of increasing interest as a potential treatment modality for cocaine and other substance use disorders [6,9,10,11,12,13,14,15,16,17,18]. This review highlights key considerations for improving CET, particularly regarding the balance between standardised and personalised cues. While standardised cues enhance replicability, personalisation may increase ecological validity and treatment effectiveness. A practical approach to achieving both would be integrating common themes, environments, contexts and languages informed by individuals with lived experience. For instance, cues could incorporate local slang, images of commonly known drug-use locations or familiar architecture, making the cues more relatable and immersive for participants.
Patient and public involvement (PPI) was not explicitly mentioned in the reviewed studies, but its inclusion could improve cue design, ensuring relevance across socioeconomic groups. Individuals from lower SES backgrounds may not typically use drugs in settings such as pubs or nightclubs due to financial constraints, whereas higher SES groups may report substance use in these environments or at work-related events. Personalised cues tailored to these distinctions could improve the inclusivity and effectiveness of cue-based interventions [266].
Finally, this review identified three studies which used VR to deliver virtual drug cues [6,106,108]. Technological advances allow for new modes to deliver CET, for example, the use of VR headsets, controllers or the use of other technology, for example, 360 videos (immersive videos which capture a panoramic sphere in all directions). Evidence suggests that technology delivered CET may improve the efficacy of the treatment intervention; however, there also other challenges when implementing technology in treatment, including training, costs, hardware and software infrastructure, and ethical considerations [268].
4.5. Recommendations
Future research should prioritise the use of personalised cues to enhance ecological validity and treatment effectiveness. The level of immersion required should be carefully considered, as more immersive and individually relevant cues may elicit stronger craving responses, potentially improving the efficacy of CET. In addition, multi-sensory cue presentations, incorporating visual, auditory, tactile, olfactory, and gustatory stimuli, should be further explored to determine their potential benefits in real-world applications.
A major challenge identified in this review was the lack of detailed descriptions of cues used in many studies. While cues were often broadly categorised as drug-related, critical information, such as the specific content of scripts, the visual and auditory details of videos, and the nature of the environments depicted, was frequently missing. This lack of specificity hinders the ability of future researchers to replicate and adapt these cues for their own studies, ultimately limiting progress in the field. To address this issue, future research should provide more comprehensive descriptions of the cues used, including their sensory characteristics and contextual details, to improve reproducibility, comparability, and the development of more effective CET protocols.
There is also a need for greater standardisation in cue exposure research. Establishing international consensus on the selection and categorisation of cues, particularly in relation to their level of immersiveness and personalisation, would help to improve methodological consistency across studies. Standardised categories could be developed to distinguish between cues used in experimental cue exposure research and those employed in CET interventions. Using open-source methodologies or equivalent initiatives could provide a transparent framework for researchers to share details of their cue exposure paradigms. Such an approach would facilitate collaboration, enhance methodological rigour, and accelerate advancements in CET research.
4.6. Limitations
Although we reviewed a large body of evidence, publication bias may still be present, as studies with positive findings are more likely to be published in peer-reviewed journals or English-language journals. Relevant studies published in non-English literature may have been missed. In addition, this review is limited by the scope of this work. The results presented describe the characteristics of studies involving cue exposure and measurements of craving. However, it does not provide a critical appraisal or address specific questions about the included studies, effects of cue exposure, effectiveness of cocaine cues, or whether features or cue modalities are more impactful than others.
5. Conclusions
This review identified a range of sensory cues used in studies to elicit cocaine craving, encompassing all the senses: visual, tactile, auditory, olfactory, and gustatory. The number and type of cues varied across the studies, but all included at least one cocaine-specific cue. Common examples included images of substances representing cocaine, paraphernalia, cocaine preparation items, talk of cocaine use, and images or videos of others using cocaine.
Due to the laboratory settings for most of these studies and the use of standardised cues for all participants, personalisation was limited, with many cues sourced from stock images. As some research suggests that CET might be effective for substance use disorders [6,9,10,11,12,13,14,15,16,17,18] and specifically for cocaine [6,15,22], further research should investigate some critical aspects that may influence its effectiveness, including the role of multiple sensory cues, personalisation, and ecological validity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/brainsci15060626/s1, File S1: Included studies.
Author Contributions
E.B.: Methodology, resources, data curation, visualisation, drafted and revised the article. N.L.: Data curation, visualisation, drafted and revised the article. M.C.: Funding acquisition, conceptualisation, methodology and revised the article. A.C.: Funding acquisition, conceptualisation, methodology and revised the article. S.C.: Funding acquisition, conceptualisation, methodology and revised the article. J.D.P.: Funding acquisition, conceptualisation, methodology and revised the article. C.D.: Funding acquisition, conceptualisation, methodology and revised the article S.G.: Funding acquisition, conceptualisation, methodology and revised the article. C.K.: Conceptualisation, methodology and revised the article. T.P.: Funding acquisition, conceptualisation, methodology and revised the article. D.S.: Funding acquisition, conceptualisation, methodology and revised the article. S.S.: Conceptualisation and methodology. L.V.: Funding acquisition, conceptualisation, methodology and revised the article. C.W.: Conceptualisation and methodology. P.D.: Funding acquisition, conceptualisation, methodology, data curation and revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This project is funded by the National Institute for Health and Care Research (NIHR) under its Invention for Innovation (i4i) Programme (Grant Reference Number NIHR206721). PD, DS and CD were supported by the NIHR Maudsley Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King’s College London. PD, CD, TP, and SC were supported by the NIHR Mental Health Research Groups (MHRG) programme through the Centre for Addiction and Mental Health Research at the University of Hull. CD was also supported by the NIHR Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Acknowledgments
The authors also acknowledge the project PPI Advisory Board’s contribution to the review design: Lee Beddows, Nigel Critchley, Adrian Esdaille, Sophie Quick-Collier, Shelley Starr, John Usher, Colin Williams, Paul York.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| CBT | Cognitive behavioural therapy |
| CET | Cue exposure therapy |
| DCS | d-cycloserine |
| MI | Motivational interviewing |
| MET | Motivational enhancement therapy |
| OST | Opioid substitution treatment |
| OUD | Opioid use disorder |
| PPI | Patient and public involvement |
| PRISMA-P | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | Prospective Register of Systematic Review |
| SUD | Substance use disorder |
| US | United States |
| UK | United Kingdom |
| VR | Virtual reality |
References
- Office for National Statistics. Deaths Related to Drug Poisoning in England and Wales 2022 Registrations; Office for National Statistics: Newport, UK, 2023. [Google Scholar]
- Penberthy, J.K.; Ait-Daoud, N.; Vaughan, M.; Fanning, T. Review of treatment for cocaine dependence. Curr. Drug Abus. Rev. 2010, 3, 49–62. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Global Status Report on Alcohol and Health and Treatment of Substance Use Disorders; WHO (World Health Organization): Geneva, Switzerland, 2024. [Google Scholar]
- UNODC (United Nations Office on Drugs and Crime). Global Report on Cocaine 2023 Local Dynamics, Global Challenges; UNODC (United Nations Office on Drugs and Crime): Vienna, Austria, 2023. [Google Scholar]
- Office for National Statistics (ONS). Deaths Related to Drug Poisoning in England and Wales: 2023 Registrations; Office for National Statistics: Newport, UK, 2024. [Google Scholar]
- Lehoux, T.; Porche, C.N.; Capobianco, A.; Gervilla, M.; Lecuyer, F.; Anthouard, J.; Weiner, L. Towards virtual reality exposure therapy for cocaine use disorder: A feasibility study of inducing cocaine craving through virtual reality. Addict. Behav. Rep. 2024, 19, 100549. [Google Scholar] [CrossRef] [PubMed]
- Drummond, D.C.; Cooper, T.; Glautier, S.P. Conditioned learning in alcohol dependence: Implications for cue exposure treatment. Br. J. Addict. 1990, 85, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Drummond, D.C. Theories of drug craving, ancient and modern. Addiction 2001, 96, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Conklin, C.A.; Tiffany, S.T. Applying extinction research and theory to cue-exposure addiction treatments. Addiction 2002, 97, 155–167. [Google Scholar] [CrossRef]
- Culbertson, C.S.; Shulenberger, S.; De, R.; Garza, L.; Newton, T.F.; Brody, A.L. Virtual reality cue exposure therapy for the treatment of tobacco dependence. J. Cyber Ther. Rehabil. 2012, 5, 57. [Google Scholar]
- Lee, J.H.; Kwon, H.; Choi, J.; Yang, B.H. Cue-exposure therapy to decrease alcohol craving in virtual environment. Cyberpsychol. Behav. 2007, 10, 617–623. [Google Scholar] [CrossRef]
- Lee, J.; Lim, Y.; Graham, S.J.; Kim, G.; Wiederhold, B.K.; Wiederhold, M.D.; Kim, I.Y.; Kim, S.I. Nicotine Craving and Cue Exposure Therapy by Using Virtual Environments. Cyberpsychol. Behav. 2004, 7, 705–713. [Google Scholar] [CrossRef]
- Hernández-Serrano, O.; Ghiţă, A.; Figueras-Puigderrajols, N.; Fernández-Ruiz, J.; Monras, M.; Ortega, L.; Mondon, S.; Teixidor, L.; Gual, A.; Ugas-Ballester, L.; et al. Predictors of changes in alcohol craving levels during a virtual reality cue exposure treatment among patients with alcohol use disorder. J. Clin. Med. 2020, 9, 3018. [Google Scholar] [CrossRef]
- Coffey, S.F.; Saladin, M.E.; Drobes, D.J.; Brady, K.T.; Dansky, B.S.; Kilpatrick, D.G. Trauma and substance cue reactivity in individuals with comorbid posttraumatic stress disorder and cocaine or alcohol dependence. Drug Alcohol Depend. 2002, 65, 115–127. [Google Scholar] [CrossRef]
- Hone-Blanchet, A.; Wensing, T.; Fecteau, S. The use of virtual reality in craving assessment and cue-exposure therapy in substance use disorders. Front. Hum. Neurosci. 2014, 8, 844. [Google Scholar] [CrossRef] [PubMed]
- Havermans, R.C.; Mulkens, S.; Nederkoorn, C.; Jansen, A. The efficacy of cue exposure with response prevention in extinguishing drug and alcohol cue reactivity. Behav. Interv. 2007, 22, 121–135. [Google Scholar] [CrossRef]
- Mihindou, C.; Vouillac, C.; Koob, G.F.; Ahmed, S.H. Preclinical validation of a novel cocaine exposure therapy for relapse prevention. Biol. Psychiatry 2011, 70, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Marissen, M.A.E.; Franken, I.H.A.; Blanken, P.; Van Den Brink, W.; Hendriks, V.M. Cue exposure therapy for the treatment of opiate addiction: Results of a randomized controlled clinical trial. Psychother. Psychosom. 2007, 76, 97–105. [Google Scholar] [CrossRef]
- Drummond, C.; Tiffany, S.; Glautier, S.; Remington, B. Cue Exposure in UNDERSTANDING and Treating Addictive Behaviours; John Wiley & Sons: Hoboken, NJ, USA, 1995. [Google Scholar]
- Kiyak, C.; Simonetti, M.E.; Norton, S.; Deluca, P. The efficacy of cue exposure therapy on alcohol use disorders: A quantitative meta-analysis and systematic review. Addict. Behav. 2023, 139, 107578. [Google Scholar] [CrossRef]
- Kwon, H.; Choi, J.; Roh, S.; Yang, B.H.; Lee, J.H. Application of Virtual Reality-Cue Exposure Therapy for Reducing Alcohol Craving. Annu. Rev. CyberTher. Telemed. 2006, 4, 161–166. [Google Scholar]
- Prisciandaro, J.J.; Myrick, H.; Henderson, S.; McRae-Clark, A.L.; Ana, E.J.S.; Saladin, M.E.; Brady, K.T. Impact of DCS-facilitated cue exposure therapy on brain activation to cocaine cues in cocaine dependence. Drug Alcohol Depend. 2013, 132, 195–201. [Google Scholar] [CrossRef][Green Version]
- Martin, T.; Larowe, S.D.; Malcolm, R. Progress in Cue Exposure Therapy for the Treatment of Addictive Disorders: A Review Update. Open Addict. J. 2013, 3, 92–101. [Google Scholar] [CrossRef][Green Version]
- Vafaie, N.; Kober, H. Association of Drug Cues and Craving with Drug Use and Relapse: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022, 79, 641–650. [Google Scholar] [CrossRef]
- Mellentin, A.I.; Skøt, L.; Nielsen, B.; Schippers, G.M.; Nielsen, A.S.; Stenager, E.; Juhl, C. Cue exposure therapy for the treatment of alcohol use disorders: A meta-analytic review. Clin. Psychol. Rev. 2017, 57, 195–207. [Google Scholar] [CrossRef]
- Ghiţă, A.; Hernández-Serrano, O.; Fernández-Ruiz, J.; Moreno, M.; Monras, M.; Ortega, L.; Mondon, S.; Teixidor, L.; Gual, A.; Gacto-Sanchez, M.; et al. Attentional Bias, Alcohol Craving, and Anxiety Implications of the Virtual Reality Cue-Exposure Therapy in Severe Alcohol Use Disorder: A Case Report. Front. Psychol. 2021, 12, 543586. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Jinks, A.; Cotton, A.; Rylance, R. Obesity interventions for people with a learning disability: An integrative literature review. J. Adv. Nurs. 2011, 67, 460–471. [Google Scholar] [CrossRef]
- Clark, A.K.; Wilder, C.M.; Winstanley, E.L. A systematic review of community opioid overdose prevention and naloxone distribution programs. J. Addict. Med. 2014, 8, 153–163. [Google Scholar] [CrossRef]
- Kranzler, H.R.; Bauer, L.O. Bromocriptine and cocaine cue reactivity in cocaine-dependent patients. Br. J. Addict. 1992, 87, 1537–1548. [Google Scholar] [CrossRef]
- Engeli, E.J.; Russo, A.G.; Ponticorvo, S.; Zoelch, N.; Hock, A.; Hulka, L.M.; Kirschner, M.; Preller, K.H.; Seifritz, E.; Quednow, B.B.; et al. Accumbal-thalamic connectivity and associated glutamate alterations in human cocaine craving: A state-dependent rs-fMRI and 1H-MRS study. Neuroimage Clin. 2023, 39, 103490. [Google Scholar] [CrossRef]
- Smelson, D.A.; Ziedonis, D.; Williams, J.; Losonczy, M.F.; Williams, J.; Steinberg, M.L.; Kaune, M. The efficacy of olanzapine for decreasing cue-elicited craving in individuals with schizophrenia and cocaine dependence: A preliminary report. J. Clin. Psychopharmacol. 2006, 26, 9–12. [Google Scholar] [CrossRef]
- Robbins, S.J.; Ehrman, R.N.; Childress, A.R.; O’brien, C.P. Using cue reactivity to screen medications for cocaine abuse: A test of amantadine hydrochloride. Addict. Behav. 1992, 17, 491–499. [Google Scholar] [CrossRef]
- Mayer, A.R.; Wilcox, C.E.; Dodd, A.B.; Klimaj, S.D.; Dekonenko, C.J.; Claus, E.D.; Bogenschutz, M. The efficacy of attention bias modification therapy in cocaine use disorders. Am. J. Drug Alcohol Abus. 2016, 42, 459–468. [Google Scholar] [CrossRef]
- Schulte, M.H.J.; Kaag, A.M.; Boendermaker, W.J.; van den Brink, W.; Goudriaan, A.E.; Wiers, R.W. The effect of N-acetylcysteine and working memory training on neural mechanisms of working memory and cue reactivity in regular cocaine users. Psychiatry Res. Neuroimaging. 2019, 287, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, P.F.; Daniels, S.; Lundahl, L.H.; Rogers, V.; Lukas, S.E. Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: A preliminary report. Psychopharmacology 1999, 142, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.C.; Sofuoglu, M.; Morgan, P.T.; Tuit, K.L.; Sinha, R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: Impact of gender and cue type. Psychoneuroendocrinology 2013, 38, 1532–1544. [Google Scholar] [CrossRef]
- Dakwar, E.; Levin, F.; Foltin, R.W.; Nunes, E.V.; Hart, C.L. The effects of subanesthetic ketamine infusions on motivation to quit and cue-induced craving in cocaine-dependent research volunteers. Biol. Psychiatry 2014, 76, 40–46. [Google Scholar] [CrossRef]
- Dackis, C.A.; Gold, M.S.; Sweeney, D.R.; Byron, J.P.; Climko, R. Single-Dose Bromocriptine Reverses Cocaine Craving. Psychiatry Res. 1987, 20, 261–264. [Google Scholar] [CrossRef]
- Milivojevic, V.; Fox, H.C.; Jayaram-Lindstrom, N.; Hermes, G.; Sinha, R. Sex differences in guanfacine effects on stress-induced stroop performance in cocaine dependence. Drug Alcohol Depend. 2017, 179, 275–279. [Google Scholar] [CrossRef]
- Fox, H.C.; Morgan, P.T.; Sinha, R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology 2014, 39, 1527–1537. [Google Scholar] [CrossRef]
- Johnson, M.W.; Bruner, N.R.; Johnson, P.S.; Silverman, K.; Berry, M.S. Randomized Controlled Trial of D-Cycloserine in Cocaine Dependence: Effects on Contingency Management and Cue-Induced Cocaine Craving in a Naturalistic Setting. Exp. Clin. Psychopharmacol. 2020, 28, 157–168. [Google Scholar] [CrossRef]
- Prisciandaro, J.J.; Myrick, H.; Henderson, S.; McRae-Clark, A.L.; Brady, K.T. Prospective associations between brain activation to cocaine and no-go cues and cocaine relapse. Drug Alcohol Depend. 2013, 131, 44–49. [Google Scholar] [CrossRef]
- Milivojevic, V.; Charron, L.; Fogelman, N.; Hermes, G.; Sinha, R. Pregnenolone Reduces Stress-Induced Craving, Anxiety, and Autonomic Arousal in Individuals with Cocaine Use Disorder. Biomolecules 2022, 12, 1593. [Google Scholar] [CrossRef]
- Young, K.A.; Franklin, T.R.; Roberts, D.C.; Jagannathan, K.; Suh, J.J.; Wetherill, R.R.; Wang, Z.; Kampman, K.M.; O’Brien, C.P.; Childress, A.R. Nipping cue reactivity in the bud: Baclofen prevents limbic activation elicited by subliminal drug cues. J. Neurosci. 2014, 34, 5038–5043. [Google Scholar] [CrossRef] [PubMed]
- Rosse, R.B.; Alim, T.N.; Fay-McCarthy, M.; Collins, J.P., Jr.; Vocci, F.J., Jr.; Lindquist, T.; Deutsch, S.I. Nimodipine pharmacotherapeutic adjuvant therapy for inpatient treatment of cocaine dependence. Clin. Neuropharmacol. 1994, 17, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Goudriaan, A.E.; Veltman, D.J.; Van Den Brink, W.; Dom, G.; Schmaal, L. Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: A randomized placebo-controlled cross-over study using pharmacological fMRI. Addict. Behav. 2013, 38, 1509–1517. [Google Scholar] [CrossRef]
- Joseph, J.E.; McRae-Clark, A.; Sherman, B.J.; Baker, N.L.; Moran-Santa Maria, M.; Brady, K.T. Neural correlates of oxytocin and cue-reactivity in cocaine-dependent men and women with and without childhood trauma HHS Public Access. Psyhcopharmacology 2019, 237, 249–261. [Google Scholar] [CrossRef]
- Verveer, I.; van der Veen, F.M.; Shahbabaie, A.; Remmerswaal, D.; Franken, I.H.A. Multi-session electrical neuromodulation effects on craving, relapse and cognitive functions in cocaine use disorder: A randomized, sham-controlled tDCS study. Drug Alcohol Depend. 2020, 217, 108429. [Google Scholar] [CrossRef]
- Maria, M.M.M.-S.; Sherman, B.J.; Brady, K.T.; Baker, N.L.; Hyer, J.M.; Ferland, C.; McRae-Clark, A.L. Impact of endogenous progesterone on reactivity to yohimbine and cocaine cues in cocaine-dependent women. Pharmacol. Biochem. Behav. 2018, 165, 63–69. [Google Scholar] [CrossRef]
- Rohsenow, D.J.; Monti, P.M.; Martin, R.A.; Colby, S.M.; Myers, M.G.; Gulliver, S.B.; Brown, R.A.; Mueller, T.I.; Gordon, A.; Abrams, D.B. Motivational enhancement and coping skills training for cocaine abusers: Effects on substance use outcomes. Addiction 2004, 99, 862–874. [Google Scholar] [CrossRef]
- Marsden, J.; Goetz, C.; Meynen, T.; Mitcheson, L.; Stillwell, G.; Eastwood, B.; Strang, J.; Grey, N. Memory-Focused Cognitive Therapy for Cocaine Use Disorder: Theory, Procedures and Preliminary Evidence From an External Pilot Randomised Controlled Trial. EBioMedicine 2018, 29, 177–189. [Google Scholar] [CrossRef]
- Moran-Santa Maria, M.M.; Baker, N.L.; Ramakrishnan, V.; Brady, K.T.; McRae-Clark, A. Impact of acute guanfacine administration on stress and cue reactivity in cocaine-dependent individuals. Am. J. Drug Alcohol Abus. 2015, 41, 146–152. [Google Scholar] [CrossRef]
- Fox, H.C.; Seo, D.; Tuit, K.; Hansen, J.; Kimmerling, A.; Morgan, P.T.; Sinha, R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: Preliminary findings. J. Psychopharmacol. 2012, 26, 958–972. [Google Scholar] [CrossRef]
- Sterling, R.C.; Dean, J.; Weinstein, S.P.; Murphy, J.; Gottheil, E. Gender differences in cue exposure reactivity and 9-month outcome. J. Subst. Abus. Treat. 2004, 27, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.S.; Batki, S.L.; Berger, S.P. Fluoxetine attenuates adrenocortical but not subjective responses to cocaine cues. Am. J. Drug Alcohol Abus. 2004, 30, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Kilgus, M.; Pumariega, A. Experimental Manipulation of Cocaine Craving by Videotaped Environmental Cues. South. Med. J. 1994, 87, 1138–1140. [Google Scholar] [CrossRef] [PubMed]
- Milivojevic, V.; Fox, H.C.; Sofuoglu, M.; Covault, J.; Sinha, R. Effects of progesterone stimulated allopregnanolone on craving and stress response in cocaine dependent men and women. Psychoneuroendocrinology 2016, 65, 44–53. [Google Scholar] [CrossRef]
- Jobes, M.L.; Aharonovich, E.; Epstein, D.H.; Phillips, K.A.; Reamer, D.; Anderson, M.; Preston, K.L. Effects of Prereactivation Propranolol on Cocaine Craving Elicited by Imagery Script/Cue Sets in Opioid-dependent Polydrug Users: A Randomized Study. J. Addict. Med. 2015, 9, 491–498. [Google Scholar] [CrossRef]
- Modesto-Lowe, V.; Burleson, J.A.; Hersh, D.; Bauer, L.O.; Kranzler, H.R. Effects of naltrexone on cue-elicited craving for alcohol and cocaine. Drug Alcohol Depend. 1997, 49, 9–16. [Google Scholar] [CrossRef]
- Mayer, A.R.; Dodd, A.B.; Wilcox, C.E.; Klimaj, S.D.; Claus, E.D.; Bryan, A.D. Effects of attentional bias modification therapy on the cue reactivity and cognitive control networks in participants with cocaine use disorders. Am. J. Drug Alcohol Abus. 2020, 46, 357–367. [Google Scholar] [CrossRef]
- Liu, S.; Lane, S.D.; Schmitz, J.M.; Cunningham, K.A.; John, V.P.; Moeller, F.G. Effects of escitalopram on attentional bias to cocaine-related stimuli and inhibitory control in cocaine-dependent subjects. J. Psychopharmacol. 2013, 27, 801–807. [Google Scholar] [CrossRef]
- DeVito, E.E.; Kiluk, B.D.; Nich, C.; Mouratidis, M.; Carroll, K.M. Drug Stroop: Mechanisms of response to computerized cognitive behavioral therapy for cocaine dependence in a randomized clinical trial. Drug Alcohol Depend. 2018, 183, 162–168. [Google Scholar] [CrossRef]
- Alim, T.; Rosse, R.; Vocci, F., Jr.; Lindquist, T.; Deutsch, S. Diethylpropion Pharmacotherapeutic Adjuvant Therapy for Inpatient Treatment of Cocaine Dependence: A Test of the Cocaine-Agonist Hypothesis. Clin. Neuropharmacol. 1995, 18, 183–195. [Google Scholar] [CrossRef]
- Santa Ana, E.J.; Prisciandaro, J.J.; Saladin, M.E.; McRae-Clark, A.L.; Shaftman, S.R.; Nietert, P.J.; Brady, K.T. D-cycloserine combined with cue exposure therapy fails to attenuate subjective and physiological craving in cocaine dependence. Am. J. Addict. 2015, 24, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.L.; Moscon, J.A.; Fregni, F.; Nitsche, M.A.; Nakamura-Palacios, E.M. Cognitive related electrophysiological changes induced by non-invasive cortical electrical stimulation in crack-cocaine addiction. Int. J. Neuropsychopharmacol. 2014, 17, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Price, K.L.; McRae-Clark, A.L.; Saladin, M.E.; Maria, M.M.M.-S.; DeSantis, S.M.; Back, S.E.; Brady, K.T. D-cycloserine and cocaine cue reactivity: Preliminary findings. Am. J. Drug Alcohol Abus. 2009, 35, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Kosten, T.R.; Scanley, B.E.; Tucker, K.A.; Oliveto, A.; Prince, C.; Sinha, R.; Potenza, M.N.; Skudlarski, P.; Wexler, B.E. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology 2006, 31, 644–650. [Google Scholar] [CrossRef]
- Callans, L.S.; Philogene-Khalid, H.; Jagannathan, K.; Cunningham, R.; Yu, D.; Lu, X.; Walters, M.I.; Morrison, M.F. Clavulanic Acid Decreases Cocaine Cue Reactivity in Addiction-Related Brain Areas, a Randomized fMRI Pilot Study. Psychopharmacol. Bull. 2024, 8, 8–14. [Google Scholar]
- Jobes, M.L.; Ghitza, U.E.; Epstein, D.H.; Phillips, K.A.; Heishman, S.J.; Preston, K.L. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology 2011, 218, 83–88. [Google Scholar] [CrossRef]
- De Meneses-Gaya, C.; Crippa, J.A.; Hallak, J.E.; Miguel, A.Q.; Laranjeira, R.; Bressan, R.A.; Zuardi, A.W.; Lacerda, A.L. Cannabidiol for the treatment of crack-cocaine craving: An exploratory double-blind study. Braz. J. Psychiatry 2021, 43, 467–476. [Google Scholar] [CrossRef]
- Hersh, D.; Bauer, L.O.; Kranzler, H.R. Carbamazepine and cocaine-cue reactivity. Drug Alcohol Depend. 1995, 39, 213–221. [Google Scholar] [CrossRef]
- Mongeau-Pérusse, V.; Brissette, S.; Bruneau, J.; Conrod, P.; Dubreucq, S.; Gazil, G.; Stip, E.; Jutras-Aswad, D. Cannabidiol as a treatment for craving and relapse in individuals with cocaine use disorder: A randomized placebo-controlled trial. Addiction 2021, 116, 2431–2442. [Google Scholar] [CrossRef]
- Lowry, N.; Marsden, J.; Clydesdale, B.; Eastwood, B.; Havelka, E.M.; Goetz, C. Acute impact of self-guided mental imagery on craving in cocaine use disorder: A mixed-methods analysis of a randomized controlled trial. Addiction 2021, 116, 2418–2430. [Google Scholar] [CrossRef]
- Petrakis, I.L.; Satel, S.L.; Stine, S.; Kosten, T.R.; Namanworth, S.N.; Charney, D.S.; Krystal, J.H. AMPT Effects on Cue-Induced Craving for Cocaine. Am. J. Addict. 1996, 5, 313–320. [Google Scholar]
- Stauffer, C.S.; Musinipally, V.; Suen, A.; Lynch, K.L.; Shapiro, B.; Woolley, J.D. A two-week pilot study of intranasal oxytocin for cocaine-dependent individuals receiving methadone maintenance treatment for opioid use disorder. Addict. Res. Theory 2016, 24, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cunningham, K.A.; Anastasio, N.C.; Bjork, J.M.; Taylor, B.A.; Arias, A.J.; Riley, B.P.; Snyder, A.D.; Moeller, F.G. A serotonergic biobehavioral signature differentiates cocaine use disorder participants administered mirtazapine. Transl. Psychiatry 2022, 12, 187. [Google Scholar] [CrossRef]
- Smelson, D.; Chen, K.W.; Ziedonis, D.; Andes, K.; Lennox, A.; Callahan, L.; Rodrigues, S.; Eisenberg, D. A pilot study of qigong for reducing cocaine craving early in recovery. J. Altern. Complement. Med. 2013, 19, 97–101. [Google Scholar] [CrossRef]
- Price, K.L.; Baker, N.L.; McRae-Clark, A.L.; Saladin, M.E.; DeSantis, S.M.; Ana, E.J.S.; Brady, K.T. A randomized, placebo-controlled laboratory study of the effects of d-cycloserine on craving in cocaine-dependent individuals. Psychopharmacology 2013, 226, 739–746. [Google Scholar] [CrossRef]
- Saladin, M.E.; Gray, K.M.; McRae-Clark, A.L.; LaRowe, S.D.; Yeatts, S.D.; Baker, N.L.; Hartwell, K.J.; Brady, K.T. A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. Psychopharmacology 2013, 226, 721–737. [Google Scholar] [CrossRef]
- Alcorn, J.L.; Pike, E.; Stoops, W.S.; Lile, J.A.; Rush, C.R. A pilot investigation of acute inhibitory control training in cocaine users. Drug Alcohol Depend. 2017, 174, 145–149. [Google Scholar] [CrossRef]
- Smelson, D.A.; Williams, J.; Ziedonis, D.; Sussner, B.D.; Losonczy, M.F.; Engelhart, C.; Kaune, M. A double-blind placebo-controlled pilot study of risperidone for decreasing cue-elicited craving in recently withdrawn cocaine dependent patients. J. Subst. Abus. Treat. 2004, 27, 45–49. [Google Scholar] [CrossRef]
- Bordnick, P.S.; Elkins, R.L.; Orr, T.E.; Walters, P.; Thyer, B.A. Evaluating the relative effectiveness of three aversion therapies designed to reduce craving among cocaine abusers. Behav. Interv. 2004, 19, 1–24. [Google Scholar] [CrossRef]
- Ehrman, R.N.; Robbins, S.J.; Cornish, J.W.; Childress, A.R.; O’brien, C.P. Failure of ritanserin to block cocaine cue reactivity in humans. Drug Alcohol Depend. 1996, 42, 167–174. [Google Scholar] [CrossRef]
- Berger, S.; Reid, M.; Delucchi, K.; Hall, S.; Hall, S.; Mickalian, J.; Crawford, C. Haloperidol antagonism of cue-elicited cocaine craving. J. Clin. Immunol. 1987, 139, 121–148. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S.; Mickalian, J.D.; Delucchi, K.L.; Paul Berger, S. A Nicotine Antagonist, Mecamylamine, Reduces Cue-Induced Cocaine Craving in Cocaine-Dependent Subjects. Neuropsychopharmacology 1999, 20, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S.; Mickalian, J.D.; Delucchi, K.L.; Hall, S.M.; Berger, S.P. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998, 49, 95–104. [Google Scholar] [CrossRef]
- Haeny, A.M.; Chowdhary, A.; King, J.; Sypher, I.; O’Malley, S.S.; Sinha, R. A thematic analysis of stress, substance-cue, and neutral/relaxing events to inform approaches for improving treatment among Black adults who use substances. J. Subst. Use Addict. Treat. 2024, 156, 209184. [Google Scholar] [CrossRef]
- Shulman, G.D. Experience with the cocaine trigger inventory. Adv. Alcohol Subst. Abus. 1989, 8, 71–85. [Google Scholar] [CrossRef]
- Alves, G.S.L.; Araujo, R.B. The use of cooperative games to treat crack-dependent patients hospitalized at a detoxifcation unit. Rev. Bras. Med. Esporte 2012, 18, 77–80. [Google Scholar] [CrossRef]
- Araujo, R.B.; Castro, M.d.G.T.d.; Pedroso, R.S.; Lucena-Santos, P.; Balbinot, A.D.; Fischer, V.J.; Marques, A.C.P.R. Induction and comparison of craving for tobacco, marijuana and crack. Arch. Clin. Psychiatry 2015, 42, 117–121. [Google Scholar] [CrossRef]
- Chaplin, T.M.; Hong, K.; Fox, H.C.; Siedlarz, K.M.; Bergquist, K.; Sinha, R. Behavioral arousal in response to stress and drug cue in alcohol and cocaine addicted individuals versus healthy controls. Hum. Psychopharmacol. 2010, 25, 368–376. [Google Scholar] [CrossRef]
- DiGirolamo, G.J.; Gonzalez, G.; Smelson, D.; Guevremont, N.; Andre, M.I.; Patnaik, P.O.; Zaniewski, Z.R. Increased Depression and Anxiety Symptoms are Associated with More Breakdowns in Cognitive Control to Cocaine Cues in Veterans with Cocaine Use Disorder. J. Dual Diagn. 2017, 13, 298–304. [Google Scholar] [CrossRef]
- Duncan, E.; Boshoven, W.; Harenski, K.; Fiallos, A.; Tracy, H.; Jovanovic, T.; Hu, X.; Drexler, K.; Kilts, C. An fMRI study of the interaction of stress and cocaine cues on cocaine craving in cocaine-dependent men. Am. J. Addict. 2007, 16, 174–182. [Google Scholar] [CrossRef]
- Fotros, A.; Casey, K.F.; Larcher, K.; Verhaeghe, J.A.; Cox, S.M.; Gravel, P.; Reader, A.J.; Dagher, A.; Benkelfat, C.; Leyton, M. Cocaine cue-induced dopamine release in amygdala and hippocampus: A high-resolution PET 18Fallypride study in cocaine dependent participants. Neuropsychopharmacology 2013, 38, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Garavan, H.; Pankiewicz, J.; Bloom, A.; Cho, J.-K.; Sperry, L.; Ross, T.J.; Salmeron, B.J.; Risinger, R.; Kelley, D.; Stein, E.A. Cue-Induced Cocaine Craving: Neuroanatomical Specificity for Drug Users and Drug Stimuli. Am. J. Psychiatry 2000, 157, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Moscon, J.A.; Conti, C.L.; Nakamura-Palacios, E.M. Increased electroencephalographic activity in crack-cocaine users visualizing crack cues. J. Psychiatr. Res. 2016, 83, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Haney, M.; Hanson, C.; Biswal, B.; Hanson, S.J. Modeling Causal Relationship between Brain Regions Within the Drug-Cue Processing Network in Chronic Cocaine Smokers. Neuropsychopharmacology 2015, 40, 2960–2968. [Google Scholar] [CrossRef]
- Reid, M.S.; Prichep, L.S.; Ciplet, D.; O’Leary, S.; Tom, M.; Howard, B.; John, E.R. Quantitative Electroencephalographic Studies of Cue-Induced Cocaine Craving. Clin. Electroencephalogr. 2003, 34, 110–123. [Google Scholar] [CrossRef]
- Reid, M.S.; Flammino, F.; Howard, B.; Nilsen, D.; Prichep, L.S. Topographic imaging of quantitative EEG in response to smoked cocaine self-administration in humans. Neuropsychopharmacology 2006, 31, 872–884. [Google Scholar] [CrossRef]
- Reid, M.S.; Flammino, F.; Howard, B.; Nilsen, D.; Prichep, L.S. Cocaine cue versus cocaine dosing in humans: Evidence for distinct neurophysiological response profiles. Pharmacol. Biochem. Behav. 2008, 91, 155–164. [Google Scholar] [CrossRef]
- Reid, M.S.; Thakkar, V. Valproate treatment and cocaine cue reactivity in cocaine dependent individuals. Drug Alcohol Depend. 2009, 102, 144–150. [Google Scholar] [CrossRef]
- Rosse, R.B.; Alim, T.N.; Johri, S.K.; Hess, A.L.; Deutsch, S.I. Anxiety and pupil reactivity in cocaine dependent subjects endorsing cocaine-induced paranoia: Preliminary report. Addiction 1995, 90, 981–984. [Google Scholar]
- Rosse, R.B.; Kendrick, K.; Anemarie Hess, M.L.; Tanya Aiim, B.N.; Miller, M.; Stephen Deutsch, B.I. Preattentive and Attentive Eye Movements During Visual Scanning of a Cocaine Cue: Correlation With Intensity of Cocaine C ravings. J. Neuropsychiatr. 1997, 9, 91–93. [Google Scholar]
- Saladin, M.E.; Brady, K.T.; Graap, K.; Rothbaum, B.O. A preliminary report on the use of virtual reality technology to elicit craving and cue reactivity in cocaine dependent individuals. Addict. Behav. 2006, 31, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.J.; Marshalek, P.J.; Rezai, A.R.; Lander, L.R.; Berry, J.H.; Haut, M.W. A case report illustrating the effects of repetitive transcranial magnetic stimulation on cue-induced craving in an individual with opioid and cocaine use disorder. Exp. Clin. Psychopharmacol. 2020, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gervilla, M.; Lécuyer, F.; Lehoux, T.; Anthouard, J.; Weiner, L.; Porche, C.; Capobianco, A. Design of a Virtual Cocaine Consumption Scenario for Craving Study. In Proceedings of the The 10th IEEE International Conference on Healthcare Informatics, Rochester, MN, USA, 11–14 June 2022; Available online: https://vimeo.com/568320310 (accessed on 12 June 2024).
- Zhang, S.; Zhornitsky, S.; Angarita, G.A.; Li, C.R. Hypothalamic response to cocaine cues and cocaine addiction severity. Addict. Biol. 2020, 25, e12682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhornitsky, S.; Wang, W.; Dhingra, I.; Le, T.M.; Li, C.-S.R. Cue-elicited functional connectivity of the periaqueductal gray and tonic cocaine craving. Drug Alcohol Depend. 2020, 216, 108240. [Google Scholar] [CrossRef]
- Zhang, S.; Zhornitsky, S.; Le, T.M.; Li, C.S.R. Hypothalamic Responses to Cocaine and Food Cues in Individuals with Cocaine Dependence. Int. J. Neuropsychopharmacol. 2019, 22, 754–764. [Google Scholar] [CrossRef]
- Wilcox, C.E.; Teshiba, T.M.; Merideth, F.; Ling, J.; Mayer, A.R. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011, 115, 137–144. [Google Scholar] [CrossRef]
- Elton, A.; Smitherman, S.; Young, J.; Kilts, C.D. Effects of childhood maltreatment on the neural correlates of stress- and drug cue-induced cocaine craving. Addict. Biol. 2015, 20, 820–831. [Google Scholar] [CrossRef]
- Bell, R.P.; Garavan, H.; Foxe, J.J. Neural correlates of craving and impulsivity in abstinent former cocaine users: Towards biomarkers of relapse risk. Neuropharmacology 2014, 85, 461–470. [Google Scholar] [CrossRef]
- Konova, A.B.; Parvaz, M.A.; Bernstein, V.; Zilverstand, A.; Moeller, S.J.; Delgado, M.R.; Alia-Klein, N.; Goldstein, R.Z. Neural mechanisms of extinguishing drug pleasant cue associations in human addiction: Role of the VMPFC. Addict. Biol. 2019, 24, 88–99. [Google Scholar] [CrossRef]
- Rohsenow, D.J.; Martin, R.A.; Eaton, C.A.; Monti, P.M. Cocaine Craving as a Predictor of Treatment Attrition and Outcomes After Residential Treatment for Cocaine Dependence*. J. Stud. Alcohol Drugs 2007, 68, 641–648. [Google Scholar] [CrossRef]
- Kilts, C.D.; Gross, R.E.; Timothy Ely, B.D.; Karen Drexler, B.P. The Neural Correlates of Cue-Induced Craving in Cocaine-Dependent Women. Am. J. Psychiatry 2004, 161, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Tap, S.; Van Stipriaan, E.; Goudriaan, A.E.; Kaag, A.M. Sex-Dependent Differences in the Neural Correlates of Cocaine and Emotional Cue-Reactivity in Regular Cocaine Users and Non-Drug-Using Controls: Understanding the Role of Duration and Severity of Use. Eur. Addict. Res. 2024, 30, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.J.; Parvaz, M.A.; Shumay, E.; Beebe-Wang, N.; Konova, A.B.; Alia-Klein, N.; Volkow, N.D.; Goldstein, R.Z. Gene × abstinence effects on drug cue reactivity in addiction: Multimodal evidence. J. Neurosci. 2013, 33, 10027–10036. [Google Scholar] [CrossRef] [PubMed]
- Horrell, T.; El-Baz, A.; Baruth, J.; Tasman, A.; Sokhadze, G.; Stewart, C.; Sokhadze, E. Neurofeedback effects on evoked and induced EEG gamma band reactivity to drug-related Cues in Cocaine addiction. J. Neurother. 2010, 14, 195–216. [Google Scholar] [CrossRef]
- Kilts, C.D.; Schweitzer, J.B.; Quinn, C.K.; Gross, R.E.; Faber, T.L.; Muhammad, F.; Ely, T.D.; Hoffman, J.M.; Drexler, K.P.G. Neural Activity Related to Drug Craving in Cocaine Addiction. Arch. Gen. Psychiatry 2001, 58, 334–341. [Google Scholar] [CrossRef]
- Hester, R.; Garavan, H. Neural mechanisms underlying drug-related cue distraction in active cocaine users. Pharmacol. Biochem. Behav. 2009, 93, 270–277. [Google Scholar] [CrossRef]
- Parvaz, M.A.; Malaker, P.; Zilverstand, A.; Moeller, S.J.; Alia-Klein, N.; Goldstein, R.Z. Attention bias modification in drug addiction: Enhancing control of subsequent habits. Proc. Natl. Acad. Sci. USA 2021, 118, e2012941118. [Google Scholar] [CrossRef]
- Kearney-Ramos, T.E.; Dowdle, L.T.; Lench, D.H.; Mithoefer, O.J.; Devries, W.H.; George, M.S.; Anton, R.F.; Hanlon, C.A. Transdiagnostic Effects of Ventromedial Prefrontal Cortex Transcranial Magnetic Stimulation on Cue Reactivity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 599–609. [Google Scholar] [CrossRef]
- D’Amour-Horvat, V.; Cox, S.M.L.; Dagher, A.; Kolivakis, T.; Jaworska, N.; Leyton, M. Cocaine cue-induced mesocorticolimbic activation in cocaine users: Effects of personality traits, lifetime drug use, and acute stimulant ingestion. Addict. Biol. 2022, 27, e13094. [Google Scholar] [CrossRef]
- Li, C.S.R.; Kosten, T.R.; Sinha, R. Sex differences in brain activation during stress imagery in abstinent cocaine users: A functional magnetic resonance imaging study. Biol. Psychiatry 2005, 57, 487–494. [Google Scholar] [CrossRef]
- Fernández-Calderón, F.; Lozano, O.M.; Moraleda-Barreno, E.; Lorca-Marín, J.A.; Díaz-Batanero, C. Initial orientation vs maintenance of attention: Relationship with the severity of dependence and therapeutic outcome in a sample of cocaine use disorder patients. Addict. Behav. 2021, 116, 106834. [Google Scholar] [CrossRef] [PubMed]
- Childress, A.R.; Ehrman, R.N.; Wang, Z.; Li, Y.; Sciortino, N.; Hakun, J.; O’Brien, C.P. Prelude to passion: Limbic activation by “unseen” drug and sexual cues. PLoS ONE 2008, 3, e1506. [Google Scholar] [CrossRef]
- Strickland, J.C.; Marks, K.R.; Beckmann, J.S.; Lile, J.A.; Rush, C.R.; Stoops, W.W. Contribution of cocaine-related cues to concurrent monetary choice in humans. Psychopharmacology 2018, 235, 2871–2881. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bujedo, J.; Domínguez-Salas, S.; Pérez-Moreno, P.J.; Moraleda-Barreno, E.; Lozano, O.M. Reliability and validity evidence of a new interpretation bias task in patients diagnosed with drug use disorder: A preliminary study of the Word Association Task for Drug Use Disorder (WAT-DUD). Am. J. Drug Alcohol Abus. 2019, 45, 365–376. [Google Scholar] [CrossRef]
- Regier, P.S.; Jagannathan, K.; Franklin, T.R.; Wetherill, R.R.; Langleben, D.D.; Gawyrsiak, M.; Kampman, K.M.; Childress, A.R. Sustained brain response to repeated drug cues is associated with poor drug-use outcomes. Addict. Biol. 2021, 26, e13028. [Google Scholar] [CrossRef]
- Moeller, S.J.; Maloney, T.; Parvaz, M.A.; Dunning, J.P.; Alia-Klein, N.; Woicik, P.A.; Hajcak, G.; Telang, F.; Wang, G.-J.; Volkow, N.D.; et al. Enhanced Choice for Viewing Cocaine Pictures in Cocaine Addiction. Biol. Psychiatry. 2009, 66, 169–176. [Google Scholar] [CrossRef]
- Dudish-Poulsen, S.A.; Hatsukami, D.K. Dissociation between subjective and behavioral responses after cocaine stimuli presentations 1. Drug Alcohol Depend. 1997, 47, 1–9. [Google Scholar] [CrossRef]
- Penetar, D.M.; Burgos-Robles, A.; Trksak, G.H.; MacLean, R.R.; Dunlap, S.; Lee, D.Y.-W.; Lukas, S.E. Effects of transcutaneous electric acupoint stimulation on drug use and responses to cue-induced craving: A pilot study. Chin. Med. 2012, 7, 14. [Google Scholar] [CrossRef]
- Tull, M.T.; McDermott, M.J.; Gratz, K.L.; Coffey, S.F.; Lejuez, C.W. Cocaine-related attentional bias following trauma cue exposure among cocaine dependent in-patients with and without post-traumatic stress disorder. Addiction 2011, 106, 1810–1818. [Google Scholar] [CrossRef]
- Kearney-Ramos, T.E.; Dowdle, L.T.; Mithoefer, O.J.; Devries, W.; George, M.S.; Hanlon, C.A. State-dependent effects of ventromedial prefrontal cortex continuous thetaburst stimulation on cocaine cue reactivity in chronic cocaine users. Front. Psychiatry 2019, 10, 1127–1134. [Google Scholar] [CrossRef]
- Parvaz, M.A.; Moeller, S.J.; Goldstein, R.Z. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry 2016, 73, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Hochheimer, M.; Strickland, J.C.; Rabinowitz, J.A.; Ellis, J.D.; Bergeria, C.L.; Hobelmann, J.G.; Huhn, A.S. The impact of opioid-stimulant co-use on tonic and cue-induced craving. J. Psychiatr. Res. 2023, 164, 15–22. [Google Scholar] [CrossRef]
- Wang, W.; Zhornitsky, S.; Zhang, S.; Li, C.S.R. Noradrenergic correlates of chronic cocaine craving: Neuromelanin and functional brain imaging. Neuropsychopharmacology 2021, 46, 851–859. [Google Scholar] [CrossRef]
- Marks, K.R.; Pike, E.; Stoops, W.W.; Rush, C.R. Alcohol Administration Increases Cocaine Craving But Not Cocaine Cue Attentional Bias. Alcohol. Clin. Exp. Res. 2015, 39, 1823–1831. [Google Scholar] [CrossRef]
- Strickland, J.; Reynolds, A.; Stoops, W. Regulation of Cocaine Craving by Cognitive Strategies in an Online Sample of Cocaine Users. Psychol. Addict. Behav. 2016, 30, 607–612. [Google Scholar] [CrossRef]
- Prisciandaro, J.J.; McRae-Clark, A.L.; Myrick, H.; Henderson, S.; Brady, K.T. Brain activation to cocaine cues and motivation/treatment status. Addict. Biol. 2014, 19, 240–249. [Google Scholar] [CrossRef]
- Negrete, J.C.; Emil, S. Cue-evoked arousal in cocaine users: A study of variance and predictive value. Drug Alcohol Depend. 1992, 30, 187–192. [Google Scholar] [CrossRef]
- Ray, S.; Pandina, R.; Bates, M.E. Memory for drug-related visual stimuli in young adult, cocaine-dependent polydrug users. Am. J. Drug Alcohol Abus. 2014, 40, 170–175. [Google Scholar] [CrossRef]
- Hanlon, C.A.; Dowdle, L.T.; Gibson, N.B.; Li, X.; Hamilton, S.; Canterberry, M.; Hoffman, M. Cortical substrates of cue-reactivity in multiple substance dependent populations: Transdiagnostic relevance of the medial prefrontal cortex. Transl. Psychiatry 2018, 8, 186. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Logan, J.; Childress, A.-R.; Jayne, M.; Ma, Y.; Wong, C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage 2008, 39, 1266–1273. [Google Scholar] [CrossRef]
- Franken, I.H.A.; Hulstijn, K.P.; Stam, C.J.; Hendriks, V.M.; Van Den Brink, W. Two new neurophysiological indices of cocaine craving: Evoked brain potentials and cue modulated startle reflex. J. Psychopharmacol. 2004, 18, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Webber, H.E.; Kessler, D.A.; Lathan, E.C.; Wardle, M.C.; Green, C.E.; Schmitz, J.M.; Lane, S.D.; Vujanovic, A.A. Posttraumatic stress symptom clusters differentially predict late positive potential to cocaine imagery cues in trauma-exposed adults with cocaine use disorder. Drug Alcohol Depend. 2021, 227, 108929. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.R.; Roberts, W.; Stoops, W.W.; Pike, E.; Fillmore, M.T.; Rush, C.R. Fixation time is a sensitive measure of cocaine cue attentional bias. Addiction 2014, 109, 1501–1508. [Google Scholar] [CrossRef]
- Dias, N.R.; Schmitz, J.M.; Rathnayaka, N.; Red, S.D.; Sereno, A.B.; Moeller, F.G.; Lane, S.D. Anti-saccade error rates as a measure of attentional bias in cocaine dependent subjects. Behav. Brain Res. 2015, 292, 493–499. [Google Scholar] [CrossRef]
- Kaag, A.M.; Reneman, L.; Homberg, J.; van den Brink, W.; van Wingen, G.A. Enhanced amygdala-striatal functional connectivity during the processing of cocaine cues in male cocaine users with a history of childhood trauma. Front. Psychiatry. 2018, 9, 70. [Google Scholar] [CrossRef]
- Schlauch, R.C.; Breiner, M.J.; Stasiewicz, P.R.; Christensen, R.L.; Lang, A.R. Women inmate substance abusers’ reactivity to visual alcohol, cigarette, marijuana, and crack-cocaine cues: Approach and avoidance as separate dimensions of reactivity. J. Psychopathol. Behav. Assess. 2013, 35, 45–56. [Google Scholar] [CrossRef][Green Version]
- Sokhadze, E.; Singh, S.; Stewart, C.; Hollifield, M.; El-Baz, A.; Tasman, A. Attentional Bias to drug- and Stress-related pictorial cues in cocaine addiction comorbid with posttraumatic stress disorder. J. Neurother. 2008, 12, 205–225. [Google Scholar] [CrossRef]
- Marks, K.R.; Pike, E.; Stoops, W.W.; Rush, C.R. Test-retest reliability of eye tracking during the visual probe task in cocaine-using adults. Drug Alcohol Depend. 2014, 145, 235–237. [Google Scholar] [CrossRef][Green Version]
- Franken, I.H.A.; Dietvorst, R.C.; Hesselmans, M.; Franzek, E.J.; Van De Wetering, B.J.M.; Van Strien, J.W. Cocaine craving is associated with electrophysiological brain responses to cocaine-related stimuli. Addict. Biol. 2008, 13, 386–392. [Google Scholar] [CrossRef]
- Alcorn, J.L.; Strickland, J.C.; Lile, J.A.; Stoops, W.W.; Rush, C.R. Acute methylphenidate administration reduces cocaine-cue attentional bias. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 103, 109974. [Google Scholar] [CrossRef]
- Pike, E.; Stoops, W.W.; Fillmore, M.T.; Rush, C.R. Drug-related stimuli impair inhibitory control in cocaine abusers. Drug Alcohol Depend. 2013, 133, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.R.; Alcorn, J.L.; Stoops, W.W.; Rush, C.R. Cigarette cue attentional bias in cocaine- smoking and non-cocaine-using cigarette smokers. Nicotine Tob. Res. 2016, 18, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Vincent, G.M.; Cope, L.M.; King, J.; Nyalakanti, P.; Kiehl, K.A. Callous-Unemotional Traits Modulate Brain Drug Craving Response in High-Risk Young Offenders. J. Abnorm. Child. Psychol. 2018, 46, 993–1009. [Google Scholar] [CrossRef]
- Marks, K.R.; Pike, E.; Stoops, W.W.; Rush, C.R. The magnitude of drug attentional bias is specific to substance use disorder. Psychol. Addict. Behav. 2015, 29, 690–695. [Google Scholar] [CrossRef]
- Tull, M.T.; Gratz, K.L.; McDermott, M.J.; Bordieri, M.J.; Daughters, S.B.; Lejuez, C.W. The Role of Emotion Regulation Difficulties in the Relation Between PTSD Symptoms and the Learned Association Between Trauma-Related and Cocaine Cues. Subst. Use Misuse 2016, 51, 1318–1329. [Google Scholar] [CrossRef]
- Mahoney, J.J.; Haut, M.W.; Carpenter, J.; Ranjan, M.; Thompson-Lake, D.G.Y.; Marton, J.L.; Zheng, W.; Berry, J.H.; Tirumalai, P.; Mears, A.; et al. Low-intensity focused ultrasound targeting the nucleus accumbens as a potential treatment for substance use disorder: Safety and feasibility clinical trial. Front. Psychiatry 2023, 14, 1211566. [Google Scholar] [CrossRef]
- Prisciandaro, J.J.; Joseph, J.E.; Myrick, H.; McRae-Clark, A.L.; Henderson, S.; Pfeifer, J.; Brady, K.T. The relationship between years of cocaine use and brain activation to cocaine and response inhibition cues. Addiction 2014, 109, 2062–2070. [Google Scholar] [CrossRef]
- Van De Laar, M.C.; Licht, R.; Franken, I.H.A.; Hendriks, V.M. Event-related potentials indicate motivational relevance of cocaine cues in abstinent cocaine addicts. Psychopharmacology 2004, 177, 121–129. [Google Scholar] [CrossRef]
- Bardeen, J.R.; Dixon-Gordon, K.L.; Tull, M.T.; Lyons, J.A.; Gratz, K.L. An investigation of the relationship between borderline personality disorder and cocaine-related attentional bias following trauma cue exposure: The moderating role of gender. Compr. Psychiatry 2014, 55, 113–122. [Google Scholar] [CrossRef]
- Díaz-Batanero, C.; Domínguez-Salas, S.; Moraleda, E.; Fernández-Calderón, F.; Lozano, O.M. Attentional bias toward alcohol stimuli as a predictor of treatment retention in cocaine dependence and alcohol user patients. Drug Alcohol Depend. 2018, 182, 40–47. [Google Scholar] [CrossRef]
- Parvaz, M.A.; Moeller, S.J.; Malaker, P.; Sinha, R.; Alia-Klein, N.; Goldstein, R.Z. Abstinence reverses EEG-indexed attention bias between drug-related and pleasant stimuli in cocaine-addicted individuals. J. Psychiatry Neurosci. 2017, 42, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hester, R.; Dixon, V.; Garavan, H. A consistent attentional bias for drug-related material in active cocaine users across word and picture versions of the emotional Stroop task. Drug Alcohol Depend. 2006, 81, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.J.; Maloney, T.; Parvaz, M.A.; Alia-Klein, N.; Woicik, P.A.; Telang, F.; Wang, G.-J.; Volkow, N.D.; Goldstein, R.Z. Impaired insight in cocaine addiction: Laboratory evidence and effects on cocaine-seeking behaviour. Brain 2010, 133, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- LaRowe, S.; Myrick, H.; Hedden, S.; Mardikian, P.; Saladin, M.; McRae, A.; Brady, K.; Kalivas, P.; Malcolm, R. Is Cocaine Desire Reduced by N-Acetylcysteine? Am. J. Psychiatry 2007, 164, 1115–1117. [Google Scholar] [CrossRef]
- Dunning, J.P.; Parvaz, M.A.; Hajcak, G.; Maloney, T.; Alia-Klein, N.; Woicik, P.A.; Telang, F.; Wang, G.-J.; Volkow, N.D.; Goldstein, R.Z. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users—An ERP study. Eur. J. Neurosci. 2011, 33, 1716–1723. [Google Scholar] [CrossRef]
- Cope, L.M.; Vincent, G.M.; Jobelius, J.L.; Nyalakanti, P.K.; Calhoun, V.D.; Kiehl, K.A. Psychopathic traits modulate brain responses to drug cues in incarcerated offenders. Front. Hum. Neurosci. 2014, 8, 87. [Google Scholar] [CrossRef]
- Moeller, S.J.; Hajcak, G.; Parvaz, M.A.; Dunning, J.P.; Volkow, N.D.; Goldstein, R.Z. Psychophysiological prediction of choice: Relevance to insight and drug addiction. Brain 2012, 135, 3481–3494. [Google Scholar] [CrossRef]
- Webber, H.E.; de Dios, C.; Wardle, M.C.; Suchting, R.; Green, C.E.; Schmitz, J.M.; Lane, S.D.; Versace, F. Electrophysiological Responses to Emotional and Cocaine Cues Reveal Individual Neuroaffective Profiles in Cocaine Users. Exp. Clin. Psychopharmacol. 2021, 30, 514–524. [Google Scholar] [CrossRef]
- Montgomery, C.; Field, M.; Atkinson, A.M.; Cole, J.C.; Goudie, A.J.; Sumnall, H.R. Effects of alcohol preload on attentional bias towards cocaine-related cues. Psychopharmacology 2010, 210, 365–375. [Google Scholar] [CrossRef]
- Maria, M.M.M.S.; McRae-Clark, A.; Baker, N.L.; Ramakrishnan, V.; Brady, K.T. Yohimbine administration and cue-reactivity in cocaine-dependent individuals. Psychopharmacology 2014, 231, 4157–4165. [Google Scholar] [CrossRef]
- Campbell, L.; Galuska, C.; McRae-Clark, A.; Sherman, B. Cortisol reactivity and situational drug use in cocaine-dependent females. Psychiatry Res. 2019, 282, 112611. [Google Scholar] [CrossRef] [PubMed]
- Bonson, K.R.; Grant, S.J.; Contoreggi, C.S.; Links, J.M.; Metcalfe, J.; Weyl, H.L.; London, E.D. Neural Systems and Cue-Induced Cocaine Craving. Neuropsychopharmacology 2002, 26, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Milella, M.S.; Fotros, A.; Gravel, P.; Casey, K.F.; Larcher, K.; Verhaeghe, J.A.; Cox, S.M.; Reader, A.J.; Dagher, A.; Benkelfat, C.; et al. Cocaine cue-induced dopamine release in the human prefrontal cortex. J. Psychiatry Neurosci. 2016, 41, 322–330. [Google Scholar] [CrossRef]
- Robbins, S.J.; Ehrman, R.N.; Childress, A.R.; O’brien, C.P. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999, 53, 223–230. [Google Scholar] [CrossRef]
- Satel, S.L.; Krystal, J.H.; Delgado, P.L.; Kosten, T.R.; Charney, D.S. Tryptophan Depletion and Attenuation of Cue-Induced Craving for Cocaine. Am. J. Psychiatry 1995, 152, 778–783. [Google Scholar]
- DeSantis, S.M.; Bandyopadhyay, D.; Back, S.E.; Brady, K.T. Non-treatment laboratory stress- and cue-reactivity studies are associated with decreased substance use among drug-dependent individuals. Drug Alcohol Depend. 2009, 105, 227–233. [Google Scholar] [CrossRef][Green Version]
- Waldrop, A.E.; Price, K.L.; DeSantis, S.M.; Simpson, A.N.; Back, S.E.; McRae, A.L.; Spratt, E.G.; Kreek, M.J.; Brady, K.T. Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues. Psychoneuroendocrinology 2010, 35, 798–806. [Google Scholar] [CrossRef]
- Back, S.E.; Hartwell, K.; DeSantis, S.M.; Saladin, M.; McRae-Clark, A.L.; Price, K.L.; Maria, M.M.M.-S.; Baker, N.L.; Spratt, E.; Kreek, M.J.; et al. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010, 106, 21–27. [Google Scholar] [CrossRef]
- Bauer, L.O.; Kranzler, H.R. Electroencephalographic Activity and Mood in Cocaine-Dependent Outpatients: Effects of Cocaine Cue Exposure. Biol. Psychiatry 1994, 36, 189–197. [Google Scholar] [CrossRef]
- Robbins, S.J.; Ehrman, R.N.; Childress, A.R.; Cornish, J.W.; O’brien, C.P. Mood state and recent cocaine use are not associated with levels of cocaine cue reactivity. Drug Alcohol Depend. 2000, 59, 33–42. [Google Scholar] [CrossRef]
- Kelly Avants, S.; Margolin, A.; Kosten, T.R.; Cooney, N.L. Differences between responders and nonresponders to cocaine cues in the laboratory. Addict. Behav. 1995, 20, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.J.; Ehrman, R.N.; Childress, A.R.; O’brien, C.P. Relationships Among Physiological and Self-report Responses Produced by Cocaine Related Cues. Addict. Behav. 1997, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.S.; Ciplet, D.; O’Leary, S.; Branchey, M.; Buydens-Branchey, L.; Angrist, B. Sensitization to the psychosis-inducing effects of cocaine compared with measures of cocaine craving and cue reactivity. Am. J. Addict. 2004, 13, 305–315. [Google Scholar] [CrossRef]
- Ehrman, R.N.; Robbins, S.J.; Childress, A.R.; Goehl, L.; Hole, A.V.; O’brien, C.P. Laboratory Exposure to Cocaine Cues Does Not Increase Cocaine Use by Outpatient Subjects. J. Subst. Abus. Treat. 1998, 15, 431–435. [Google Scholar] [CrossRef]
- Smelson, D.; Yu, L.; Buyske, S.; Gonzalez, G.; Tischfield, J.; Deutsch, C.K.; Ziedonis, D. Genetic association of GABA-A receptor alpha-2 and Mu opioid receptor with cocaine Cue-reactivity: Evidence for inhibitory synaptic neurotransmission involvement in cocaine dependence. Am. J. Addict. 2012, 21, 411–415. [Google Scholar] [CrossRef]
- Ehrman, R.N.; Robbins, S.J.; Childress, A.R.; O’brien, C.P. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology 1992, 107, 523–529. [Google Scholar] [CrossRef]
- Cox, S.M.L.; Yau, Y.; Larcher, K.; Durand, F.; Kolivakis, T.; Delaney, J.S.; Dagher, A.; Benkelfat, C.; Leyton, M. Cocaine cue-induced dopamine release in recreational cocaine users. Sci. Rep. 2017, 7, srep46665. [Google Scholar] [CrossRef]
- Johnson, B.A.; Chen, Y.R.; Schmitz, J.; Bordnick, P.; Shafer, A. Cue reactivity in cocaine-dependent subjects: Effects of cue type and cue modality. Addict. Behav. 1998, 23, 7–15. [Google Scholar] [CrossRef]
- Killeen, T.K.; Brady, K.T. Skin conductance hypo-responding in recently abstinent cocaine dependent inpatients. Am. J. Addict. 2000, 9, 154–162. [Google Scholar] [CrossRef]
- Volkow, N.D.; Tomasi, D.; Wang, G.-J.; Logan, J.; Alexoff, D.L.; Jayne, M.; Fowler, J.S.; Wong, C.; Yin, P.; Du, C. Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers. Mol. Psychiatry 2014, 19, 1037–1043. [Google Scholar] [CrossRef]
- Childress, A.R.; David Mozley, P.; Mcelgin, W.; Fitzgerald, J.; Reivich, M.; O’brien, C.P. Limbic Activation During Cue-Induced Cocaine Craving. Am. J. Psychiatry 1999, 156, 11–18. [Google Scholar] [CrossRef] [PubMed]
- De La Garza, R.; Newton, T.F.; Kalechstein, A.D. Risperidone diminishes cocaine-induced craving. Psychopharmacology 2005, 178, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.F.; Kuwabara, H.; Schretlen, D.J.; Bonson, K.R.; Zhou, Y.; Nandi, A.; Brašić, J.R.; Kimes, A.S.; A Maris, M.; Kumar, A.; et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 2006, 31, 2716–2727. [Google Scholar] [CrossRef]
- Antons, S.; Yip, S.W.; Lacadie, C.M.; Dadashkarimi, J.; Scheinost, D.; Brand, M.; Potenza, M.N. Connectome-based prediction of craving in gambling disorder and cocaine use disorder. Dialogues Clin. Neurosci. 2023, 25, 33–42. [Google Scholar] [CrossRef]
- Giasson-Gariépy, K.; Potvin, S.; Ghabrash, M.; Bruneau, J.; Jutras-Aswad, D. Cannabis and cue-induced craving in cocaine-dependent individuals: A pilot study. Addict. Behav. 2017, 73, 4–8. [Google Scholar] [CrossRef]
- Volkow, N.D.; Tomasi, D.; Wang, G.-J.; Fowler, J.S.; Telang, F.; Goldstein, R.Z.; Alia-Klein, N.; Wong, C. Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PLoS ONE 2011, 6, e16573. [Google Scholar] [CrossRef]
- Kober, H.; Lacadie, C.M.; Wexler, B.E.; Malison, R.T.; Sinha, R.; Potenza, M.N. Brain Activity during Cocaine Craving and Gambling Urges: An fMRI Study. Neuropsychopharmacology 2016, 41, 628–637. [Google Scholar] [CrossRef]
- Denomme, W.J.; Shane, M.S. History of withdrawal modulates drug- and food-cue reactivity in cocaine dependent participants. Drug Alcohol Depend. 2020, 208, 107815. [Google Scholar] [CrossRef]
- Vaccaro, A.G.; Lacadie, C.M.; Potenza, M.N. Intrinsic connectivity demonstrates a shared role of the posterior cingulate for cue reactivity in both gambling and cocaine use disorders. Addict. Behav. 2024, 155, 108027. [Google Scholar] [CrossRef]
- Margolin, A.; Kelly Avants, S.; Kosten, T.R. Cue-Elicited Cocaine Craving and Autogenic Relaxation Association With Treatment Outcome. J. Subst. Abus. Treat. 1994, 11, 549–552. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Logan, J.; Childress, A.-R.; Jayne, M.; Ma, Y.; Wong, C. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J. Neurosci. 2006, 26, 6583–6588. [Google Scholar] [CrossRef] [PubMed]
- Scala, S.G.; Kang, M.S.; Cox, S.M.L.; Rosa-Neto, P.; Massarweh, G.; Leyton, M. Mesocorticolimbic function in cocaine polydrug users: A multimodal study of drug cue reactivity and cognitive regulation. Addict. Biol. 2024, 29, e13358. [Google Scholar] [CrossRef]
- Martinotti, G.; Pettorruso, M.; Montemitro, C.; Spagnolo, P.A.; Martellucci, C.A.; Di Carlo, F.; Fanella, F.; di Giannantonio, M. Repetitive transcranial magnetic stimulation in treatment-seeking subjects with cocaine use disorder: A randomized, double-blind, sham-controlled trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 116, 110513. [Google Scholar] [CrossRef]
- Smelson, D.A.; Losonczy, M.F.; Davis, C.W.; Kaune, M.; Williams, J.; Ziedonis, D. Risperidone Decreases Craving and Relapses in Individuals with Schizophrenia and Cocaine Dependence. Can. J. Psychiatry 2002, 47, 671–675. [Google Scholar] [CrossRef]
- Lam, S.C.B.; Wang, Z.; Li, Y.; Franklin, T.; O’brien, C.; Magland, J.; Childress, A.R. Wavelet-transformed temporal cerebral blood flow signals during attempted inhibition of cue-induced cocaine craving distinguish prognostic phenotypes. Drug Alcohol Depend. 2013, 128, 140–147. [Google Scholar] [CrossRef][Green Version]
- Wexler, B.E.; Gottschalk, C.H.; Fulbright, R.K.; Prohovnik, I.; Lacadie, C.M.; Rounsaville, B.J.; Gore, J.C. Functional Magnetic Resonance Imaging of Cocaine Craving. Am. J. Psychiatry 2001, 158, 86–95. [Google Scholar] [CrossRef]
- Maas, L.C.; Lukas, S.E.; Kaufman, M.J.; Weiss, R.D.; Daniels, S.L.; Rogers, V.W.; Kukes, T.J.; Renshaw, P.F. Functional Magnetic Resonance Imaging of Human Brain Activation During Cue-Induced Cocaine Craving. Am. J. Psychiatry 1998, 155, 124–126. [Google Scholar] [CrossRef]
- Smelson, D.; Losonczy, M.; Kilker, C.; Kind, J.; Williams, J.; Ziedonis, D. An analysis of cue reactivity among persons with and without schizophrenia who are addicted to cocaine. Psychiatr. Serv. 2002, 53, 1612–1616. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Telang, F.; Logan, J.; Jayne, M.; Ma, Y.; Pradhan, K.; Wong, C.; Swanson, J.M. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage 2010, 49, 2536–2543. [Google Scholar] [CrossRef]
- Smelson, D.A.; Roy, M.; Roy, A.; Santana, S. Electroretinogram in withdrawn cocaine-dependent subjects: Relationship to cue-elicited craving. Br. J. Psychiatry 1998, 172, 537–539. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Tomasi, D.; Telang, F.; Fowler, J.S.; Pradhan, K.; Jayne, M.; Logan, J.; Goldstein, R.Z.; Alia-Klein, N.; et al. Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers. PLoS ONE 2010, 5, e11509. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Berman, J.; Gonzalez, B.; Roy, M. Cerebrospinal fluid monoamine metabolites in cocaine patients no relationship to cue-induced craving. J. Psychopharmacol. 2002, 16, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Pettorruso, M.; Martinotti, G.; Santacroce, R.; Montemitro, C.; Fanella, F.; di Giannantonio, M. rTMS Reduces Psychopathological Burden and Cocaine Consumption in Treatment-Seeking Subjects With Cocaine Use Disorder: An Open Label, Feasibility Study. Front. Psychiatry 2019, 10, 621. [Google Scholar] [CrossRef]
- Smelson, D.; Roy, A.; Roy, M. Risperidone Diminishes Cue-Elicited Craving in Withdrawn Cocaine-Dependent Patients. Can. J. Psychiatry 1997, 42, 984. [Google Scholar] [CrossRef]
- Foltin, R.W.; Fischman, M.W. Residual effects of repeated cocaine smoking in humans. Drug Alcohol Depend. 1997, 47, 117–124. [Google Scholar] [CrossRef]
- Liu, X.; Vaupel, D.B.; Grant, S.; London, E.D. Effect of Cocaine-Related Environmental Stimuli on the Spontaneous Electroencephalogram in Polydrug Abusers. Neuropsychopharmacology 1998, 19, 10–17. [Google Scholar]
- DiGirolamo, G.J.; Smelson, D.; Guevremont, N. Cue-induced craving in patients with cocaine use disorder predicts cognitive control deficits toward cocaine cues. Addict. Behav. 2015, 47, 86–90. [Google Scholar] [CrossRef]
- Leyton, M.; Casey, K.F.; Delaney, J.S.; Kolivakis, T.; Benkelfat, C. Cocaine craving, euphoria, and self-administration: A preliminary study of the effect of catecholamine precursor depletion. Behav. Neurosci. 2005, 119, 1619–1627. [Google Scholar] [CrossRef]
- Haney, M.; Rubin, E.; Denson, R.K.; Foltin, R.W. Modafinil reduces smoked cocaine self-administration in humans: Effects vary as a function of cocaine ‘priming’ and cost. Drug Alcohol Depend. 2021, 221, 108554. [Google Scholar] [CrossRef]
- Grant, S.; London, E.D.; Newlin, D.B.; Villemagne, V.L.; Liu, X.; Contoreggi, C.; Phillips, R.L.; Kimes, A.S.; Margolin, A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. USA 1996, 93, 12040–12045. [Google Scholar] [CrossRef]
- Copersino, M.L.; Serper, M.R.; Vadhan, N.; Goldberg, B.R.; Richarme, D.; Chou, J.C.-Y.; Stitzer, M.; Cancro, R. Cocaine craving and attentional bias in cocaine-dependent schizophrenic patients. Psychiatry Res. 2004, 128, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Land, M.A.; Ramesh, D.; Miller, A.L.; Pyles, R.B.; Cunningham, K.A.; Moeller, F.G.; Anastasio, N.C. Methylation Patterns of the HTR2A Associate With Relapse-Related Behaviors in Cocaine-Dependent Participants. Front. Psychiatry 2020, 11, 532. [Google Scholar] [CrossRef] [PubMed]
- Anastasio, N.C.; Liu, S.; Maili, L.; Swinford, S.E.; Lane, S.D.; Fox, R.G.; Hamon, S.C.; A Nielsen, D.; A Cunningham, K.; Moeller, F.G. Variation within the serotonin (5-HT) 5-HT2C receptor system aligns with vulnerability to cocaine cue reactivity. Transl. Psychiatry 2014, 4, e369. [Google Scholar] [CrossRef]
- Ceceli, A.; A Parvaz, M.; King, S.; Schafer, M.; Malaker, P.; Sharma, A.; Alia-Klein, N.; Goldstein, R.Z. Altered prefrontal signaling during inhibitory control in a salient drug context in cocaine use disorder. Cereb. Cortex 2023, 33, 597–611. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Tomasi, D.; Alia-Klein, N.; Carrillo, J.H.; Maloney, T.; Woicik, P.A.; Wang, R.; Telang, F.; Volkow, N.D. Dopaminergic response to drug words in cocaine addiction. J. Neurosci. 2009, 29, 6001–6006. [Google Scholar] [CrossRef]
- Franken, I.H.A.; Kroon, L.Y.; Hendriks, V.M. Influences of individual differences in craving and obsessive cocaine thoughts on attentional processes in cocaine abuse pateints. Addict. Behav. 2000, 25, 99–102. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Woicik, P.A.; Maloney, T.; Tomasi, D.; Alia-Klein, N.; Shan, J.; Honorio, J.; Samaras, D.; Wang, R.; Telang, F.; et al. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc. Natl. Acad. Sci. USA 2010, 107, 16667–16672. [Google Scholar] [CrossRef]
- Goldstein, R.Z.; Alia-Klein, N.; Tomasi, D.; Carrillo, J.H.; Maloney, T.; Woicik, P.A.; Wang, R.; Telang, F.; Volkow, N.D. Anterior cingulate cortex hypoactivations to an emotionally salient task in cocaine addiction. Proc. Natl. Acad. Sci. USA 2009, 106, 9453–9458. [Google Scholar] [CrossRef]
- Ma, L.; Steinberg, J.L.; Cunningham, K.A.; Bjork, J.M.; Lane, S.D.; Schmitz, J.M.; Burroughs, T.; Narayana, P.A.; Kosten, T.R.; Bechara, A.; et al. Altered anterior cingulate cortex to hippocampus effective connectivity in response to drug cues in men with cocaine use disorder. Psychiatry Res. Neuroimaging 2018, 271, 59–66. [Google Scholar] [CrossRef]
- Smith, D.G.; Simon Jones, P.; Bullmore, E.T.; Robbins, T.W.; Ersche, K.D. Enhanced orbitofrontal cortex function and lack of attentional bias to cocaine cues in recreational stimulant users. Biol. Psychiatry 2014, 75, 124–131. [Google Scholar] [CrossRef]
- Carpenter, K.M.; Martinez, D.; Vadhan, N.P.; Barnes-Holmes, D.; Nunes, E.V. Measures of attentional bias and relational responding are associated with behavioral treatment outcome for cocaine dependence. Am. J. Drug Alcohol Abus. 2012, 38, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; N’Diaye, K.; Fortias, M.; Mallet, L.; Vorspan, F. I can’t get it off my mind: Attentional bias in former and current cocaine addiction. J. Psychopharmacol. 2020, 34, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.Z.; Woicik, P.A.; Lukasik, T.; Maloney, T.; Volkow, N.D. Drug Fluency: A Potential Marker for Cocaine Use Disorders. Drug Alcohol Depend. 2007, 89, 97–101. [Google Scholar] [CrossRef][Green Version]
- Gardini, S.; Caffarra, P.; Venneri, A. Decreased drug-cue-induced attentional bias in individuals with treated and untreated drug dependence. Acta Neuropsychiatr. 2009, 21, 179–185. [Google Scholar] [CrossRef]
- Vadhan, N.P.; Carpenter, K.M.; Copersino, M.L.; Hart, C.L.; Foltin, R.W.; Nunes, E.V. Attentional bias towards cocaine-related stimuli: Relationship to treatment-seeking for cocaine dependence. Am. J. Drug Alcohol Abus. 2007, 33, 727–736. [Google Scholar] [CrossRef]
- Liu, S.; Lane, S.D.; Schmitz, J.M.; Waters, A.J.; Cunningham, K.A.; Moeller, F.G. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am. J. Drug Alcohol Abus. 2011, 37, 117–122. [Google Scholar] [CrossRef]
- Goldstein, R.; Tomasi, D.; Rajaram, S.; Cottone, L.; Zhang, L.; Maloney, T.; Telang, F.; Alia-Klein, N.; Volkow, N. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience 2007, 144, 1153–1159. [Google Scholar] [CrossRef]
- Kennedy, A.P.; Gross, R.E.; Ely, T.; Drexler, K.P.G.; Kilts, C.D. Clinical correlates of attentional bias to drug cues associated with cocaine dependence. Am. J. Addict. 2014, 23, 478–484. [Google Scholar] [CrossRef]
- Waters, A.J.; Marhe, R.; Franken, I.H.A. Attentional bias to drug cues is elevated before and during temptations to use heroin and cocaine. Psychopharmacology 2012, 219, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.C.; D’Sa, C.; Kimmerling, A.; Siedlarz, K.M.; Tuit, K.L.; Stowe, R.; Sinha, R. Immune system inflammation in cocaine dependent individuals: Implications for medications development. Hum. Psychopharmacol. 2012, 27, 156–166. [Google Scholar] [CrossRef]
- Li, C.S.R.; Kemp, K.; Milivojevic, V.; Sinha, R. Neuroimaging Study of Sex Differences in the Neuropathology of Cocaine Abuse. Gend. Med. 2005, 2, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Potenza, M.N.; Hong, K.I.A.; Lacadie, C.M.; Fulbright, R.K.; Tuit, K.L.; Sinha, R. Neural correlates of stress-induced and cue-induced drug craving: Influences of sex and cocaine dependence. Am. J. Psychiatry 2012, 169, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Fox, H.C.; Talih, M.; Malison, R.; Anderson, G.M.; Kreek, M.J.; Sinha, R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology 2005, 30, 880–891. [Google Scholar] [CrossRef]
- Sinha, R.; Fox, H.; Hong, K.I.; Sofuoglu, M.; Morgan, P.T.; Bergquist, K.T. Sex Steroid Hormones, Stress Response, and Drug Craving in Cocaine-Dependent Women: Implications for Relapse Susceptibility. Exp. Clin. Psychopharmacol. 2007, 15, 445–452. [Google Scholar] [CrossRef]
- Fox, H.C.; Tuit, K.L.; Sinha, R. Stress system changes associated with marijuana dependence may increase craving for alcohol and cocaine. Hum. Psychopharmacol. 2013, 28, 40–53. [Google Scholar] [CrossRef]
- Smith, K.; Lacadie, C.M.; Milivojevic, V.; Fogelman, N.; Sinha, R. Sex differences in neural responses to stress and drug cues predicts future drug use in individuals with substance use disorder. Drug Alcohol Depend. 2023, 244, 109794. [Google Scholar] [CrossRef]
- Sinha, R.; Fuse, T.; Aubin, L.R.; O’Malley, S.S. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology 2000, 152, 140–148. [Google Scholar] [CrossRef]
- Fox, H.C.; Garcia, M.; Kemp, K.; Milivojevic, V.; Kreek, M.J.; Sinha, R. Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology 2006, 185, 348–357. [Google Scholar] [CrossRef]
- Fox, H.C.; Hong, K.I.A.; Siedlarz, K.; Sinha, R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology 2008, 33, 796–805. [Google Scholar] [CrossRef]
- Sinha, R.; Garcia, M.; Paliwal, P.; Kreek, M.J.; Rounsaville, B.J. Stress-Induced Cocaine Craving and Hypothalamic-Pituitary-Adrenal Responses Are Predictive of Cocaine Relapse Outcomes. Arch. Gen. Psychiatry 2006, 63, 324–331. [Google Scholar] [CrossRef]
- Xu, K.; Seo, D.; Hodgkinson, C.; Hu, Y.; Goldman, D.; Sinha, R. A variant on the kappa opioid receptor gene (OPRK1) is associated with stress response and related drug craving, limbic brain activation and cocaine relapse risk. Transl. Psychiatry 2013, 3, e292. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.E.; Price, J.L.; Leonard, D.; Suris, A.; Kandil, E.; Shaw, M.; Kroener, S.; Brown, E.S.; Adinoff, B. The Efficacy of Lidocaine in Disrupting Cocaine Cue-Induced Memory Reconsolidation. Drug Alcohol Depend. 2020, 212, 108062. [Google Scholar] [CrossRef]
- Sinha, R.; Catapano, D.; O’malley, S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology 1999, 142, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, K.L.; Fox, H.C.; Sinha, R. Self-reports of interoceptive responses during stress and drug cue-related experiences in cocaine- and alcohol-dependent individuals. Exp. Clin. Psychopharmacol. 2010, 18, 229–237. [Google Scholar] [CrossRef]
- Fox, H.C.; Hong, K.-I.A.; Siedlarz, K.M.; Bergquist, K.; Anderson, G.; Kreek, M.J.; Sinha, R. Sex-specific dissociations in autonomic and HPA responses to stress and cues in alcohol-dependent patients with cocaine abuse. Alcohol Alcohol. 2009, 44, 575–585. [Google Scholar] [CrossRef]
- Sinha, R.; Lacadie, C.; Skudlarski, P.; Fulbright, R.K.; Rounsaville, B.J.; Kosten, T.R.; Wexler, B.E. Neural activity associated with stress-induced cocaine craving: A functional magnetic resonance imaging study. Psychopharmacology 2005, 183, 171–180. [Google Scholar] [CrossRef]
- Sinha, R.; Talih, M.; Malison, R.; Cooney, N.; Anderson, G.M.; Kreek, M.J. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology 2003, 170, 62–72. [Google Scholar] [CrossRef]
- Yalachkov, Y.; Kaiser, J.; Naumer, M.J. Sensory and motor aspects of addiction. Behav. Brain Res. 2010, 207, 215–222. [Google Scholar] [CrossRef]
- Weiss, F.; Maldonado-Vlaar, C.S.; Parsons, L.H.; Kerr, T.M.; Smith, D.L.; Ben-Shahar, O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: Effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc. Natl. Acad. Sci. USA 1999, 97, 4321–4326. [Google Scholar] [CrossRef]
- Agarwal, K.; McDuffie, C.; Manza, P.; Joseph, P.V. Taste and smell alterations and substance use disorders. In Sensory Science and Chronic Diseases: Clinical Implications and Disease Management; Springer International Publishing: Cham, Switzerland, 2022; pp. 159–179. [Google Scholar]
- Bilehsavar, S.H.; Batouli, S.A.; Soukhtanlou, M.; Alavi, S.; Oghabian, M. Different Olfactory Perception in Heroin Addicts Using Functional Magnetic Resonance Imaging. Basic. Clin. Neurosci. 2022, 13, 257–268. [Google Scholar] [CrossRef]
- Thaysen-Petersen, D.; Hammerum, S.K.; Düring, S.W.; Larsen, P.V.; Fink-Jensen, A.; Mellentin, A.I. The efficacy of conventional and technology assisted cue exposure therapy for treating substance use disorders: A qualitative systematic review. Front. Psychiatry 2025, 16, 1544763. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).