Uncomfortable Paresthesia and Dysesthesia Following Tonic Spinal Cord Stimulator Implantation
Abstract
1. Introduction
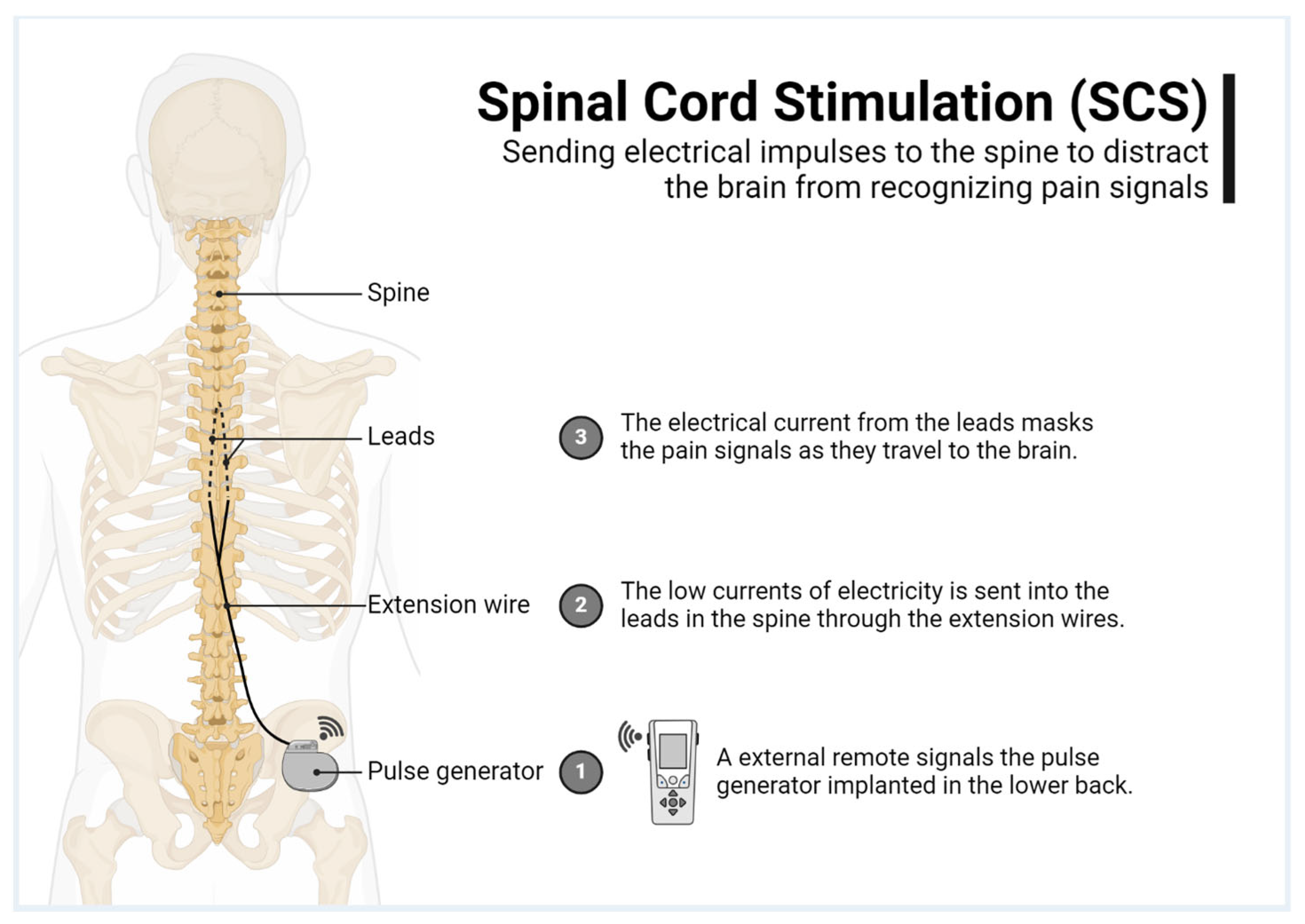
2. Materials and Methods

3. Results
3.1. Demographics
3.2. Effectiveness of SCS Therapy
3.3. Summary of Complications
3.3.1. Need for Revision
3.3.2. Uncomfortable Paresthesia/Dysesthesia
3.3.3. Explantation and Failure of Treatment
3.3.4. Lead Migration
3.3.5. Infection/Seroma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCS | Spinal cord stimulator |
| CRPS | Complex regional pain syndrome |
| PSPS | Persistent spinal pain syndrome |
| NRS | Numerical Rating Scale |
| LBP | Low back pain |
References
- Harmsen, I.E.; Hasanova, D.; Elias, G.J.; Boutet, A.; Neudorfer, C.; Loh, A.; Germann, J.; Lozano, A.M. Trends in clinical trials for spinal cord stimulation. Ster. Funct. Neurosurg. 2021, 99, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Francio, V.; Barndt, B.S.; Davani, S.; Towery, C.; Allen, T.R. Poster 118: Clinical Indications and Guidelines for the Implantation of Spinal Cord Stimulators (SCS) in Pain Management: A Narrative Review for Clinicians. PM&R 2018, 10, S43. [Google Scholar] [CrossRef]
- Kumar, K.; Nath, R.; Wyant, G.M. Treatment of chronic pain by epidural spinal cord stimulation: A 10-year experience. J. Neurosurg. 1991, 75, 402–407. [Google Scholar] [CrossRef]
- Shealy, C.N.; Mortimer, J.T.; Reswick, J.B. Electrical inhibition of pain by stimulation of the dorsal columns: Preliminary clinical report. Anesth. Analg. 1967, 46, 489–491. [Google Scholar] [CrossRef]
- Willis, W.D. The pain system. The neural basis of nociceptive transmission in the mammalian nervous system. Pain Headache 1985, 8, 1–346. [Google Scholar]
- Sdrulla, A.D.; Guan, Y.; Raja, S.N. Spinal cord stimulation: Clinical efficacy and potential mechanisms. Pain. Pract. 2018, 18, 1048–1067. [Google Scholar] [CrossRef]
- Leung, N.; Tsourmas, N.F.; Yuspeh, L.; Kalia, N.; Lavin, R.A.; Tao, X.; Bernacki, E.J. Increased Spinal Cord Stimulator Use and Continued Opioid Treatment Among Injured Workers. J. Occup. Environ. Med. 2020, 62, e436–e441. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, T.; Aner, M.; Sharma, S.; Ghosh, P.; Gill, J.S. Explantation of percutaneous spinal cord stimulator devices: A retrospective descriptive analysis of a single-center 15-year experience. Pain. Med. 2019, 20, 1355–1361. [Google Scholar] [CrossRef]
- Pope, J.E.; Deer, T.R.; Falowski, S.; Provenzano, D.; Hanes, M.; Hayek, S.M.; Amrani, J.; Carlson, J.; Skaribas, I.; Parchuri, K.; et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation Technol. Neural Interface 2017, 20, 543–552. [Google Scholar] [CrossRef]
- Negoita, S.; Duy, P.Q.; Mahajan, U.V.; Anderson, W.S. Timing and prevalence of revision and removal surgeries after spinal cord stimulator implantation. J. Clin. Neurosci. 2019, 62, 80–82. [Google Scholar] [CrossRef]
- Papadopoulos, D.V.; Suk, M.S.; Andreychik, D.; Nikolaou, V.; Haak, M. Rates and Causes of Reoperations Following Spinal Cord Stimulation Within a 2–12 year Period. Glob. Spine J. 2023, 15, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Leplus, A.; Voirin, J.; Cuny, E.; Onno, M.; Billot, M.; Rigoard, P.; Fontaine, D. Is Spinal Cord Stimulation Still Effective After One or More Surgical Revisions? Neuromodulation Technol. Neural Interface 2023, 26, 1102–1108. [Google Scholar] [CrossRef]
- Peeters, J.-B.; Raftopoulos, C. Tonic, burst, high-density, and 10-kHz high-frequency spinal cord stimulation: Efficiency and patients’ preferences in a failed back surgery syndrome predominant population. Review of literature. World Neurosurg. 2020, 144, e331–e340. [Google Scholar] [CrossRef]
- Piedade, G.S.; Gillner, S.; Slotty, P.J.; Vesper, J. Combination of waveforms in modern spinal cord stimulation. Acta Neurochir. 2022, 164, 1187–1191. [Google Scholar] [CrossRef]
- Sinclair, C.; Verrills, P.; Barnard, A. A review of spinal cord stimulation systems for chronic pain. J. Pain. Res. 2016, 9, 481–492. [Google Scholar] [CrossRef]
- Luecke, T.; Edgar, D.; Huse, D. 10 kHz spinal cord stimulation for the treatment of chronic back and/or leg pain: Summary of clinical studies. SAGE Open Med. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Kapural, L.; Yu, C.; Doust, M.W.; Gliner, B.E.; Vallejo, R.; Sitzman, B.T.; Amirdelfan, K.; Morgan, D.M.; Yearwood, T.L.; Bundschu, R.; et al. Comparison of 10-kHz high-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery 2016, 79, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Chan, C.; Cheung, C. Spinal cord stimulation for chronic non-cancer pain: A review of current evidence and practice. Hong Kong Med. J. 2017, 23, 517–523. [Google Scholar] [CrossRef]
- Gill, J.S.; Kohan, L.R.; Hasoon, J.; Urits, I.; Viswanath, O.; Cai, V.L.; Yazdi, C.; Aner, M.M.; Kaye, A.D.; Simopoulos, T.T. A survey on the choice of spinal cord stimulation parameters and implantable pulse generators and on reasons for explantation. Orthop. Rev. 2022, 14, 39648. [Google Scholar] [CrossRef]
- Al-Kaisy, A.; Royds, J.; Al-Kaisy, O.; Palmisani, S.; Pang, D.; Smith, T.; Padfield, N.; Harris, S.; Wesley, S.; Yearwood, T.L.; et al. Explant rates of electrical neuromodulation devices in 1177 patients in a single center over an 11-year period. Reg. Anesth. Pain. Med. 2020, 45, 883–890. [Google Scholar] [CrossRef]
- Deer, T.; Slavin, K.V.; Amirdelfan, K.; North, R.B.; Burton, A.W.; Yearwood, T.L.; Tavel, E.; Staats, P.; Falowski, S.; Pope, J.; et al. Success using neu-romodulation with BURST (SUNBURST) study: Results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation Technol. Neural Interface 2018, 21, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, B.C.; Bendel, M.A.; Deer, T.R.; Eldrige, J.S.; Walega, D.R.; Wang, Z.; Costandi, S.; Azer, G.; Qu, W.; Falowski, S.M.; et al. Spinal Cord Stimulator Implant Infection Rates and Risk Factors: A Multicenter Retrospective Study. Neuromodulation Technol. Neural Interface 2017, 20, 558–562. [Google Scholar] [CrossRef]
- Mekhail, N.; Azer, G.; Saweris, Y.; Mehanny, D.S.; Costandi, S.; Mao, G. The Impact of Tobacco Cigarette Smoking on Spinal Cord Stimulation Effectiveness in Chronic Spine–Related Pain Patients. Reg. Anesth. Pain. Med. 2018, 43, 768–775. [Google Scholar] [CrossRef]
- Mekhail, N.; Costandi, S.; Mehanny, D.S.; Armanyous, S.; Saied, O.; Taco-Vasquez, E.; Saweris, Y. The Impact of Tobacco Smoking on Spinal Cord Stimulation Effectiveness in Complex Regional Pain Syndrome Patients. Neuromodulation Technol. Neural Interface 2020, 23, 133–139. [Google Scholar] [CrossRef]
- Choi, H.; Gaiha, R.; Moeschler, S.M.; Bendel, M.A.; McCormick, Z.L.; Teramoto, M.; Rosenow, J.M.; Kielb, S.; Avram, M.J.; Walega, D.R. Factors Associated with Implantable Pulse Generator Site Pain: A Multicenter Cross-Sectional Study. Neuromodulation Technol. Neural Interface 2021, 24, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Salokangas, R.K.; Vilkman, H.; Ilonen, T.; Taiminen, T.; Bergman, J.; Haaparanta, M.; Solin, O.; Alanen, A.; Syvã«¡Hti, E.; Hietala, J. High levels of dopamine activity in the basal ganglia of cigarette smokers. Am. J. Psychiatry 2000, 157, 632–634. [Google Scholar] [CrossRef]
- Tracy, L.M.; Ioannou, L.; Baker, K.S.; Gibson, S.J.; Georgiou-Karistianis, N.; Giummarra, M.J. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 2016, 157, 7–29. [Google Scholar] [CrossRef]
- Ditre, J.W.; Brandon, T.H.; Zale, E.L.; Meagher, M.M. Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychol. Bull. 2011, 137, 1065–1093. [Google Scholar] [CrossRef] [PubMed]
- Herbsleb, M.; Schulz, S.; Ostermann, S.; Donath, L.; Eisenträger, D.; Puta, C.; Voss, A.; Gabriel, H.W.; Bär, K.-J. The relation of autonomic function to physical fitness in patients suffering from alcohol dependence. Drug Alcohol. Depend. 2013, 132, 505–512. [Google Scholar] [CrossRef]
- De Vita, M.J.; Maisto, S.A.; Ansell, E.B.; Zale, E.L.; Ditre, J.W. Pack-years of tobacco cigarette smoking as a predictor of spontaneous pain reporting and experimental pain reactivity. Exp. Clin. Psychopharmacol. 2019, 27, 552–560. [Google Scholar] [CrossRef]
- Oura, P.; Hautala, A.; Kiviniemi, A.; Auvinen, J.; Puukka, K.; Tulppo, M.; Huikuri, H.; Seppänen, T.; Karppinen, J. Musculoskeletal pains and cardiovascular autonomic function in the general Northern Finnish population. BMC Musculoskelet. Disord. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Goudman, L.; Brouns, R.; Linderoth, B.; Moens, M. Effects of Spinal Cord Stimulation on Heart Rate Variability in Patients with Failed Back Surgery Syndrome: Comparison Between a 2-lead ECG and a Wearable Device. Neuromodulation Technol. Neural Interface 2021, 24, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.P.; Eldabe, S.; Buchser, E.; Johanek, L.M.; Guan, Y.; Linderoth, B. Parameters of Spinal Cord Stimulation and Their Role in Electrical Charge Delivery: A Review. Neuromodulation Technol. Neural Interface 2016, 19, 373–384. [Google Scholar] [CrossRef]
- Butler, B.; Acosta, G.; Shi, R. Exogenous Acrolein intensifies sensory hypersensitivity after spinal cord injury in rat. J. Neurol. Sci. 2017, 379, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lalkhen, A.G.; Chincholkar, M.; Patel, J. Microbiological Evaluation of the Extension Wire and Percutaneous Epidural Lead Anchor Site Following a “2-Stage Cut-Down” Spinal Cord Stimulator Procedure. Pain Pract. 2017, 17, 886–891. [Google Scholar] [CrossRef]
- Falowski, S.M.; Provenzano, D.A.; Xia, Y.; Doth, A.H. Spinal Cord Stimulation Infection Rate and Risk Factors: Results From a United States Payer Database. Neuromodulation Technol. Neural Interface 2019, 22, 279–289. [Google Scholar] [CrossRef]
- Mekhail, N. Where does the balance lie between doing what’s right for our patients and patients’ rights? Spinal cord stimulation in chronic pain smokers. Reg. Anesth. Pain. Med. 2019, 44, 421–422. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D. Should spinal cord stimulation be abandoned in smoking patients with chronic pain? Reg. Anesth. Pain. Med. 2019, 44, 420–421. [Google Scholar] [CrossRef]



| Population Characteristics (n = 103) | |
|---|---|
| Age (years) | |
| (Mean, SD) | 55.48 ± 13.08 |
| Sex | |
| Male | 34 (33%) |
| Female | 69 (67%) |
| Occupation | |
| Working | 36 (34.95%) |
| Disability | 23 (22.33%) |
| Modified Duty | 6 (5.83%) |
| Retired | 23 (22.33%) |
| Unemployed | 15 (14.56%) |
| Indication of SCS | |
| PSPS | 39 (37.86%) |
| CRPS | 14 (13.60%) |
| LBP | 35 (34%) |
| Other | 15 (14.54%) |
| SCS implantation region | |
| Cervical | 9 (8.74%) |
| Upper thoracic | 4 (3.88%) |
| Lower thoracic | 80 (77.67%) |
| Other | 10 (9.71%) |
| Months of follow up | |
| (Median, IQR) | 20 (28) |
| Complications | Active Tobacco Users (n = 30) | Never Tobacco Users (n = 61) | Former Tobacco Users (n = 12) | Total (n =103) | p Value |
|---|---|---|---|---|---|
| Infection | 1 (3.33%) | 2 (3.27%) | 1 (8.33%) | 6 (5.82%) | 0.69 |
| Lead Migration | 5 (16.67%) | 3 (4.91%) | 1 (8.33%) | 9 (8.73%) | 0.17 |
| Revision | 16 (53.33%) | 16 (26.22%) | 4 (33.33%) | 36 (34.95%) | 0.03 |
| Explantation | 6 (20%) | 2 (3.27%) | 5 (41.67%) | 13 (12.62%) | 0.09 |
| Dysesthesia/Paresthesia | 10 (33.33%) | 4 (6.55%) | 3 (25%) | 17 (27.86%) | 0.04 |
| Loss of Effect | 2 (6.66%) | 0 | 2 (16.67%) | 4 (3.88%) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethi, Z.; Aijaz, T.; Ortega-Camacho, A.; Nasr, N.F.; Knezevic, N.N. Uncomfortable Paresthesia and Dysesthesia Following Tonic Spinal Cord Stimulator Implantation. Brain Sci. 2025, 15, 616. https://doi.org/10.3390/brainsci15060616
Sethi Z, Aijaz T, Ortega-Camacho A, Nasr NF, Knezevic NN. Uncomfortable Paresthesia and Dysesthesia Following Tonic Spinal Cord Stimulator Implantation. Brain Sciences. 2025; 15(6):616. https://doi.org/10.3390/brainsci15060616
Chicago/Turabian StyleSethi, Zubin, Tabish Aijaz, Alvaro Ortega-Camacho, Ned F. Nasr, and Nebojsa Nick Knezevic. 2025. "Uncomfortable Paresthesia and Dysesthesia Following Tonic Spinal Cord Stimulator Implantation" Brain Sciences 15, no. 6: 616. https://doi.org/10.3390/brainsci15060616
APA StyleSethi, Z., Aijaz, T., Ortega-Camacho, A., Nasr, N. F., & Knezevic, N. N. (2025). Uncomfortable Paresthesia and Dysesthesia Following Tonic Spinal Cord Stimulator Implantation. Brain Sciences, 15(6), 616. https://doi.org/10.3390/brainsci15060616






